Simmons Citrate Agar is a chemically defined medium in which sodium citrate act as the only carbon source and ammonium dihydrogen phosphate act as the only nitrogen source. It is the medium developed as a modification of Koser’s citrate medium and it is used mainly to differentiate members of Enterobacteriaceae.
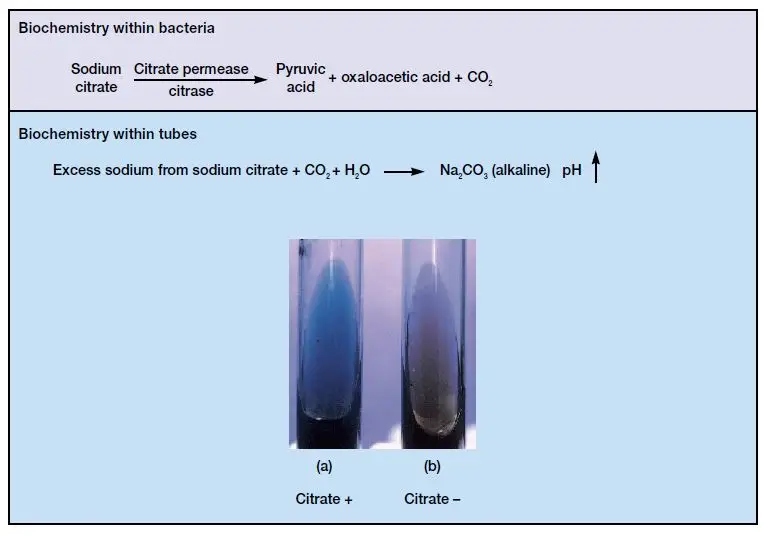
It is the process in which the organisms capable of using citrate as the sole carbon compound will grow on this medium, while the organisms which cannot use citrate will not grow. The medium contain bromothymol blue indicator which remain green at neutral pH, and it is changed into blue when the pH becomes alkaline.
The alkalinity is produced when the organism metabolises citrate and break down the ammonium salts releasing alkaline compounds. It is this characteristic property that helps in identifying citrate positive organisms like Klebsiella and Enterobacter from citrate negative organisms like Escherichia coli.
The Simmons Citrate Agar Test is the biochemical test used to check whether the organism can utilise citrate as the only carbon source. It is the test where the organism is inoculated lightly on the Simmons citrate slant and incubated at 35–37°C for 24–48 hours. If the organism is able to transport citrate inside the cell with the help of citrate permease, it grows on the medium and the utilisation of citrate results in production of alkaline by-products which turn the medium blue. This is referred to as a positive citrate test. The growth without any colour change is also taken as positive because the organism is still able to use citrate. When there is no growth and the medium remain green, it indicates the absence of citrate utilisation and it is called a negative test.
It is important that the inoculum is kept light to avoid false positive results, because the nutrients carried over from rich media may support growth even if the organism cannot utilise citrate.
Principle of Simmons Citrate Agar

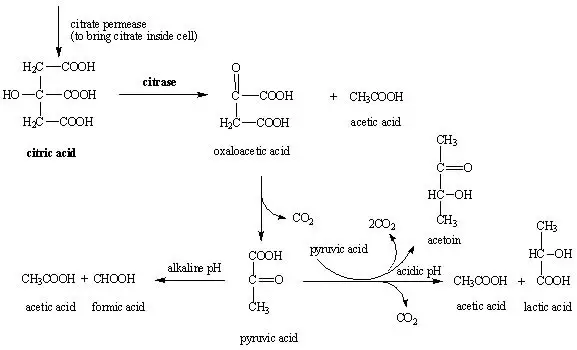
The principle of the Simmons citrate agar test is based on the ability of an organism to utilise citrate as the only carbon source when no other organic compound is available. It is the process in which the medium supply sodium citrate as the sole carbon source and ammonium dihydrogen phosphate as the only nitrogen source, so only those organisms that possess the enzyme citrate permease can survive in this restricted environment. It is this enzyme that helps in transporting citrate inside the bacterial cell, where the citrate is further cleaved by citrate lyase into oxaloacetate and acetate. The oxaloacetate is changed into pyruvate and carbon dioxide, and these compounds enter into normal metabolic reactions for energy production. At the same time, the utilisation of ammonium salts results in the release of ammonia, and the carbon dioxide produced react with sodium ions and water to form sodium carbonate. These reactions produce alkaline conditions in the medium.
The agar contains bromothymol blue indicator which is green at neutral pH and it becomes blue when the pH shifts towards alkaline values. It is the increase in pH due to citrate metabolism that changes the colour from green to blue. The growth of the organism on the slant with this colour change indicates a positive citrate test, while the absence of growth and retention of the green colour indicates a negative result. It is the growth on this selective medium that confirms the organism has used citrate as the only carbon source.
Composition of Simmons Citrate Agar
Composition of Simmons Citrate Agar (per 1 litre)
- Sodium chloride (NaCl) – 5.0 g
- Sodium citrate (dihydrate) – 2.0 g
- Ammonium dihydrogen phosphate (NH₄H₂PO₄) – 1.0 g
- Dipotassium phosphate (K₂HPO₄) – 1.0 g
- Magnesium sulfate (MgSO₄·7H₂O) – 0.2 g
- Bromothymol blue – 0.08 g
- Agar – 15.0 g
- Distilled or deionized water – 1000 ml
Final property–
- Final pH is adjusted to 6.9 ± 0.2 at 25°C.
Requirements for Simmons Citrate Agar test
- Simmons citrate agar slants prepared with the defined medium.
- Medium having sodium citrate as the only carbon source and ammonium dihydrogen phosphate as the only nitrogen source.
- Bromothymol blue indicator and agar base present in the medium.
- The pH of the medium adjusted to around 6.9 at 25°C.
- Sterile distilled water or saline for preparing a light inoculum if needed.
- A sterile inoculating needle for transferring the culture.
- Bunsen burner or alcohol lamp for sterilising the needle and maintaining aseptic conditions.
- An incubator maintained at 35–37°C for normal incubation.
- Pure culture of the organism that is 16–24 hours old.
- Aerobic condition maintained by keeping the tube caps loose.
- Suitable control organisms like Klebsiella pneumoniae for positive control and Escherichia coli for negative control.
Preparation of Simmons Citrate Agar

- The medium is prepared by taking the required ingredients per 1 litre of distilled water. It is the composition where agar (15 g), Sodium chloride (5 g), sodium citrate (2 g), ammonium dihydrogen phosphate (1 g), dipotassium phosphate (1 g), magnesium sulfate (0.2 g) and bromothymol blue (0.08 g) is used.
- It is the process where all the salts is dissolved in distilled water. When dehydrated commercial powder is used, then about 24.28 g powder is suspended in 1000 ml water.
- The pH is adjusted to around 6.9 (±0.2) at 25°C.
- Agar and the indicator is added if these are not included in the dehydrated mixture.
- The medium is heated gently and mixed so that complete dissolution of agar and salts is occurred.
- About 4–5 ml of the prepared solution is dispensed into test tubes.
- The tubes is then sterilized in an autoclave at 121°C for 15 minutes under 15 psi pressure.
- After sterilization, the tubes is kept in a slanted position during cooling. It forms a long slant with a shallow butt, which is required for proper growth surface.
- The medium is stored in refrigerator (2–8°C). It is the final stage where the uninoculated slants appear deep forest green and it can be stored for about 6–8 weeks.
Procedure of Simmons Citrate Agar

- It is this process a fresh pure culture (about 16–24 hours) is selected from a solid medium. A single isolated colony is taken with a sterile inoculating needle as it helps in taking a light inoculum. Sometimes a light suspension is made in sterile water or saline which is helpful in preventing carryover nutrients that may give false positive reaction.
- The light inoculum is now transferred aseptically to the Simmons citrate agar slant. The surface of the slant is streaked in a zig-zag manner. It is important that the butt is not stabbed because citrate utilization is an aerobic process.
- The tube cap is kept loosened slightly so that proper gas exchange is occurred. The tubes is incubated aerobically at about 35–37°C. Some organisms is incubated at 30°C depending on their requirement. Incubation is usually for 24–48 hours, and slow organisms can be kept for 4–7 days.
- It is then observed for the change of colour from green to blue on the slant which indicates positive reaction. Growth on the slant even without blue colour is also considered positive. When no growth is seen and the medium remains forest green, then it is the negative result.
Result of Simmons citrate test

Positive result with blue colour–
It is observed when the slant changes from green to deep blue and growth is present. The organism is able to utilize citrate as the only carbon source and ammonium salt as nitrogen source, producing alkaline by-products which increases the pH. This reaction is seen in organisms like Klebsiella pneumoniae, Enterobacter spp., Citrobacter freundii, Serratia marcescens and Salmonella enteritidis.
Positive result with growth but no colour change–
Visible growth appears on the slant while the medium remains green. It is considered positive because the organism has citrate permease and survives in this medium. Sometimes colour change is not seen when incubation time is not enough or the organism is slow growing. When growth is present the test is taken as positive although repeating the test can be done.
Negative result with no growth–
The medium stays forest green with no visible growth on the slant. It shows that the organism cannot utilize citrate and the enzyme citrate permease is absent. This type of result is usually seen in Escherichia coli, Shigella spp., Morganella morganii and Yersinia enterocolitica.
False reactions and mistakes–
False negative reaction is occurred when the tube cap is too tight and air cannot enter because citrate utilization is an aerobic process. False positive reaction is occurred when heavy inoculum is used and nutrients from the previous medium is carried over. A light inoculum or saline suspension is used to avoid this problem.

Uses of Simmons Citrate Agar
- It is used for differentiation of Enterobacteriaceae based on the ability of the organism to utilize citrate as the only carbon source.
- It helps in distinguishing fecal coliforms like Escherichia coli (citrate negative) from non-fecal or environmental coliforms like Enterobacter aerogenes (citrate positive).
- It is an important part of the IMViC test series, where the citrate test is used together with indole, methyl red and Voges–Proskauer tests for identifying coliform bacteria.
- The medium is also used to differentiate citrate positive Salmonella species such as S. enteritidis from citrate negative ones like S. typhi, S. paratyphi A, S. pullorum and S. gallinarum.
- It is the process used in food and water testing for detecting fecal contamination by observing the presence of coliforms.
- Some closely related organisms is also differentiated by this test such as–
– Serratia proteamaculans (positive) from Yersinia pseudotuberculosis and Y. enterocolitica (negative)
– Proteus rettgeri (positive) from Morganella morganii (negative)
– Edwardsiella species (negative) from Salmonella species (positive)
– Leminorella grimontii (positive) from Leminorella richardii (negative)
– Acidovorax delafieldii (positive) from Acidovorax facilis and A. temperans (negative)
Advantages of Simmons Citrate Agar
- One major advantage is that the medium contains bromothymol blue indicator, so the colour change from green to blue is very clear. It is easier to interpret than earlier citrate broths where only turbidity was seen and results were often confusing.
- It helps in proper differentiation of fecal coliforms like E. coli which is citrate negative from non-fecal coliforms like Enterobacter and Klebsiella which is citrate positive. This makes the medium useful in environmental and public health studies.
- The medium is used for differentiating various Salmonella species, as citrate positive forms like S. enteritidis can be separated from citrate negative ones like S. typhi and S. paratyphi A.
- Other Enterobacteriaceae is also separated by this test such as Serratia proteamaculans (positive) from Yersinia pseudotuberculosis (negative) and Proteus rettgeri (positive) from Morganella morganii (negative).
- It is a chemically defined medium with citrate as the only carbon source and ammonium salts as the only nitrogen source. It is the process where strict nutritional selection is imposed so only organisms with citrate permease can grow, reducing unnecessary growth by other pathways.
- It is cost-effective, easy to prepare and gives a rapid metabolic indication which helps in routine laboratory identification work, acting as an important preliminary test.
- The agar slant provides a proper aerobic surface which is essential for citrate oxidation. It supports better growth conditions compared to liquid medium where oxygen supply is limited.
Limitations of Simmons Citrate Agar
- One limitation is that nutrients from the primary medium can be carried over during inoculation. Even very small nutrient carryover is enough to support growth and this may give a false positive result on the citrate medium.
- When a heavy inoculum is used, dead cells or organic material is transferred to the medium. These materials can support some growth without real citrate utilization. For this reason a light inoculum taken with a needle is preferred.
- The test requires proper oxygen supply because citrate utilization occurs under aerobic condition. When the tube cap is tightly closed the air cannot enter and a false negative result is obtained. The cap is kept loose for correct result.
- Some organisms can grow but they do not produce enough alkaline products to change the colour immediately. Depending only on colour can cause wrong interpretation because visible growth without colour change is also positive.
- The test alone cannot identify the organism up to species level. It must be used together with other biochemical tests like Indole, Methyl red and Voges–Proskauer for complete identification.
- The medium is sensitive to pH and drying. If the pH is not around 6.5–7.0 during preparation or the slant becomes dry then it becomes difficult to interpret the reaction properly.
- When several biochemical tests are inoculated from the same culture, this medium should be inoculated first or the needle must be flamed before touching it. This prevents accidental transfer of glucose or other nutrients from previous media.
- Sometimes equivocal results appear as faint colour change or weak growth. These results cannot be taken as final and the test should be repeated for confirmation.
List of Bacteria which gives positive and negative result in Simmons citrate test
Citrate Positive Bacteria
These organisms have citrate permease, so citrate is transported into the cell and used as the only carbon source. Growth is seen on the slant and alkaline products make the medium blue, although growth without colour change is also taken as positive.
- Klebsiella pneumoniae (most Klebsiella species is positive)
- Enterobacter aerogenes
- Enterobacter cloacae
- Citrobacter freundii
- Serratia marcescens
- Serratia proteamaculans
- Salmonella enteritidis
- Other Salmonella subgenus II, III and IV species
- Proteus mirabilis
- Proteus rettgeri
- Providencia species
- Yokenella regensburgei
- Leminorella grimontii
- Acidovorax delafieldii
Citrate Negative Bacteria
These organisms lack citrate permease, so citrate cannot be transported or utilized. No growth is seen and the medium remains forest green.
- Escherichia coli (wild type is negative)
- Shigella species (S. flexneri, S. sonnei, S. dysenteriae)
- Salmonella typhi
- Salmonella paratyphi A
- Salmonella pullorum
- Salmonella gallinarum
- Yersinia enterocolitica
- Yersinia pseudotuberculosis
- Morganella morganii
- Edwardsiella species
- Hafnia alvei
- Leminorella richardii
- Acidovorax facilis
- Acidovorax temperans
Key Differentiating Pairs
- Escherichia coli (–) vs Enterobacter aerogenes (+)
- Salmonella typhi (–) vs Salmonella enteritidis (+)
- Yersinia pseudotuberculosis (–) vs Serratia proteamaculans (+)
- Morganella morganii (–) vs Proteus rettgeri (+)
Important Exception– Some evolved E. coli strains can become citrate positive under long-term selective pressure, but these are not typical clinical isolates.
FAQ
1. What is Simmons Citrate Agar used for?
It is used for differentiating Enterobacteriaceae based on the ability of the organism to utilize citrate as the only carbon source.
2. What is the principle of Simmons Citrate Agar?
It is the principle where organisms that have citrate permease can use citrate and produce alkaline products. These products increase the pH and change the colour of the indicator.
3. How does Simmons Citrate Agar work?
The medium provides citrate as the carbon source and ammonium salt as the nitrogen source. When the organism grows, alkaline compounds is produced which turns the indicator blue.
4. What color indicates a positive result on Simmons Citrate Agar?
A positive reaction is indicated by blue colour on the slant or visible growth even without colour change.
5. What color indicates a negative result on Simmons Citrate Agar?
A negative reaction is shown when the medium remains forest green with no growth.
6. What are the components of Simmons Citrate Agar?
It contains sodium citrate, ammonium dihydrogen phosphate, dipotassium phosphate, sodium chloride, magnesium sulfate, agar and bromothymol blue indicator.
7. Which organisms give a positive result on Simmons Citrate Agar?
Organisms like Klebsiella pneumoniae, Enterobacter aerogenes, Citrobacter freundii, Serratia marcescens, Salmonella enteritidis, Proteus rettgeri, Providencia and others is positive.
8. Which organisms give a negative result on Simmons Citrate Agar?
Organisms like Escherichia coli, Shigella species, Salmonella typhi, Yersinia enterocolitica, Morganella morganii and Edwardsiella species is negative.
9. What is the pH indicator in Simmons Citrate Agar?
The indicator used in the medium is bromothymol blue.
10. Why does Simmons Citrate Agar change color?
It changes colour because alkaline products like ammonia are produced during citrate utilization which increases the pH of the medium.
11. How do you inoculate Simmons Citrate Agar?
A light inoculum is taken with a sterile needle and streaked on the surface of the slant in a zig-zag manner without stabbing the butt.
12. What is the incubation time and temperature for Simmons Citrate Agar?
It is incubated at around 35–37°C for 24–48 hours. Slow organisms may need longer incubation.
13. Is Simmons Citrate Agar a selective or differential medium?
It is a differential medium because it separates organisms based on citrate utilization.
14. Who developed Simmons Citrate Agar?
The medium was developed by Simmons as a modification of Koser’s citrate medium.
15. What is the initial color of uninoculated Simmons Citrate Agar?
The uninoculated medium appears deep forest green.
- Aryal, S. (2022, January 8). Simmons Citrate Agar- Composition, Principle, Preparation, Results, Uses. Microbe Notes. https://microbenotes.com/simmons-citrate-agar/
- Aryal, S. (2022, August 10). Citrate Utilization Test- Principle, Media, Procedure and Result. Microbe Online. https://microbeonline.com/citrate-utilization-test-principle-procedure-interpretation/
- Aryal, S. (2022, August 10). Simmons Citrate Agar- Composition, Principle, Uses, Preparation and Result Interpretation. Microbiology Info. https://microbiologyinfo.com/simmons-citrate-agar-composition-principle-uses-preparation-and-result-interpretation/
- Carolina Biological Supply Company. (2025). Bacteria: The Citrate Test. https://www.carolina.com/teacher-resources/Interactive/bacteria-citrate-test/tr32901.tr
- Comenius University Bratislava. (n.d.). ENTEROBACTERIACEAE, CAMPYLOBACTER, HELICOBACTER. Jessenius Faculty of Medicine. https://www.jfmed.uniba.sk/fileadmin/jlf/Pracoviska/ustav-mikrobiologie-a-imunologie/ENTEROBACTERIACEAE__CAMPYLOBACTER__HELICOBACTER.pdf
- Guentzel, M. N. (1996). Escherichia, Klebsiella, Enterobacter, Serratia, Citrobacter, and Proteus. In S. Baron (Ed.), Medical Microbiology (4th ed.). University of Texas Medical Branch at Galveston. https://www.ncbi.nlm.nih.gov/books/NBK8035/
- Hartline, R. (2023, February 18). 1.20: Citrate Test. Biology LibreTexts. https://bio.libretexts.org/Bookshelves/Microbiology/Microbiology_Laboratory_Manual_(Hartline)/01%3A_Labs/1.20%3A_Citrate_Test
- MacKenzie, E. (2025, August 12). 31.12: Simmon’s Citrate Agar Test. Biology LibreTexts. https://bio.libretexts.org/Courses/Irvine_Valley_College/IVC_Microbiology_Lab_Manual/31%3A_METABOLIC_TESTING/31.12%3A_Simmons_Citrate_Agar_Test
- Smith, M., & Selby, S. (2021, March 19). 3.9: Simmons Citrate Agar. Biology LibreTexts. https://bio.libretexts.org/Learning_Objects/Laboratory_Experiments/Microbiology_Labs/Microbiology_for_Allied_Health_Students%3A_Lab_Manual/3.09%3A_Simmons_Citrate_Agar
- The Simmons Citrate Utilization Test: Principles, Methodology, and Diagnostic Significance in Clinical Microbiology. (n.d.).
- Van Hofwegen, D. J., Hovde, C. J., & Minnich, S. A. (2016). Rapid Evolution of Citrate Utilization by Escherichia coli by Direct Selection Requires citT and dctA. Journal of Bacteriology, 198(7), 1022–1034. https://doi.org/10.1128/JB.00831-15
- VUMIE. (n.d.). Citrate Utilization Test (Simmons). http://vumicro.com/help/content/citrate_utilization_test.htm
- Wikipedia. (n.d.). Simmons’ citrate agar. Retrieved October 10, 2025, from https://en.wikipedia.org/wiki/Simmons%27_citrate_agar
Thank you