The Novobiocin Susceptibility Test is a biochemical diagnostic test that is used for the differentiation of certain species of coagulase negative staphylococci. It is mainly used to identify Staphylococcus saprophyticus from other coagulase negative staphylococci such as Staphylococcus epidermidis. This test is commonly performed in microbiology laboratory for organisms isolated from urinary tract infections.
It is based on the principle that Staphylococcus saprophyticus shows intrinsic resistance to novobiocin antibiotic, whereas most of the other coagulase negative staphylococci are sensitive to this drug. Novobiocin is an aminocoumarin antibiotic which inhibits bacterial DNA synthesis by acting on the enzyme DNA gyrase. Due to inhibition of this enzyme, DNA replication is affected and bacterial growth is inhibited in susceptible organisms.
In this test, a pure culture of the organism is inoculated evenly on the surface of Mueller-Hinton agar or blood agar plate. A novobiocin disc containing 5 µg of antibiotic is placed on the agar surface. The plate is then incubated aerobically at 35–37°C for a period of 18 to 24 hours. After incubation, the zone of inhibition around the disc is observed and measured.
If the zone of inhibition is more than 16 mm, the organism is considered sensitive to novobiocin. If the zone diameter is 16 mm or less, the organism is considered resistant, which is indicative of Staphylococcus saprophyticus. However, some other species such as Staphylococcus cohnii and Staphylococcus xylosus may also show resistance, therefore this test is mainly used for presumptive identification and may require further confirmatory tests.
Objectives of Novobiocin Susceptibility Test
- To determine the susceptibility of bacterial isolate against novobiocin antibiotic.
- To identify Staphylococcus saprophyticus presumptively from urine culture samples, as this organism is resistant to novobiocin.
- To differentiate Staphylococcus saprophyticus from other coagulase negative staphylococci which are sensitive to novobiocin.
- To classify coagulase negative staphylococci into novobiocin sensitive group and novobiocin resistant group based on their reaction.
Principle of Novobiocin Susceptibility Test
The principle of the novobiocin susceptibility test is based on the intrinsic resistance shown by Staphylococcus saprophyticus to the antibiotic novobiocin. Most of the other clinically important coagulase negative staphylococci are sensitive to this drug. This difference in susceptibility is used to differentiate Staphylococcus saprophyticus from other coagulase negative staphylococci such as Staphylococcus epidermidis.
Novobiocin acts by inhibiting bacterial DNA synthesis. It binds to the GyrB subunit of the enzyme DNA gyrase and blocks the ATP hydrolysis that is required for DNA supercoiling and replication. Due to this action, growth of susceptible bacteria is inhibited. However, Staphylococcus saprophyticus possess a modified form of the GyrB protein which prevents effective binding of the antibiotic, and therefore the organism remains resistant.
During the test, novobiocin diffuses from the antibiotic disc into the agar medium. In susceptible organisms, bacterial growth is inhibited around the disc forming a clear zone of inhibition. In resistant organisms such as Staphylococcus saprophyticus, growth occurs close to the disc or a small zone of inhibition is observed. A zone diameter of 16 mm or less is considered resistant and is used for presumptive identification of the organism.
Requirements for Novobiocin Susceptibility Test
Culture Media
- Plating media – Mueller Hinton Agar (MHA), Tryptic Soy Agar (TSA), or Blood Agar plates (usually with 5% sheep blood).
- Suspension media – Tryptic Soy Broth (TSB), Brain Heart Infusion (BHI) broth, or sterile distilled water used for preparing the inoculum.
Reagents
- Antibiotic disks – Paper disks containing 5 µg novobiocin.
- Turbidity standard – 0.5 McFarland standard used for standardization of inoculum density.
Equipment and Supplies
- Incubator – Aerobic incubator adjusted at 35–37°C.
- Applicators – Sterile cotton swabs or inoculating loop used for spreading culture.
- Forceps – Sterile forceps for aseptic placement of novobiocin disk on agar surface.
- Measuring tools – Metric ruler or sliding calipers used to measure zone of inhibition.
- Sterilization source – Bunsen burner or incinerator.
Bacterial Isolates
- Test organism – Pure culture (18–72 hours old) which is Gram positive, catalase positive, coagulase negative cocci.
- Quality control strains –
- Staphylococcus saprophyticus ATCC 15305 (resistant control).
- Staphylococcus aureus ATCC 25923 or Staphylococcus epidermidis ATCC 12228 (susceptible control).
Procedure of Novobiocin Susceptibility Test
Method 1: Disk Diffusion (Plate) Method
- Pure culture colonies (18–72 hours old) are selected and a bacterial suspension is prepared in broth (Tryptic Soy Broth) or sterile water. The turbidity is adjusted to match 0.5 McFarland standard.
- A sterile swab is dipped into the suspension and excess fluid is removed. The swab is streaked uniformly over the entire surface of Mueller Hinton Agar or Blood Agar plate in three different directions to obtain a confluent lawn growth.
- The inoculated agar surface is allowed to dry for few minutes (not more than 15 minutes). A 5 µg novobiocin disk is then placed aseptically on the agar surface and pressed gently to ensure proper contact with medium.
- The plate is inverted and incubated aerobically at 35–37°C for 18–24 hours (in some cases 16–18 hours is sufficient).
- After incubation the diameter of zone of inhibition around the disk is measured in millimeters using a ruler or calipers. A zone of 16 mm or less indicates resistance, while a zone more than 16 mm indicates susceptibility.
Method 2: Broth (Tube) Method (Rapid)
- A pure culture (18–24 hours old) is taken and a light inoculum is prepared in a tube containing 3 mL of Tryptic Soy Broth. The broth should appear clear without visible turbidity initially.
- One 5 µg novobiocin disk is aseptically added into the inoculated broth tube and the tube is shaken gently for about 10 seconds.
- A control tube is prepared simultaneously with the same inoculum but without addition of novobiocin disk.
- Both tubes are incubated aerobically at 35–37°C for up to 5 hours or until the control tube attains turbidity equivalent to 0.5 McFarland standard.
- The turbidity of test tube is compared with control. Presence of turbidity in disk-containing tube indicates resistance, while absence of turbidity indicates susceptibility.
Result of Novobiocin Susceptibility Test
Disk Diffusion Method (Mueller Hinton Agar)
- Susceptible (Sensitive) – zone of inhibition more than 16 mm.
- Resistant – zone of inhibition equal to or less than 16 mm.
Disk Diffusion Method (Blood Agar)
- Susceptible (Sensitive) – zone of inhibition equal to or more than 12 mm.
- Resistant – zone of inhibition less than 12 mm.
Tube (Broth) Method
- Susceptible (Sensitive) – no visible turbidity is seen in the broth.
- Resistant – visible turbidity is observed indicating growth.
Clinical Interpretation
- Resistant result – organism is presumptively identified as Staphylococcus saprophyticus though other coagulase negative staphylococci may also show resistance.
- Susceptible result – organism is likely Staphylococcus epidermidis, Staphylococcus haemolyticus or Staphylococcus aureus.
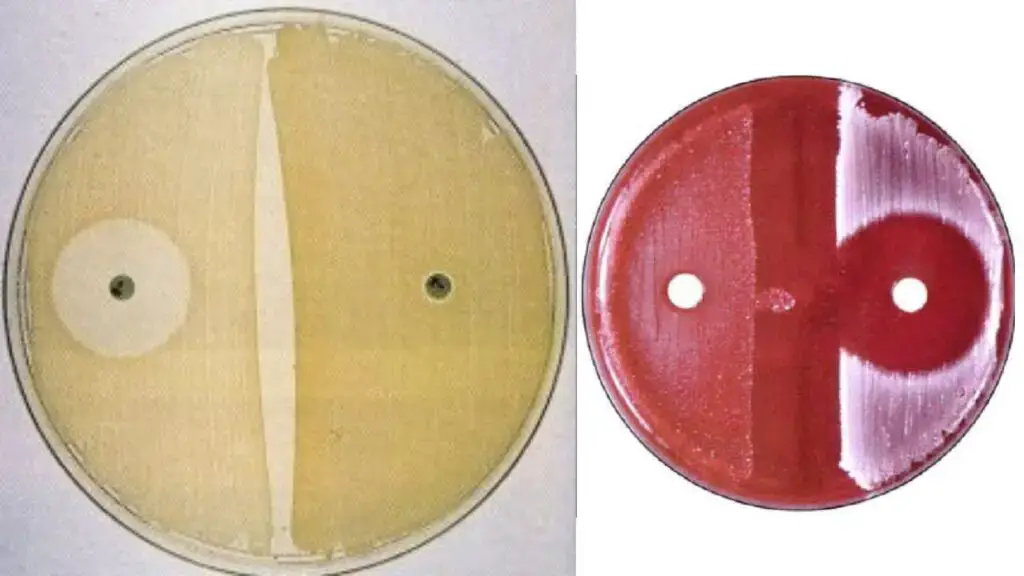
Organisms Showing Positive and Negative Result
Novobiocin Resistant (Positive Result)
- Staphylococcus saprophyticus
- Staphylococcus cohnii (subsp. cohnii and urealyticus)
- Staphylococcus xylosus
- Staphylococcus borealis
- Staphylococcus sciuri (subsp. sciuri carnaticus and rodentium)
- Staphylococcus hominis subsp. novobiosepticus
- Staphylococcus lentus
- Staphylococcus vitulinus (also known as S. pulvereri)
- Staphylococcus kloosii
- Staphylococcus equorum
- Staphylococcus arlettae
- Staphylococcus gallinarum
- Staphylococcus nepalensis
- Staphylococcus succinus
- Staphylococcus fleurettii
- Staphylococcus stepanovicii
Novobiocin Susceptible (Negative Result)
- Staphylococcus epidermidis
- Staphylococcus aureus
- Staphylococcus haemolyticus
- Staphylococcus hominis subsp. hominis
- Staphylococcus capitis (subsp. capitis and urealyticus)
- Staphylococcus lugdunensis
- Staphylococcus warneri
- Staphylococcus simulans
- Staphylococcus caprae
- Staphylococcus auricularis
- Staphylococcus carnosus
- Staphylococcus pasteuri
- Staphylococcus pettenkoferi
- Staphylococcus saccharolyticus
- Staphylococcus chromogenes
Uses of Novobiocin Susceptibility Test
- It is used for presumptive identification of Staphylococcus saprophyticus from urine samples as this organism is intrinsically resistant to novobiocin.
- It is used to differentiate Staphylococcus saprophyticus (resistant) from Staphylococcus epidermidis and other coagulase negative staphylococci which are usually susceptible.
- It helps in differentiation of subspecies of Staphylococcus hominis as S. hominis subsp. novobiosepticus shows resistance while S. hominis subsp. hominis is susceptible.
- It is used for taxonomic grouping of coagulase negative staphylococci into novobiocin susceptible group and novobiocin resistant group.
- It is applied in veterinary and food microbiology for detection of novobiocin resistant staphylococci from animal skin milk and meat products.
- It is also useful in screening of Staphylococcus borealis which shows phenotypic resistance to novobiocin and is isolated from human skin and elderly infections.
Advantages of Novobiocin Susceptibility Test
- It is a reliable method for presumptive identification of Staphylococcus saprophyticus as this organism shows intrinsic resistance to novobiocin.
- It is a simple practical and cost effective test which can be easily performed in routine microbiology laboratory.
- It helps in differentiation of Staphylococcus saprophyticus from other coagulase negative staphylococci which are usually novobiocin susceptible.
- It provides guidance for proper antibiotic therapy as identification of S. saprophyticus helps in selecting suitable drugs for urinary tract infection.
- It gives rapid results when tube method is used and results may be obtained within few hours.
- It shows higher accuracy as compared to some automated identification systems and chromogenic media.
- It is also useful for identification of other novobiocin resistant staphylococci such as S. cohnii, S. xylosus, S. sciuri and S. hominis subsp. novobiosepticus.
Limitation of Novobiocin Susceptibility Test
- It gives only presumptive identification of Staphylococcus saprophyticus and not a confirmatory diagnosis.
- It lacks specificity as several other coagulase negative staphylococci are also intrinsically resistant to novobiocin.
- It cannot differentiate Staphylococcus saprophyticus from Staphylococcus borealis which also shows novobiocin resistance.
- It is reliable mainly for isolates obtained from urine samples and results may be misleading when applied to other clinical specimens.
- It is time consuming when plate method is used as it requires 18–24 hours of incubation.
- It cannot be performed directly on primary specimens and requires isolated colonies from a pure culture.
- It needs additional biochemical tests for final identification as novobiocin resistance alone is not sufficient for species differentiation.
Quality Control
- Staphylococcus saprophyticus ATCC 15305 – used as novobiocin resistant (positive) control.
- Staphylococcus saprophyticus ATCC 13518 – may also be used as resistant control in some laboratories.
- Staphylococcus epidermidis ATCC 12228 – used as novobiocin susceptible (negative) control.
- Staphylococcus aureus ATCC 25923 – used as susceptible control strain.
- Staphylococcus epidermidis ATCC 14990 – used as novobiocin susceptible control.
Precautions of Novobiocin Susceptibility Test
- Standard laboratory precautions should be followed and all specimens should be handled as potentially infectious.
- Gloves and laboratory coat should be worn and all used media and materials should be properly sterilized after use.
- Novobiocin discs should be stored at 2–8°C in original container and protected from light heat and moisture.
- The discs should be allowed to attain room temperature before use to prevent condensation.
- Discs showing discoloration or damaged desiccant should not be used.
- The test should be performed only on isolated colonies from a pure culture and not for primary isolation.
- The test organism should be 18–72 hours old as older cultures may give incorrect results.
- The organism should be confirmed as Gram positive catalase positive and coagulase negative before performing the test.
- The inoculum should be standardized to 0.5 McFarland turbidity standard.
- The novobiocin disc should be placed within 15 minutes of inoculating the agar plate and pressed gently to ensure proper contact.
- Plates should be incubated aerobically at 35–37°C for appropriate time period.
- Results should be interpreted cautiously as the test gives only presumptive identification and other novobiocin resistant staphylococci may be present.
- Confirmatory biochemical or molecular tests should be performed when definitive identification is required.
- Abdulhusein, H. S., & Kadim, B. M. (2024). Detection and identification of Staphylococcus species isolated from skin wounds of cow in Al-Shatrah city-Southern Iraq. Journal of Education for Pure Science-University of Thi-Qar, 14(4), 115–128. https://doi.org/10.32792/jeps.v14i4.516
- Abraham, A. (2025, October 23). Novobiocin: Unlocking its mechanism of action. Abraham Entertainment.
- Aryal, S. (2022, August 10). Novobiocin susceptibility test – Principle, procedure, uses and interpretation. Microbiology Info. https://microbiologyinfo.com/novobiocin-susceptibility-test/
- Becker, K., Heilmann, C., & Peters, G. (2014). Coagulase-negative staphylococci. Clinical Microbiology Reviews, 27(4), 870–926. https://doi.org/10.1128/CMR.00109-13
- Brenhouse, L. (n.d.). Staphylococcus saprophyticus. Mechanisms of Pathogenicity.
- Chamberlain, N. R. (2016). Urinary tract infections (urethritis, cystitis, pyelonephritis). ATSU.
- Clinical and Laboratory Standards Institute. (2024). Breakpoints eliminated from CLSI document M100 since 2010.
- Clinical and Laboratory Standards Institute. (2024). QC ranges eliminated from CLSI document M100 since 2010.
- Clinical and Laboratory Standards Institute. (2025). Breakpoint implementation toolkit (BIT).
- Clinical and Laboratory Standards Institute. (2025). Performance standards for antimicrobial susceptibility testing (35th ed.). CLSI supplement M100.
- Dahal, P. (2023, March 6). Novobiocin susceptibility test- Principle, procedure, results. Microbe Notes. https://microbenotes.com/novobiocin-susceptibility-test-principle-procedure-and-results/
- Dalynn Biologicals. (2014, October). Novobiocin disks [Instructions for use].
- Dubois, D., Leyssene, D., Chacornac, J. P., Kostrzewa, M., Schmit, P. O., Talon, R., Bonnet, R., & Delmas, J. (2010). Identification of a variety of Staphylococcus species by matrix-assisted laser desorption ionization-time of flight mass spectrometry. Journal of Clinical Microbiology, 48(3), 941–945. https://doi.org/10.1128/JCM.00413-09
- Ehlers, S., & Merrill, S. A. (2023, June 26). Staphylococcus saprophyticus infection. In StatPearls. StatPearls Publishing.
- Gao, Y., Qiang, L., Wu, N., Tan, R., Sun, Y., Li, Z., Shen, X., & Cai, Y. (2024). Study on the potential probiotics isolated from marine aquaculture system and evaluation for aquaculture application. Aquaculture Research, 2024, 9555271. https://doi.org/10.1155/2024/9555271
- Gilad, J., & Schwartz, D. (2007). Identification of Staphylococcus species with the VITEK 2 system: The case of Staphylococcus hominis. Journal of Clinical Microbiology, 45(2), 685–686. https://doi.org/10.1128/JCM.02228-06
- Hardy Diagnostics. (2020). Novobiocin differentiation disks [Instructions for use].
- Hedman, P., Ringertz, O., Lindström, M., & Olsson, K. (1993). The origin of Staphylococcus saprophyticus from cattle and pigs. Scandinavian Journal of Infectious Diseases, 25(1), 57–60. https://pubmed.ncbi.nlm.nih.gov/8460350/
- Humphries, R., Bobenchik, A. M., Hindler, J. A., & Schuetz, A. N. (2021). Overview of changes to the Clinical and Laboratory Standards Institute performance standards for antimicrobial susceptibility testing, M100, 31st edition. Journal of Clinical Microbiology, 59(12), e00213-21. https://doi.org/10.1128/JCM.00213-21
- Iorio, N. L. P., Ferreira, R. B. R., Schuenck, R. P., Malvar, K. L., Brilhante, A. P., Nunes, A. P. F., Bastos, C. C. R., & dos Santos, K. R. N. (2007). Simplified and reliable scheme for species-level identification of Staphylococcus clinical isolates. Journal of Clinical Microbiology, 45(8), 2564–2569. https://doi.org/10.1128/JCM.00679-07
- Key Scientific Products. (n.d.). Novobiocin antibiotic 5 ug.
- Kovařovic, V., Finstrlová, A., Sedláček, I., Petráš, P., Švec, P., Mašlaňová, I., Neumann-Schaal, M., Šedo, O., Botka, T., Staňková, E., Doškař, J., & Pantůček, R. (2023). Staphylococcus brunensis sp. nov. isolated from human clinical specimens with a staphylococcal cassette chromosome-related genomic island outside of the rlmH gene bearing the ccrDE recombinase gene complex. Microbiology Spectrum, 11(5), e01342-23. https://doi.org/10.1128/spectrum.01342-23
- Latham, R. H., Running, K., & Stamm, W. E. (1983). Urinary tract infections in young adult women caused by Staphylococcus saprophyticus. JAMA, 250(22), 3063–3066.
- Lawal, O. U., Fraqueza, M. J., Bouchami, O., Worning, P., Bartels, M. D., Gonçalves, M. L., Paixão, P., Gonçalves, E., Toscano, C., Empel, J., Urbaś, M., Domínguez, M. A., Westh, H., de Lencastre, H., & Miragaia, M. (2021). Foodborne origin and local and global spread of Staphylococcus saprophyticus causing human urinary tract infections. Emerging Infectious Diseases, 27(3), 880–893. https://doi.org/10.3201/eid2703.200852
- Lee, T.-F., Lee, H., Chen, C.-M., Du, S.-H., Cheng, Y.-C., Hsu, C.-C., Chung, M.-Y., Teng, S.-H., Teng, L.-J., & Hsueh, P.-R. (2013). Comparison of the accuracy of matrix-assisted laser desorption ionization–time of flight mass spectrometry with that of other commercial identification systems for identifying Staphylococcus saprophyticus in urine. Journal of Clinical Microbiology, 51(5), 1563–1566. https://doi.org/10.1128/JCM.00261-13
- Mårdh, P.-A., Hovelius, B., Hovelius, K., & Nilsson, P. O. (1978). Coagulase-negative, novobiocin-resistant staphylococci on the skin of animals and man, on meat and in milk. Acta Veterinaria Scandinavica, 19(2), 243–253.
- Martins, K. B., Ferreira, A. M., Mondelli, A. L., Rocchetti, T. T., & da Cunha, M. L. R. S. (2018). Evaluation of MALDI-TOF VITEK®MS and VITEK® 2 system for the identification of Staphylococcus saprophyticus. Future Microbiology, 13, 1603–1609. https://doi.org/10.2217/fmb-2018-0195
- Namavar, F., De Graaff, J., De With, C., & Maclaren, D. M. (1978). Novobiocin resistance and virulence of strains of Staphylococcus saprophyticus isolated from urine and skin. Journal of Medical Microbiology, 11(3), 243–254. https://doi.org/10.1099/00222615-11-3-243
- Prinzi, A. (2023, December 13). Updating breakpoints in antimicrobial susceptibility testing. American Society for Microbiology.
- Prorok, P., Bierowiec, K., Skrok, M., Karwańska, M., Siedlecka, M., Miszczak, M., Książczyk, M., Kapczyńska, K., & Rypuła, K. (2025). Characteristics of Staphylococcus saprophyticus isolated from humans and animals. International Journal of Molecular Sciences, 26(14), 6885. https://doi.org/10.3390/ijms26146885
- Public Health Agency of Canada. (2024, February). Staphylococcus saprophyticus: Infectious substances pathogen safety data sheet. Government of Canada.
- Raz, R., Colodner, R., & Kunin, C. M. (2005). Who are you— Staphylococcus saprophyticus? Clinical Infectious Diseases, 40(6), 896–898. https://doi.org/10.1086/428353
- Remel. (n.d.). Novobiocin disk [Instructions for use]. Thermo Fisher Scientific.
- Taylor & Francis. (n.d.). Novobiocin. Taylor & Francis Knowledge.
- UiT The Arctic University of Norway. (2025, May 10). Discovery of antibiotic resistance in newly identified bacterium. ScienceDaily.
- Vickers, A. A., Chopra, I., & O’Neill, A. J. (2007). Intrinsic novobiocin resistance in Staphylococcus saprophyticus. Antimicrobial Agents and Chemotherapy, 51(12), 4484–4485. https://doi.org/10.1128/AAC.00708-07
- VUMIE. (2022). Novobiocin susceptibility test [Virtual Microbiology Lab Simulator].
- Widerström, M., Wiström, J., Ferry, S., Karlsson, C., & Monsen, T. (2007). Molecular epidemiology of Staphylococcus saprophyticus isolated from women with uncomplicated community-acquired urinary tract infection. Journal of Clinical Microbiology, 45(5), 1561–1564. https://doi.org/10.1128/JCM.02071-06
- Wikipedia. (n.d.). Staphylococcus.
- Xie, W., Luo, D., & Wang, Z. (2025). Metabolic analysis of the mode of action and mode of resistance for novobiocin in Staphylococcus aureus. Zoonoses, 5(1). https://doi.org/10.15212/ZOONOSES-2024-0035
- Text Highlighting: Select any text in the post content to highlight it
- Text Annotation: Select text and add comments with annotations
- Comment Management: Edit or delete your own comments
- Highlight Management: Remove your own highlights
How to use: Simply select any text in the post content above, and you'll see annotation options. Login here or create an account to get started.