What is Tautomer?
- A tautomer refers to a specific type of structural isomerism found in chemical compounds, wherein the isomers can readily interconvert through a chemical reaction known as tautomerization. This phenomenon involves the relocation of a hydrogen atom within the compound, resulting in two distinct chemical species that possess different atomic connectivities, molecular geometries, and physicochemical and spectroscopic properties.
- It is important to note that tautomers should not be confused with the concept of “contributing structures” in chemical resonance. While resonance forms are alternative depictions of a single chemical species, tautomers are genuinely different chemical entities with their own unique characteristics. The true structure of a molecule exhibiting resonance is a quantum superposition, representing an average of the idealized, hypothetical geometries depicted by the contributing structures.
- Tautomerism finds relevance in various areas of chemistry and biology, particularly in relation to amino acids and nucleic acids. These molecules are fundamental building blocks of life and display tautomeric behavior, which impacts their reactivity, stability, and overall function. Understanding tautomerism is crucial for comprehending the complex behavior of these biomolecules and their role in biochemical processes.
- The interconversion between tautomers can occur spontaneously or be induced by external factors such as temperature, pH, or the presence of specific catalysts. The equilibrium between tautomers is governed by the relative stability of each form, determined by factors such as electronic effects, steric hindrance, and intramolecular interactions. In some cases, one tautomer may be significantly favored over the other, resulting in a dominant form under specific conditions.
- The study of tautomerism involves experimental techniques and theoretical calculations to determine the relative energies and properties of different tautomeric forms. Spectroscopic methods, such as nuclear magnetic resonance (NMR) spectroscopy and infrared spectroscopy, are often employed to identify and characterize tautomers based on their distinct spectral signatures.
- In summary, tautomers are a type of isomer that exhibit the ability to interconvert through tautomerization. They are distinct chemical species with differing atomic connectivities, molecular geometries, and physicochemical properties. Tautomerism plays a significant role in the behavior of biomolecules and is an essential concept in understanding the complexity of chemical and biological systems.
Tautomer definition
A tautomer is a type of isomer that exists in multiple interconvertible structures, differing in the relative position of a specific atomic nucleus, typically hydrogen.
What Is Tautomerism?
- Tautomerism is a fascinating phenomenon observed in certain chemical compounds where a single molecule can exist in multiple interconvertible structures known as tautomers. These tautomers differ in the relative position of one atomic nucleus, typically hydrogen. The primary distinction between tautomers lies in the arrangement of electrons and protons within the molecule. Tautomeric compounds exist in a state of dynamic equilibrium, constantly interconverting between their different forms.
- The interconversion of tautomers usually involves the transfer of protons. In the presence of an acid catalyst, the process begins with protonation, where a hydrogen ion attaches to a specific location within the molecule, resulting in the formation of a cation. Subsequently, deprotonation occurs in an adjacent position to the cation, leading to the conversion of the molecule into a different tautomeric form.
- On the other hand, when a base catalyst is present, deprotonation takes place as the initial step. This involves the removal of a proton from a specific site within the molecule. Instead of cation delocalization as observed in acid-catalyzed tautomerism, anion delocalization occurs in base-catalyzed tautomerism. The resulting anion undergoes further transformations, ultimately leading to protonation at a different position, resulting in the formation of a distinct tautomeric structure.
- Tautomerism is also referred to as desmotropism, highlighting the dynamic nature of these isomeric compounds. The equilibrium between tautomeric forms can be influenced by various factors, such as temperature, pH, and the presence of catalysts. The relative stability of each tautomer plays a crucial role in determining its prevalence under specific conditions.
- Understanding tautomerism is essential in fields such as organic chemistry, biochemistry, and medicinal chemistry. It has significant implications for the reactivity, properties, and behavior of molecules in these disciplines. Researchers utilize various experimental techniques and theoretical calculations to study and characterize tautomeric systems. Spectroscopic methods, such as nuclear magnetic resonance (NMR) spectroscopy and infrared spectroscopy, can provide valuable insights into the identification and analysis of tautomers based on their unique spectral signatures.
- In summary, tautomerism is a phenomenon where a single chemical compound can exist in multiple interconvertible structures known as tautomers. These isomers differ in the relative position of a specific atomic nucleus, often hydrogen. Tautomeric compounds exhibit dynamic equilibrium and undergo proton transfer during interconversion. The presence of acid or base catalysts can facilitate these transformations. Understanding tautomerism is essential for comprehending the behavior and properties of molecules in various scientific fields.
Structural Requirement of Tautomerism
Tautomerism, the phenomenon of interconversion between different isomeric forms of a compound, has specific structural requirements. Here are the key factors that influence tautomerism:
- Presence of Polar Molecules and Weakly Acidic Functional Groups: Tautomerism is commonly observed in compounds that contain polar molecules and functional groups with weak acidity. These functional groups may include carbonyl groups (such as ketones and aldehydes), hydroxyl groups, and amino groups. The presence of such groups facilitates the formation and transfer of protons during tautomerization.
- Change in the Position of an Atom: Tautomerism involves a change in the relative position of an atom within the molecule. The most common atom that undergoes positional change is hydrogen. In tautomeric transformations, a hydrogen atom typically shifts between different atoms, such as carbon, oxygen, or nitrogen, leading to the interconversion of tautomeric forms.
- Minimal Effects on Bond Length and Structural Features: Tautomerism does not significantly affect bond lengths or other structural features of the molecule. The interconversion between tautomeric forms primarily involves the rearrangement of bonds and the movement of protons. The overall molecular structure and bond lengths remain relatively unchanged during this process.
- Planar or Non-Planar Molecules: Tautomerism can occur in both planar and non-planar molecules, although it is more commonly observed in planar systems. The planarity or non-planarity of a molecule depends on its molecular geometry, which is influenced by factors such as bond angles and steric interactions. The presence of conjugated systems or delocalized electron density often contributes to the planarity of tautomeric compounds.
By fulfilling these structural requirements, a compound can exhibit tautomeric behavior, allowing for the interconversion between different isomeric forms. Tautomerism plays a significant role in various chemical processes, including enzymatic reactions, organic synthesis, and the behavior of biomolecules such as amino acids and nucleic acids. Understanding the structural aspects of tautomerism is essential for elucidating the reactivity and properties of compounds undergoing tautomeric transformations.
Types of Tautomerism
Tautomerism refers to the phenomenon of a chemical compound existing in multiple interconvertible forms. Here are some of the common types of tautomerism:
- Keto-Enol Tautomerism: This is the most important and widely studied type of tautomerism. In keto-enol tautomerism, one form is a ketone (keto) and the other is an enol. The interconversion between these two forms involves the migration of a hydrogen atom and a double bond. Acid or base catalysts can facilitate this conversion, which is known as enolization.
- Prototropy: Prototropy is a type of tautomerism that occurs due to the acid-base behavior of the compound. The two tautomeric forms differ only in the position of a proton. Both forms have the same empirical formula and overall charge.
- Annular Tautomerism: Annular tautomerism occurs when a proton can occupy multiple positions within a heterocyclic system. This type of tautomerism involves the conversion of an open structure to a ring structure. When this conversion takes place, it is referred to as ring-chain tautomerism. An example of annular tautomerism is seen in glucose, which can exist as ring-chain tautomers.
- Valence Tautomerism: Valence tautomerism is characterized by the continuous formation and breaking of single and double bonds within a compound, without any migration of groups or atoms. It differs from other types of tautomerism in that there is a change in the geometrical structure, but no change in the canonical resonance structure or mesomers. Valence tautomers undergo rapid interconversion.
These are just a few examples of the types of tautomerism observed in chemical compounds. Tautomerism plays a crucial role in understanding the reactivity, properties, and behavior of molecules in various fields of chemistry. The ability of a compound to exist in different tautomeric forms significantly influences its chemical behavior and biological activity.
Prototropy
- Prototropy is a common form of tautomerism that involves the relocation of a hydrogen atom within a molecule. It is considered a subset of acid-base behavior, where tautomers are sets of isomeric protonation states with the same empirical formula and total charge.
- Prototropic tautomerism can occur through two catalytic processes: with bases and with acids. In the case of bases, the mechanism involves several steps. Firstly, deprotonation occurs, leading to the formation of a delocalized anion, such as an enolate. Then, protonation takes place at a different position of the anion, resulting in the formation of a different tautomeric form. On the other hand, in the presence of acids, the mechanism follows a series of steps starting with protonation, followed by the formation of a delocalized cation, and finally deprotonation at a different position adjacent to the cation.
- One example of prototropy is seen in the molecule glucose, which can exist in both a straight-chain form and a ring form (typically in pyranose or furanose forms). The conversion between these forms involves the movement of a proton and a simultaneous change from an open chain to a cyclic structure. This tautomeric shift can be described as H−O ⋅ C=O ⇌ O−C−O−H, where the “⋅” indicates the initial absence of a bond.
- Within prototropic tautomerism, there are two specific subcategories: annular tautomerism and ring-chain tautomers. Annular tautomerism occurs when a proton can occupy multiple positions within heterocyclic systems found in certain drugs, such as imidazole or triazole. Ring-chain tautomers, on the other hand, involve the movement of a proton accompanied by a transformation from an open chain to a cyclic structure, as observed in the interconversion between straight-chain and cyclic hemiacetal forms of sugars.
- In summary, prototropy in tautomerism involves the relocation of a hydrogen atom and can be catalyzed by bases or acids. It plays a role in various chemical reactions and structural transformations, including the conversion between different forms of molecules like glucose and the interconversion of open chain and cyclic structures.
Catalization of Tautomerization is catalyzed
Tautomerization is catalyzed by the presence of either bases or acids, depending on the specific reaction conditions. The mechanism of tautomerization can be described in two scenarios based on the type of catalyst involved.
In the presence of bases, the tautomerization mechanism involves the following steps:
- Deprotonation: A base catalyst removes a proton from a specific site within the molecule, resulting in the formation of a negatively charged species or an anion.
- Formation of an anion delocalized: The negative charge or anion formed in the previous step undergoes delocalization, spreading the charge over multiple atoms in the molecule.
- Protonation at a different position of the anion: The anion, with its charge delocalized, can now be protonated at a different location within the molecule. This protonation event leads to the formation of a different tautomeric structure.
On the other hand, in the presence of acids, the tautomerization mechanism follows a different pathway:
- Protonation: An acid catalyst donates a proton to a specific site within the molecule, resulting in the formation of a positively charged species or a cation.
- Formation of a cation delocalized: The positive charge or cation formed in the previous step undergoes delocalization, spreading the charge over multiple atoms in the molecule.
- Deprotonation at a different position of the cation: The cation, with its charge delocalized, can now undergo deprotonation at a different location within the molecule. This deprotonation event leads to the formation of a different tautomeric structure.
In both cases, the presence of a catalyst, either a base or an acid, facilitates the interconversion between tautomeric forms by providing the necessary conditions for proton transfer. The specific choice of base or acid as the catalyst depends on the reaction conditions and the nature of the molecule undergoing tautomerization.
Tautomerism in Non-Carbonyl Compounds
Tautomerism, the interconversion between different isomeric forms, is not limited to carbonyl compounds. Many non-carbonyl systems also exhibit tautomeric behavior, where different tautomers coexist in a mixture. Here are a few examples of tautomerism in non-carbonyl compounds:
- Tautomerism in Nitroso and Nitro Compounds: Nitroso compounds (-N=O) and nitro compounds (-NO2) can exist as tautomers. In the case of nitroso compounds, tautomerism involves the migration of a hydrogen atom and the rearrangement of bonds. For example, nitrosobenzene (C6H5NO) can undergo tautomerism to form its oxime tautomer (C6H5N(OH)).
- Tautomerism in Hydrazones and Azo Compounds: Hydrazones (-NHN=) and azo compounds (-N=N-) can exhibit tautomeric behavior. In hydrazones, tautomerism involves the migration of a hydrogen atom between the nitrogen atoms. Azo compounds can undergo tautomerism by the rearrangement of bonds and the movement of double bonds. These tautomeric interconversions can have implications for their reactivity and color properties.
- Tautomerism in Imidazole and Imidazole Derivatives: Imidazole, a five-membered heterocyclic compound, can exist in different tautomeric forms. Tautomerism in imidazole involves the movement of protons between nitrogen atoms and the rearrangement of bonds. The tautomeric equilibrium between different forms of imidazole is important for its biological activity and involvement in biochemical processes.
- Tautomerism in Enamines: Enamines, formed by the reaction of aldehydes or ketones with secondary amines, can undergo tautomeric interconversions. The tautomerism in enamines involves the migration of a hydrogen atom between the carbon and nitrogen atoms, resulting in different resonance structures. Enamines play a significant role in various synthetic transformations and can exhibit different reactivity depending on their tautomeric forms.
These examples highlight that tautomerism is not exclusive to carbonyl compounds but can occur in a variety of non-carbonyl systems. The interconversion between different tautomeric forms in these compounds can have implications for their chemical reactivity, physical properties, and biological activity. Understanding tautomerism in non-carbonyl compounds is essential for comprehensive studies in organic chemistry and related fields.

Tautomeric Form of Unsymmetrical Ketones
In the case of unsymmetrical ketones, which are ketones with different substituents on each side of the carbonyl group, there can exist two tautomeric forms. The tautomeric interconversion involves the movement of a hydrogen atom and a double bond within the molecule.
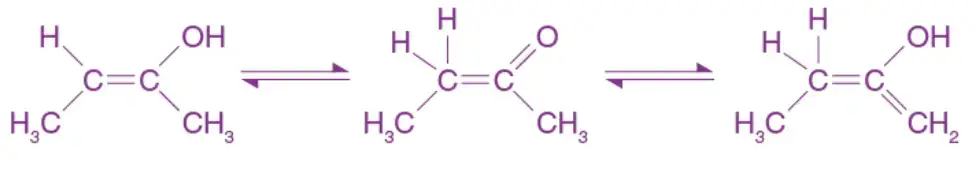
For unsymmetrical ketones, the two tautomeric forms differ in the position of the hydrogen atom attached to the carbonyl carbon. The movement of the hydrogen atom can occur either towards the more electronegative substituent (denoted as form A) or towards the less electronegative substituent (denoted as form B). This results in the shifting of the double bond to different positions within the molecule.
The tautomeric forms of unsymmetrical ketones can be represented as follows:
Form A: R1-C(=O)-R2 ⇌ R1=C(-H)-R2
Form B: R1-C(=O)-R2 ⇌ R1-C(-H)=R2
In form A, the hydrogen atom migrates towards the more electronegative substituent, while in form B, it migrates towards the less electronegative substituent. The tautomeric equilibrium between these forms is established, and the relative stability of each form depends on factors such as the nature of substituents, electronic effects, and steric hindrance.
The presence of different tautomeric forms of unsymmetrical ketones can have implications for their reactivity, chemical properties, and biological activity. Each tautomeric form may exhibit different characteristics and may participate in different reactions or interactions. The equilibrium between the tautomeric forms can be influenced by external factors such as temperature, solvent, and the presence of catalysts.
Understanding the tautomeric forms of unsymmetrical ketones is essential for studying their behavior and for designing strategies in organic synthesis, drug discovery, and other chemical applications.
Mechanism of Tautomerism Reaction
The mechanism of tautomerism reaction, specifically focusing on the acid-catalyzed keto-enol tautomerization, involves a two-step process in an aqueous solution of acid. Here is a breakdown of the mechanism:
Step 1: Protonation of the Carbonyl Oxygen In the presence of an acid catalyst, such as hydronium ion (H3O+), the carbonyl oxygen of the keto form is protonated. This protonation is facilitated by the electrophilic nature of the hydronium ion. The resulting species is an oxonium ion.
Step 2: Deprotonation and Formation of Enol In the second step, deprotonation occurs at the alpha carbon atom, which is the carbon atom adjacent to the carbonyl group. This deprotonation is favored due to the electron-withdrawing effect of the carbonyl group and the resonance stabilization of the resulting enolate anion. The deprotonation is followed by the migration of electrons, resulting in the formation of the enol tautomer.
Overall, the mechanism can be summarized as follows:
Keto Form (Ketone) ⇌ Enol Form (Enol)
The acid-catalyzed mechanism described above highlights the importance of an alpha hydrogen atom for tautomerism to occur efficiently. The presence of an alpha hydrogen atom allows for the protonation and subsequent deprotonation steps in the mechanism. In the absence of an alpha hydrogen atom, the tautomerism process can be significantly slower.
Additionally, it is important to adhere to Markovnikov’s rule for the addition of protons during the mechanism. The electrophilic nature of the hydronium ion leads to the donation of electrons from the double bond, following the principle of Markovnikov’s rule.
The understanding of the mechanism of tautomerism reactions, including the acid-catalyzed keto-enol tautomerization, provides insights into the factors that influence the equilibrium between different tautomeric forms and the conditions under which the interconversion takes place.

What is Tautomeric shift in Purine or Pyrimidine base?
Tautomeric shifts in purine or pyrimidine bases refer to the movement of hydrogen atoms within the structure of these nucleotide bases. These shifts involve the migration of protons between different positions in the base, leading to the formation of tautomeric forms. This phenomenon was recognized by Watson and Crick, who discovered that the bases in DNA are not static but can undergo dynamic changes.
Tautomeric shifts occur due to the rearrangement of atoms and the redistribution of electrons within the base molecule. As a result of these shifts, different tautomeric forms of the bases can exist, which differ in the position of hydrogen atoms. This chemical fluctuation can impact the hydrogen bonding and base pairing interactions within DNA.
The tautomeric shifts in purine and pyrimidine bases have important implications for DNA replication, transcription, and mutation. During DNA replication, if a tautomeric shift occurs in one of the template strands, it can lead to the incorporation of an incorrect nucleotide during DNA synthesis. This can result in a mutation in the newly synthesized DNA strand.
Furthermore, tautomeric shifts can affect base pairing interactions between complementary bases. For example, in the case of adenine (purine base), a tautomeric shift can lead to the formation of the rare imino form, which can pair with cytosine instead of thymine. This can introduce base mismatches and potentially alter the genetic code.
Overall, the occurrence of tautomeric shifts in purine and pyrimidine bases adds an element of chemical dynamics to the structure of DNA. These shifts contribute to the overall complexity and potential variability in DNA structure and function, playing a role in processes such as mutation and genetic diversity.
How tautomeric shift in a base in DNA may lead to mutation?
Tautomeric shifts in the bases of DNA can potentially lead to mutations. Tautomeric forms of bases are rare and less stable compared to their more common forms. These rare tautomeric forms exist only for very short periods of time.
During DNA replication or when a base is being incorporated into a growing DNA chain, if a base happens to be in its rare tautomeric form, it can pair with an incorrect complementary base. For example, the rare imino or enol forms of bases can pair with the wrong bases, such as adenine pairing with cytosine or guanine pairing with thymine.
When such mispairing occurs, the subsequent replication of the DNA strand leads to the incorporation of the mismatched base pair. This results in a base pair substitution, where an adenine-thymine (A-T) base pair may be replaced by a guanine-cytosine (G-C) base pair, or vice versa.
The consequence of this mutation is that during subsequent DNA replication or transcription, the incorrect base pairing will be perpetuated, leading to a change in the genetic code. This mutation can alter the sequence of amino acids in a protein or disrupt regulatory elements, potentially affecting the structure and function of the resulting protein or regulatory processes.
Overall, tautomeric shifts in the bases of DNA provide an opportunity for incorrect base pairing, which can introduce mutations during DNA replication. These mutations can have varying effects on the genetic information encoded in DNA and may contribute to genetic diversity, disease development, or other biological outcomes.
Examples of tautomers
Tautomeric transformations are commonly observed in organic chemistry, particularly in polar molecules and ions containing weakly acidic functional groups. Many examples of tautomers exist in pairs, where hydrogen can occupy different positions, often involving the exchange with a double bond. Some notable examples of tautomeric pairs include:
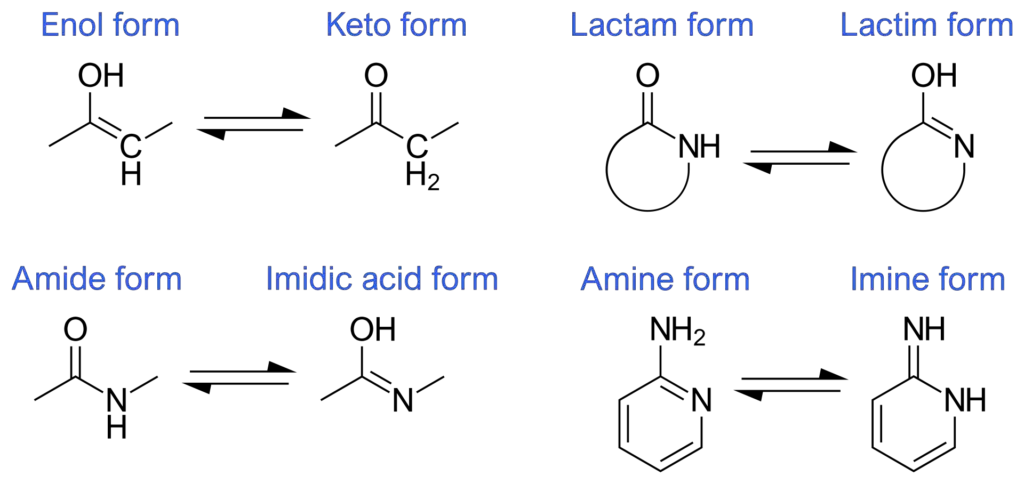
- Ketone – Enol: In keto-enol tautomerism, the hydrogen shifts between the carbonyl oxygen and a carbon atom adjacent to the carbonyl group. The tautomeric forms can be represented as H-O-C=C ⇌ O=C-C-H.
- Enamine – Imine: Tautomerism can occur between an enamine, which has a nitrogen atom bonded to a carbon-carbon double bond, and an imine, where the nitrogen is part of a carbon-nitrogen double bond. The tautomeric transformation can be depicted as H-N-C=C ⇌ N=C-C-H.
- Cyanamide – Carbodiimide: Cyanamide and carbodiimide can exist as tautomeric forms. Cyanamide contains a nitrogen atom bonded to a carbon atom and an amino group, while carbodiimide has a carbon-nitrogen double bond and two nitrogen atoms. The tautomeric interconversion is possible between these two forms.
- Guanidine – Guanidine – Guanidine: Guanidine is a molecule with a central carbon atom surrounded by three nitrogen atoms. It can undergo tautomeric transformations in three different orientations, involving the repositioning of the hydrogen atoms attached to the nitrogen atoms.
- Amide – Imidic Acid: Tautomeric conversion can occur between an amide, where a nitrogen atom is bonded to a carbonyl carbon, and an imidic acid, which has a carbon-nitrogen double bond and a hydroxyl group. The transformation can be represented as H-N-C=O ⇌ N=C-O-H.
- Lactam – Lactim: Lactam and lactim refer to the cyclic forms of amide-imidic acid tautomeric interconversion. This phenomenon is observed in compounds such as 2-pyridone and nucleobases like guanine, thymine, and cytosine.

These are just a few examples among the wide variety of tautomeric pairs that exist in organic chemistry. Tautomers play a significant role in chemical reactions, molecular structure, and the behavior of functional groups. Understanding tautomerism is crucial for grasping the reactivity and properties of these compounds.
Tautomerism vs Resonance
Tautomerism and resonance are both concepts in chemistry that involve the interconversion of different forms of a compound. However, they differ in their underlying principles and effects on the structure and stability of molecules.
Tautomerism refers to the presence of two or more forms of the same compound that can interconvert through the relocation of a proton. Tautomers exist in equilibrium with each other and can be obtained by the movement of a proton and associated electrons. Tautomerism does not affect the stability of the molecule or the bond lengths. It is a real phenomenon, meaning that tautomers exist in reality. An example of tautomerism is the keto-enol tautomerism observed in compounds containing a carbonyl group.
On the other hand, resonance is a chemical concept that describes the interaction between the lone pair of electrons and the bond pair of electrons in a compound. It involves the existence of several forms, called resonance structures, that contribute to the actual structure of a molecule. Resonance structures are not in equilibrium with each other and are hypothetical representations rather than real entities. The relocation of electrons, both in bond pairs and lone pairs, leads to the formation of resonance structures. Resonance has a profound effect on the stability of the molecule, and it affects the bond lengths. For example, in benzene, the delocalization of pi electrons across the ring contributes to its stability and equal bond lengths.
In summary, tautomerism involves the interconversion of different forms of a compound through proton relocation, while resonance describes the interaction of electrons in a molecule, leading to the existence of multiple resonance structures. Tautomerism exists in equilibrium, while resonance structures do not. Tautomerism has no effect on the stability or bond lengths of the molecule, whereas resonance significantly impacts the stability and bond lengths.
| Aspect | Tautomerism | Resonance |
|---|---|---|
| Definition | Interconversion of compounds via proton relocation | Interaction of electrons in a compound |
| Forms | Two or more forms of the same compound | Several forms (resonance structures) |
| Equilibrium | Tautomers exist in equilibrium with each other | Resonance structures do not exist in equilibrium |
| Interconversion | Relocation of a proton (and associated electrons) | Relocation of bond electrons and lone pair electrons |
| Stability | No effect on the stability of the molecule | Profound effect on the stability of the molecule |
| Bond Lengths | No effect on bond lengths | Affects bond lengths (single bond shortened, double bond lengthened) |
| Existence | Tautomers exist in reality | Resonance structures are hypothetical |
| Example | Keto-enol tautomerism in compounds with a carbonyl group | Benzene’s resonance delocalization of pi electrons |
Applications of tautomerism in various fields
Tautomerism, with its ability to interconvert between different forms, has various applications across different fields. Here are some notable applications of tautomerism:
- Pharmaceutical Industry: Tautomerism plays a crucial role in drug design and development. Understanding the different tautomeric forms of a molecule helps in optimizing drug potency, stability, and selectivity. It enables medicinal chemists to design drugs that can selectively target specific biological receptors or enzymes.
- Organic Synthesis: Tautomeric transformations are commonly employed in organic synthesis to access specific chemical intermediates or functional groups. Reversible tautomerization reactions can facilitate the formation of desired compounds, allowing chemists to access diverse molecular structures.
- Catalysis: Tautomeric shifts can serve as key steps in catalytic reactions. Enzymes, for example, often utilize tautomerization processes to facilitate biochemical reactions and promote efficient catalysis. Understanding the tautomeric behavior of catalytic intermediates can aid in the design of more effective catalysts for various chemical processes.
- Material Science: Tautomerism can influence the properties of materials and polymers. By manipulating the tautomeric equilibrium, it is possible to modulate the optical, electronic, and mechanical properties of materials. This has implications in fields such as optoelectronics, sensing, and nanotechnology.
- DNA Mutations: Tautomeric shifts in DNA bases can lead to mutation events. The rare tautomeric forms of bases can result in mismatched base pairing during DNA replication, leading to genetic mutations. Understanding the role of tautomerism in DNA helps in studying mutagenesis and genetic diseases.
- Spectroscopy: Tautomerism can affect the spectroscopic properties of molecules. Different tautomeric forms can exhibit distinct absorption or emission spectra, which can be utilized for analytical purposes. Spectroscopic techniques such as UV-visible spectroscopy and fluorescence spectroscopy can provide insights into tautomeric equilibria and molecular structure.
- Supramolecular Chemistry: Tautomerism can influence the formation of supramolecular assemblies and molecular recognition events. The dynamic nature of tautomeric interconversion allows for reversible host-guest interactions, which are important in the design of functional materials, molecular sensors, and drug delivery systems.
These are just a few examples of the diverse applications of tautomerism in various fields. The ability of compounds to exist in multiple tautomeric forms adds versatility and functionality, making tautomerism an important concept in many areas of science and technology.
FAQ
What do you mean by the term tautomer?
Tautomer refers to one of the two or more isomeric forms of a chemical compound that readily interconvert with each other. These isomers differ in the relative position of certain atoms, typically a hydrogen atom.
Explain the process of tautomerization.
The process of tautomerization involves the interconversion between different tautomeric forms of a compound. It occurs due to the migration of atoms or groups within the molecule, often accompanied by the transfer of a proton. Tautomerization is a dynamic process that can be influenced by various factors such as pH, temperature, and the presence of catalysts.
What are the structural requirements of a compound to possess tautomerization?
Structural requirements for a compound to exhibit tautomerization include:
Presence of polar functional groups, such as carbonyl (C=O), hydroxyl (OH), or amino (NH2) groups.
Possession of at least one hydrogen atom attached to an atom adjacent to the functional group.
Planar or non-planar molecular structure that allows for the necessary rearrangements.
Explain the mechanism of tautomerism in a step-by-step procedure with examples.
The mechanism of tautomerism can vary depending on the specific type of tautomeric transformation. However, a general step-by-step procedure can be outlined: a) Protonation: The compound undergoes protonation, typically at a functional group such as a carbonyl oxygen or an amino nitrogen, facilitated by an acid catalyst. b) Migration: The protonated form undergoes a rearrangement where atoms or groups shift positions, often involving the migration of a proton. c) Deprotonation: The rearranged intermediate is deprotonated, leading to the formation of a different tautomeric form.
Example: Keto-enol tautomerism involving the interconversion between a ketone (keto form) and an enol (enol form).
Which are the different types of tautomerism? How are they classified? Explain.
Different types of tautomerism include keto-enol tautomerism, prototropic tautomerism, ring-chain tautomerism, valence tautomerism, and annular tautomerism. They are classified based on the specific rearrangements and transformations occurring within the compound.
What is meant by annular tautomerism?
Annular tautomerism refers to a type of tautomerism that occurs in heterocyclic systems where a proton can occupy different positions within the ring structure. The delocalization of the proton leads to the interconversion between different tautomeric forms.
How is the ring-chain tautomerism obtained?
Ring-chain tautomerism involves the transformation of an open-chain structure to a cyclic (ring) structure, or vice versa. It occurs when the rearrangement of atoms or groups leads to the formation of a ring or the breaking of a ring.
Protonation and deprotonation are the two-essential processes in tautomerism. Justify.
Protonation and deprotonation are essential processes in tautomerism. Protonation involves the addition of a proton to a specific atom or group, while deprotonation involves the removal of a proton. These processes facilitate the rearrangement and interconversion of tautomeric forms.
What is valence tautomerism?
Valence tautomerism is a type of tautomerism where there is continuous formation and breaking of single and double bonds within a compound. It occurs without the migration of atoms or groups.
Explain the tautomerism related to the acid-base behaviour of the molecule.
Tautomerism related to the acid-base behavior of a molecule refers to the interconversion of tautomeric forms through protonation and deprotonation reactions. These reactions are influenced by the presence of acids or bases as catalysts, which facilitate the transfer of protons between different sites in the molecule.
Give some examples of tautomerism, ring-chain tautomerism, and annular tautomerism.
Examples of tautomerism include:
Keto-enol tautomerism: Acetone (keto form) ⇌ Enol form
Ring-chain tautomerism: Glucose (open-chain form) ⇌ Glucose (cyclic form)
Annular tautomerism: Pyrazole (multiple tautomers due to proton positions)
How does tautomerism depend on the catalyzation process? Which is the type of such a process? Explain briefly.
Tautomerism depends on catalyzation processes such as acid or base catalysis. Acid catalysis involves the presence of an acid catalyst that facilitates proton transfer, while base catalysis involves the presence of a base catalyst that promotes deprotonation reactions. These catalytic processes enhance the rate of tautomerization.
Write the importance of the alpha carbon atom in tautomerism.
The alpha carbon atom is important in tautomerism because it is the carbon atom adjacent to the functional group undergoing tautomerization. The presence of at least one hydrogen atom on the alpha carbon atom is crucial for the migration of the proton during the tautomeric transformation.
List the applications of tautomerism in various fields.
Applications of tautomerism in various fields include drug design and development, organic synthesis, understanding enzyme mechanisms, studying DNA base pair tautomerism and mutations, and exploring the reactivity and properties of chemical compounds.
Explain the stability of the keto-enol form of tautomer.
The stability of the keto-enol form of tautomer is influenced by several factors. The keto form is generally more stable than the enol form due to factors such as the presence of a double bond, resonance stabilization, and the absence of a labile hydrogen atom. The keto-enol equilibrium is often shifted towards the keto form, especially in neutral or acidic conditions.