The Sulfur Reduction Test is the biochemical procedure that is used to detect whether a microorganism is capable of reducing the sulfur-containing compounds to produce hydrogen sulfide (H₂S). It is the process which mainly helps in identifying members of Enterobacteriaceae where some bacteria like Salmonella, Proteus and Citrobacter are able to form the gas during their metabolic activity. It is also used in examining the fecal contamination of water because the organisms associated with pollution can release H₂S.
It is the principle that certain enzymes such as cysteine desulfurase or thiosulfate reductase act on the sulfur sources present in the medium. These are either organic amino acids like cysteine and methionine or inorganic compounds like sodium thiosulfate and sulfites. When these substrates are broken down, hydrogen sulfide gas is released which is colorless but it reacts with the heavy metal indicators in the medium. The reaction is as follows– H₂S combines with ferrous ions or lead ions forming the insoluble black precipitate (FeS or lead sulfide). It is this black coloration that indicates the presence of sulfur reduction.
The common media used in this test are SIM medium, Triple Sugar Iron (TSI) agar and Kligler’s Iron Agar (KIA) along with lead acetate paper in some cases. Among the tube media, SIM is considered more sensitive because absence of sucrose prevents the suppression of the metabolic pathway responsible for H₂S production. In this step, the organism is inoculated in the medium and incubated under suitable conditions. If the organism reduces sulfur then blackening of the butt or the line of inoculation is formed while a negative result shows no blackening.
Objectives of Sulfur Reduction Test
- To detect the hydrogen sulfide production which is released when the sulfur-containing compounds (thiosulfate, sulfates, sulfites, cysteine etc.) is metabolized by the organism.
- To differentiate the enteric bacteria because the members of Enterobacteriaceae show characteristic patterns of H₂S formation.
- To identify some of the pathogenic organisms where Salmonella and Proteus species generally show positive reaction while Shigella species is negative.
- To determine the enzymatic capability of the isolate as the enzymes like cysteine desulfurase or thiosulfate reductase is involved in the release of H₂S.
- To detect the fecal contamination in water sources as the coliforms associated with pollution is often tested for H₂S production.
- To visualize the chemical reduction because the gas reacts with the metal salts in the medium forming the black precipitate (ferrous sulfide) indicating the sulfur reduction.
Principle of Sulfur Reduction Test
The principle of the Sulfur Reduction Test is based on the ability of some microorganisms to act on the sulfur-containing compounds present in the medium and form hydrogen sulfide (H₂S). It is the process where the enzymes like cysteine desulfurase break the amino acid cysteine during protein degradation, releasing the sulfide component. In another pathway, the thiosulfate reductase enzyme reduces the inorganic sulfur compounds such as thiosulfate under anaerobic conditions. These reactions release hydrogen sulfide gas which is colorless, so an indicator is required to show the presence of the gas.
The medium contains heavy metal salts like ferrous ammonium sulfate, ferric citrate or sometimes lead acetate, and these ions react immediately with the produced H₂S. The reaction is as follows– H₂S combines with the metal ions forming the insoluble black precipitate (ferrous sulfide or lead sulfide). It is the blackening of the medium that confirms the positive sulfur reduction. Negative result is seen when no such black precipitate is formed.
Requirements for Sulfur Reduction Test
- SIM medium (Sulfide Indole Motility) which is a semi-solid deep and used because the sensitivity is high and sucrose is absent.
- Triple Sugar Iron (TSI) agar which is applied for detecting sugar fermentation along with sulfur reduction.
- Kligler’s Iron Agar (KIA) that is similar to TSI but without sucrose and is used for observing H₂S formation.
- Lead acetate agar or paper for detecting even the trace amount of hydrogen sulfide.
- Other selective or differential media like HE agar, SS agar and DCA agar used when screening enteric organisms.
- Nutrient broth or peptone water which is required mainly when the lead acetate strip method is performed.
- Sulfur source such as sodium thiosulfate, cysteine or organic sulfur amino acids present in the peptone component.
- Heavy metal salts (ferrous ammonium sulfate, ferric citrate, ferrous sulfate or lead acetate) used as the indicator to form the black precipitate.
- Inoculating needle which is a sterile straight wire used for stab inoculation in tubes.
- Incubator that can maintain the temperature around 35°C–37°C.
- Test tubes and racks required for holding the culture media.
- Bunsen burner or incinerator for sterilizing the needle and tube mouth.
- Lead acetate strips only when the paper strip technique is used.
- Test organism which must be a fresh pure culture generally 18–24 hours old.
- Positive control strains like Proteus mirabilis, Salmonella Typhimurium or Citrobacter freundii.
- Negative control strains like Shigella flexneri or Escherichia coli.
Procedure of Sulfur Reduction Test
- Pick a colony with a sterile inoculating needle from a fresh culture and touch the center of an isolated colony.
- Stab the SIM medium straight down through the center to about half or two–third depth of the tube.
- Withdraw the needle along the same insertion line so the agar is not split which may disturb the motility pattern.
- Incubate the tube at 35°C–37°C for 18–24 hours where slow-growing organisms may need longer.
- Observe the medium for the black precipitate that forms along the stab line or spreading throughout the medium.
- Pick a colony again with a sterile needle for TSI or KIA medium.
- Stab the butt of the agar deep to the bottom of the tube.
- Streak the slant surface in zig–zag manner while withdrawing the needle.
- Keep the cap loose for allowing proper air exchange needed for reaction on the slant.
- Incubate the inoculated tubes at 35°C–37°C for 18–24 hours.
- Observe the butt portion for blackening which indicates hydrogen sulfide formation.
- Inoculate a broth such as peptone water or nutrient broth when using the lead acetate paper method.
- Insert a strip of lead acetate paper into the neck of the culture tube.
- Suspend the strip so that it hangs above the medium without touching it because contact can inhibit bacterial growth.
- Secure the strip by fixing it with the cap or cotton plug.
- Incubate the tube at 35°C–37°C.
- Observe the strip daily for any blackening which indicates a positive sulfur reduction.
Results of Sulfur Reduction Test
Positive result
- Formation of the black precipitate (ferrous sulfide) indicating that hydrogen sulfide (H₂S) is produced by the organism.
- In SIM, KIA or TSI tubes the blackening may be seen along the stab line, at the junction of the slant and butt or spread throughout the butt when the production is strong.
- In strong reactions the black precipitate may cover the acid reaction so the yellow color in the butt is not visible.
- On plate media like HE agar or SS agar the colonies become black or show a black center.
- In the lead acetate method the paper strip turns brownish–black confirming the presence of H₂S.
Negative result
- Absence of any blackening in the tube or on the paper strip.
- The medium keeps its original appearance or may only show the color change related to sugar fermentation but without the formation of a black precipitate.
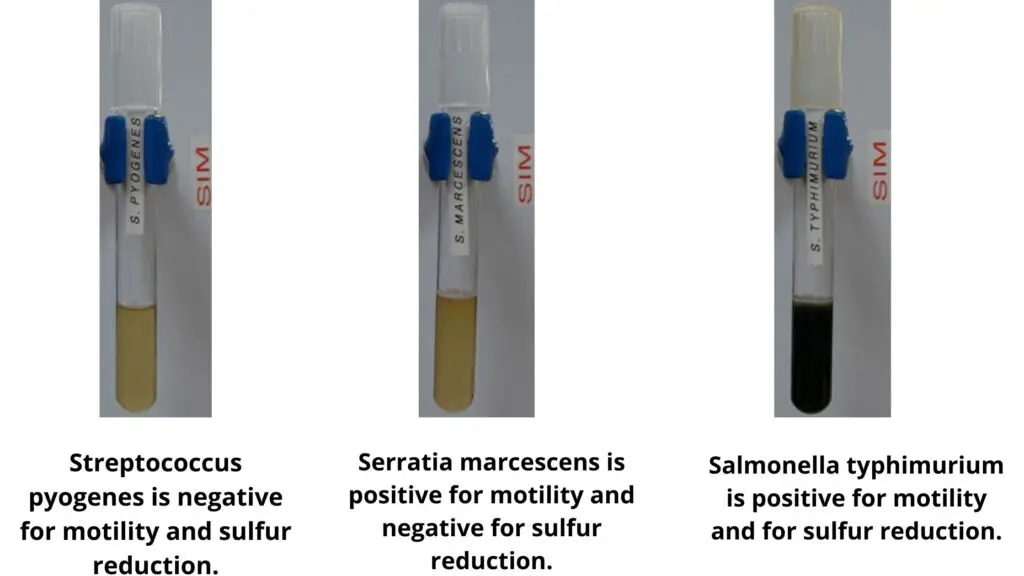
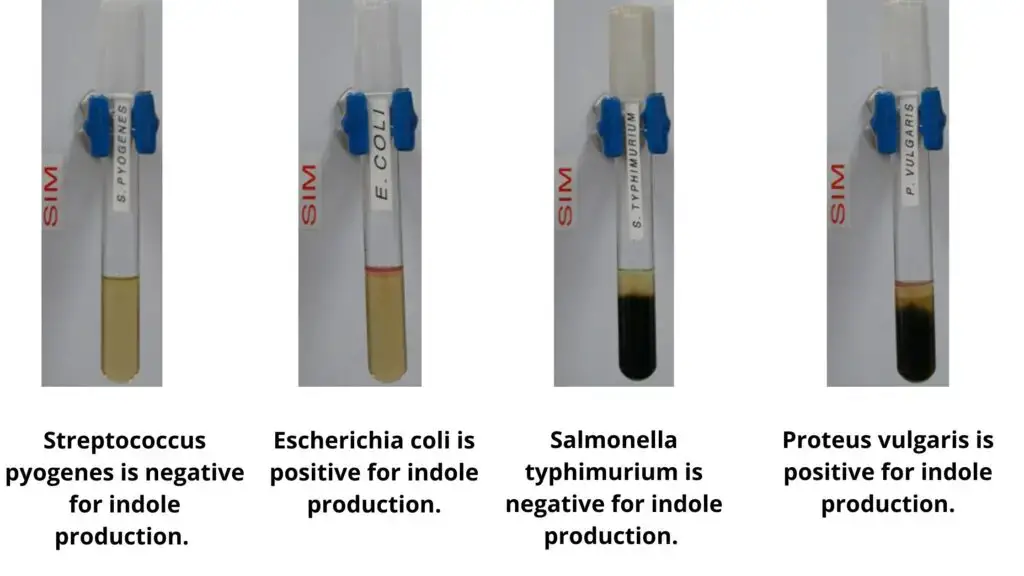
List of Sulfur Reduction Test Results of Some Common Bacteria
Sulfur–reducing bacteria (Positive reaction)
– Proteus vulgaris shows formation of black precipitate.
– Proteus mirabilis produces H₂S giving blackening of the medium.
– Salmonella Typhimurium shows clear black precipitate.
– Salmonella Enteritidis gives positive H₂S reaction.
– Salmonella Typhi may form a weak blackening or a thin black ring at the junction.
– Citrobacter freundii produces distinct blackening.
– Edwardsiella tarda forms black precipitate indicating sulfur reduction.
– Salmonella arizonae shows positive H₂S reaction.
– Campylobacter species can show sulfur reduction.
– Staphylococcus saprophyticus may give black precipitate.
– Erysipelothrix species show positive H₂S formation.
Non–sulfur–reducing bacteria (Negative reaction)
– Escherichia coli shows growth without any blackening.
– Shigella species (S. dysenteriae, S. flexneri, S. sonnei) remain negative.
– Klebsiella pneumoniae does not produce H₂S.
– Enterobacter aerogenes shows no black precipitate.
– Enterobacter cloacae remains negative.
– Pseudomonas aeruginosa grows without sulfur reduction.
– Salmonella Paratyphi A does not form blackening.
– Serratia marcescens remains negative.
– Morganella morganii shows no H₂S reaction.
– Providencia rettgeri remains negative.
– Yersinia enterocolitica does not produce black precipitate.
– Vibrio cholerae shows no H₂S.
– Staphylococcus aureus remains negative.
– Lactobacillus species show no sulfur reduction.
Quality Control of Sulfur Reduction Test
- Performance verification is required for every new lot of medium where known stock cultures is used to confirm that the medium is performing according to the expected specification.
- Positive control strains like Salmonella Typhimurium which shows growth with blackening, Proteus mirabilis which produces black precipitate in the medium or on lead acetate paper, Citrobacter freundii that gives a positive H₂S reaction and Proteus vulgaris which also forms blackening are used to check the correctness of the reaction.
- Negative control strains like Escherichia coli which grows without blackening, Shigella flexneri which also does not form any black precipitate and Pseudomonas aeruginosa which grows without producing H₂S are used to confirm the absence of reaction.
- Physical inspection of the medium is necessary where signs like discoloration, shrinking, contamination or any deterioration indicate the medium should not be used.
- The expiration date must be checked before using the medium.
- The agar tubes must be examined for cracks because these cracks can be confused with gas formation during the interpretation.
Precautions of Sulfur Reduction Test
- A straight sterile inoculating needle must be used for stabbing because a loop can split the agar and this splitting may appear like gas formation.
- The needle should be withdrawn along the same line of entry in semi–solid media so that fanning or widening of the stab does not falsely indicate motility or disturb the agar.
- The culture used must be pure because mixed cultures can produce irregular reactions that are difficult to interpret.
- The caps of TSI or KIA tubes should be kept loose during incubation to allow proper air exchange; tight caps interfere with the oxidative reactions on the slant.
- The incubation time must be followed carefully (18–24 hours) because early reading may miss H₂S or give false acid reaction, while late reading may give false alkaline result or excessive blackening.
- Lead acetate strip must not touch the culture medium as lead acetate is toxic and can inhibit the bacterial growth.
- The medium should be inspected for cracks or gaps before inoculation because cracks can be confused with gas production.
- In SIM medium the motility and H₂S reaction must be recorded before adding Kovac’s reagent since the reagent can interfere with interpretation.
- The inoculum should be taken from a solid medium because using broth culture may delay the growth and produce incorrect results.
- Sucrose in TSI medium can suppress the H₂S mechanism, so organisms like Salmonella may show negative reaction in TSI but positive in SIM or KIA.
- When the butt of TSI or KIA becomes fully black, it must be interpreted as acidic because H₂S formation requires acidic conditions.
Uses of Sulfur Reduction Test
- It is used for identifying the enteric bacteria where the species of Salmonella, Proteus, Citrobacter, Edwardsiella and Francisella is differentiated based on their H₂S formation.
- It helps in distinguishing the Salmonella species which is usually H₂S positive from the Shigella species which is generally negative.
- It is applied in water quality testing to detect the fecal contamination because H₂S–producing organisms is often associated with coliform presence.
- It is used in oral health diagnosis where bacteria such as Eikenella corrodens, Fusobacterium, Peptostreptococcus and Eubacterium species form H₂S that is related with halitosis and periodontitis.
- It assists in differentiating some non–enteric bacteria like Erysipelothrix species which is positive and Lactobacillus species which is negative, and also helps in identifying Bacteroides and Brucella.
- It is used in bioremediation where the principle is applied for precipitating heavy metals like cadmium from wastewater using bacteria that produce excessive hydrogen sulfide.
- It is used in metabolic research for studying the cysteine and sulfur metabolism in different organisms including plants and bacteria to determine whether the enzymes such as cysteine desulfurase or thiosulfate reductase is being utilized.
Advantages of Sulfur Reduction Test
- It is useful for differentiating the enteric bacteria where Salmonella and Proteus species usually show positive reaction while Shigella and Escherichia species remain negative.
- It is incorporated in combination media like SIM, TSI and KIA so that motility, indole formation and carbohydrate fermentation can be examined together in a single tube which makes the testing easier.
- It is used in detecting fecal contamination because the presence of H₂S–producing organisms indicates association with coliform bacteria in water sources.
- It provides high sensitivity when methods like lead acetate paper are used as these can detect even small amounts of hydrogen sulfide which may not be detected by iron–containing media.
- It is simple and cost–effective especially in the paper strip method where no expensive equipment is required, making it suitable for field testing.
- It gives a clear visual observation because the formation of the black precipitate (ferrous sulfide or lead sulfide) is easily recognized during a positive reaction.
Limitations of Sulfur Reduction Test
- In media containing sucrose like TSI agar, the rapid use of sucrose can suppress the pathway required for H₂S formation which may give a false–negative result even when the organism is actually positive in sucrose–free media such as SIM or KIA.
- The sensitivity of the test varies with the medium because SIM medium is more sensitive while TSI or KIA may not show clear reaction, and the lead acetate method is the most sensitive. So an organism may appear positive in one medium but negative in another.
- Heavy black precipitate formed during strong H₂S production can mask the entire butt, making it difficult to observe the color change related to fermentation though a black butt generally means the environment is acidic.
- Lead acetate is toxic and may inhibit bacterial growth, therefore the strip must not touch the medium when using the paper method.
- The test cannot distinguish whether H₂S is formed from anaerobic reduction of thiosulfate or from putrefaction of cysteine.
- There is a specific time for reading the reaction because early reading may show false acidity while late reading may give false alkaline results due to peptone use.
- Technical errors like using a loop instead of a straight needle may split the agar and create a false appearance of gas production, and tightening the tube cap too much may prevent the required air exchange.
- It gives only presumptive identification and must be supported by other biochemical or molecular tests for confirming the organism.
- Ahn, B. K., Ahn, Y. J., Lee, Y. J., Lee, Y. H., & Lee, G. J. (2022). Simple and sensitive detection of bacterial hydrogen sulfide production using a paper-based colorimetric assay. Sensors, 22(15), 5928. https://doi.org/10.3390/s22155928
- Alvarez, C., Calo, L., Romero, L. C., García, I., & Gotor, C. (2010). An O-acetylserine(thiol)lyase homolog with l-cysteine desulfhydrase activity regulates cysteine homeostasis in Arabidopsis. Plant Physiology, 152(2), 656–669. https://doi.org/10.1104/pp.109.147975
- Aryal, S. (2022, August 10). Hydrogen sulfide test – Principle, procedure, uses and interpretation. Microbiology Info. https://microbiologyinfo.com/hydrogen-sulfide-test/
- Aryal, S. (2022, August 10). Sulphur reduction test – Principle, procedure, uses and interpretation. Microbiology Info. https://microbiologyinfo.com/sulphur-reduction-test/
- Aryal, S. (2024, August 16). Triple sugar iron (TSI) agar- Composition, principle, preparation, results, uses. Microbe Notes. https://microbenotes.com/triple-sugar-iron-tsi-agar/
- Bang, S. W., Clark, D. S., & Keasling, J. D. (2000). Engineering hydrogen sulfide production and cadmium removal by expression of the thiosulfate reductase gene (phsABC) from Salmonella enterica serovar Typhimurium in Escherichia coli. Applied and Environmental Microbiology, 66(9), 3939–3944. https://doi.org/10.1128/aem.66.9.3939-3944.2000
- Biolife Italiana S.r.l. (2022, September). Triple sugar iron agar ISO [Instructions for Use].
- Biolife Italiana S.r.l. (2023, April). Triple sugar iron agar [Instructions for Use].
- Braccia, D. J., Jiang, X., Pop, M., & Hall, A. B. (2021). The capacity to produce hydrogen sulfide (H2S) via cysteine degradation is ubiquitous in the human gut microbiome. Frontiers in Microbiology, 12, 705583. https://doi.org/10.3389/fmicb.2021.705583
- Carbonero, F., Benefiel, A. C., Alizadeh-Ghamsari, A. H., & Gaskins, R. (2012). Microbial pathways in colonic sulfur metabolism and links with health and disease. Frontiers in Physiology, 3, 448. https://doi.org/10.3389/fphys.2012.00448
- Dahal, P. (2024, August 12). Hydrogen sulfide (H2S) test: Principle, procedure, results. Microbe Notes. https://microbenotes.com/hydrogen-sulfide-h2s-production-test/
- Das, M., Dewan, A., Shee, S., & Singh, A. (2021). The multifaceted bacterial cysteine desulfurases: From metabolism to pathogenesis. Antioxidants, 10(7), 997. https://doi.org/10.3390/antiox10070997
- Fang, H., Yu, Z., Xing, K., Zhou, L., Shao, Y., Zhang, X., Pei, Y., & Zhang, L. (2023). Transcriptomic analysis reveals the functions of H2S as a gasotransmitter independently of Cys in Arabidopsis. Frontiers in Plant Science, 14, 1184991. https://doi.org/10.3389/fpls.2023.1184991
- Hardy Diagnostics. (2020). SIM (sulfide, indole, motility) medium [Instructions for Use].
- Hardy Diagnostics. (2020). Triple sugar iron (TSI) agar [Instructions for Use].
- Hartline, R. (2023, February 18). 1.23: SIM deep tests. Biology LibreTexts. https://bio.libretexts.org/Bookshelves/Microbiology/Microbiology_Laboratory_Manual_(Hartline)/01%3A_Labs/1.23%3A_SIM_Deep_Tests
- Heredity Biosciences. (2024, January 30). Unlocking the secrets of microbial metabolism: The sulfur reduction test. Heredity Bioscience Blog. https://hereditybio.in/blog/unlocking-the-secrets-of-microbial-metabolism-the-sulfur-reduction-test/
- HiMedia Laboratories. (2017, February). Triple sugar iron agar [Technical Data].
- Leavitt, W. D., Cummins, R., Schmidt, M. L., Sim, M. S., Ono, S., Bradley, A. S., & Johnston, D. T. (2014). Multiple sulfur isotope signatures of sulfite and thiosulfate reduction by the model dissimilatory sulfate-reducer, Desulfovibrio alaskensis str. G20. Frontiers in Microbiology, 5, 591. https://doi.org/10.3389/fmicb.2014.00591
- Micromaster Laboratories. (n.d.). Triple sugar iron agar (DM254) [Product Specification Sheet].
- Miller, E. (2024). SIM medium. In Microbiology Laboratory Manual. Open Oregon Educational Resources. https://openoregon.pressbooks.pub/microbiologylaboratorymanual/chapter/background-theory-6/
- Oguri, T., Schneider, B., & Reitzer, L. (2012). Cysteine catabolism and cysteine desulfhydrase (CdsH/STM0458) in Salmonella enterica serovar Typhimurium. Journal of Bacteriology, 194(16), 4366–4376. https://doi.org/10.1128/JB.00729-12
- Procop, G. W., Wallace, J. D., Tuohy, M. J., LaSalvia, M. M., Addison, R. M., & Reller, L. B. (2008). A single-tube screen for Salmonella and Shigella. American Journal of Clinical Pathology, 130(2), 284–289. https://doi.org/10.1309/MTDBAXHDPKAL6GF5
- Rambach, A. (1990). New plate medium for facilitated differentiation of Salmonella spp. from Proteus spp. and other enteric bacteria. Applied and Environmental Microbiology, 56(1), 301–303.
- Remel. (2008, August 14). Triple sugar iron (TSI) agar [Instructions for Use]. Thermo Fisher Scientific.
- Sigma-Aldrich. (2018). 92499 Triple sugar iron agar (TSI agar) [Product Information]. Merck KGaA.
- Stipanuk, M. H., & Ueki, I. (2011). Dealing with methionine/homocysteine sulfur: Cysteine metabolism to taurine and inorganic sulfur. Journal of Inherited Metabolic Disease, 34(1), 17–32. https://doi.org/10.1007/s10545-009-9006-9
- Tankeshwar, A. (n.d.). Hydrogen sulfide (H₂S) production test. Microbe Online. https://microbeonline.com/hydrogen-sulfide-production-test/
- Technical analysis of the sulfur reduction test: Principles, metabolic pathways, and diagnostic applications in clinical microbiology. (n.d.). [Text provided as source].
- Thakur, S., Anokhe, A., & Kalia, V. (2021). Biochemical test for detecting hydrogen sulphide (H2S) producing bacteria. AgriCos e-Newsletter, 2(11), 53-56.
- Vlab.amrita.edu. (2011). Triple sugar iron agar (Procedure). Amrita Virtual Lab. https://vlab.amrita.edu/?sub=3&brch=76&sim=216&cnt=2
- Wang, J., Guo, X., Li, H., Qi, H., Qian, J., Yan, S., Shi, J., & Niu, W. (2019). Hydrogen sulfide from cysteine desulfurase, not 3-mercaptopyruvate sulfurtransferase, contributes to sustaining cell growth and bioenergetics in E. coli under anaerobic conditions. Frontiers in Microbiology, 10, 2357. https://doi.org/10.3389/fmicb.2019.02357
- Watson, R. (n.d.). Tests used to identify Gram negative bacteria. University of Wyoming. https://www.uwyo.edu/molb2210_lab/info/biochemical_tests.htm