What is pH Meter?
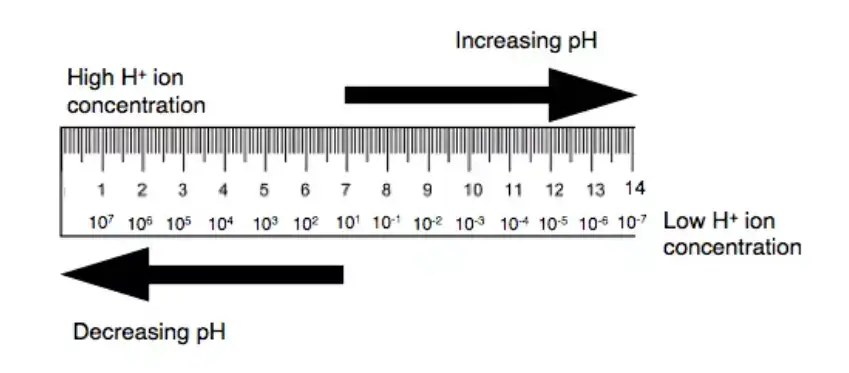
A pH meter is a critical instrument used for measuring the acidity or alkalinity of aqueous solutions, quantified in terms of pH. The pH scale ranges from 0 to 14, where a value of 7 denotes neutrality, values below 7 indicate acidity, and values above 7 denote alkalinity. This scale reflects the concentration of hydrogen ions (H⁺) in the solution: higher concentrations of hydrogen ions correspond to lower pH values, indicating greater acidity, while lower concentrations result in higher pH values, indicating greater alkalinity.
The pH meter operates by determining the difference in electrical potential between two electrodes: the pH electrode and the reference electrode. This difference in electrical potential correlates to the hydrogen ion concentration in the solution. The fundamental principle underlying the pH meter involves the logarithmic relationship between pH and hydrogen ion concentration. Specifically, pH is calculated using the formula:
\text{pH} = -\log_{10}[H^+]where [H+]represents the concentration of hydrogen ions in moles per liter.
The pH meter’s accuracy surpasses that of traditional pH test strips, which often provide less precise readings. The advent of the pH meter, with its ability to deliver more accurate measurements, was a significant advancement in analytical chemistry. The first glass electrode was introduced in 1909 by Fritz Haber and Zygmunt Klemensiewicz, laying the groundwork for modern pH measurement technology. By 1934, Arnold Beckman developed the first electronic pH meter, marking a pivotal development in the field.
In practical applications, pH meters are invaluable across various domains, including food manufacturing, chemical solution preparation, soil testing, and quality control. Their ability to deliver precise pH readings makes them essential tools in both laboratory and field settings.
Definition of pH Meter
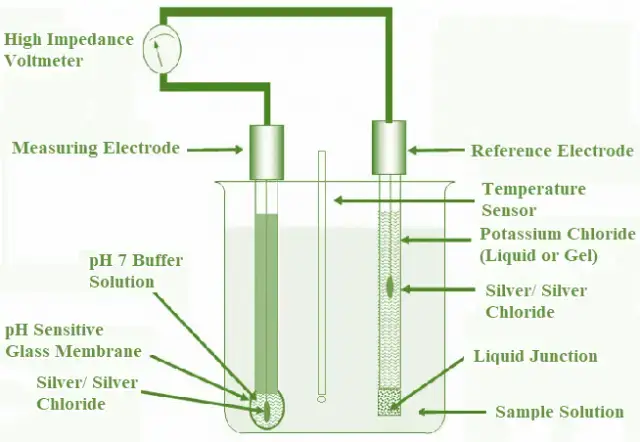
A pH meter is a scientific instrument used to measure the acidity or alkalinity of aqueous solutions by determining the hydrogen-ion activity, expressed as pH. It operates based on the difference in electrical potential between a pH electrode and a reference electrode.
Principle of pH Meter
- The principle of a pH meter is based on the interaction between a sample solution and a specially designed electrode system. This system measures the concentration of hydrogen ions in the solution, thereby determining its pH.
- The core mechanism of the pH meter involves the exchange of ions between the sample solution and the inner solution of the glass electrode. The glass electrode is typically filled with a buffer solution of pH 7, while the reference electrode is filled with a saturated potassium chloride solution. The glass electrode contains a porous glass membrane coated with metal salts and silica.
- When the electrode is immersed in the sample solution, hydrogen ions from the solution diffuse through the glass membrane and interact with the metal ions within the electrode. This ion exchange results in a potential difference across the glass membrane. The reference electrode, which maintains a stable potential, helps to measure this difference in electrical potential.
- The pH meter translates this voltage difference into a pH value using the Nernst equation. As the hydrogen ion concentration increases in an acidic solution, the voltage generated by the electrode system increases, which corresponds to a lower pH value. Conversely, in a basic solution, a higher concentration of hydroxyl ions reduces the voltage, leading to a higher pH value.
- Thus, the pH meter provides a precise measurement of pH by converting the electrical potential into a readable pH value. This conversion is crucial for accurately assessing the acidity or alkalinity of various solutions, making the pH meter an essential tool in numerous scientific and industrial applications.
Parts of a pH meter
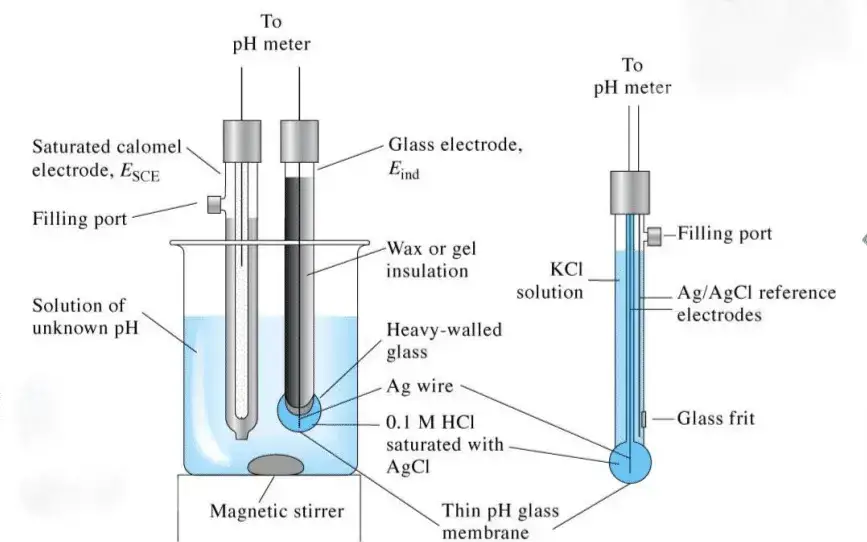
A pH meter is composed of several key components, each playing a crucial role in accurately measuring the pH of a solution. The primary parts of a pH meter include:
- High Input Impedance Meter
- Function: This component houses the microprocessor responsible for processing the minute voltages produced by the electrode system. It converts these voltages into pH readings displayed on the meter.
- Details: The microchip within the meter calculates the pH based on the measured voltage, adjusts for temperature variations, and ensures precise readings.
- Combined Electrode
- Function: This is the primary measurement component of the pH meter, integrating both the reference and measurement electrodes into a single unit.
- Details:
- Reference Electrode: Consists of a reference material, such as mercury or silver chloride, immersed in an electrolyte like potassium chloride. This electrode maintains a stable, known potential independent of the test solution.
- pH Glass Electrode: Also known as the sensor electrode, this component has a glass bulb sensitive to hydrogen ions. The electrode’s voltage changes in response to variations in hydrogen ion concentration, which is crucial for determining the pH.
- Amplifier
- Function: The amplifier enhances the voltage signal from the electrodes, improving the accuracy of the pH measurement.
- Details: It ensures that the voltage readings are accurately reflected within the pH scale (0–14), thereby providing precise measurements of acidity or alkalinity.
- Thermometer Probe
- Function: Some pH meters include a temperature probe to measure the solution’s temperature, which is essential for accurate pH readings.
- Details: The temperature probe enables Automatic Temperature Compensation (ATC), adjusting the pH reading based on the solution’s temperature, as pH can be temperature-dependent.
- Electrometer/Voltmeter
- Function: This component measures the minimal differences in electrical potential across the electrodes.
- Details: It is connected to the pH electrode and translates the electrical potential into a readable pH value.
- Filling Hole
- Function: The filling hole allows for the replenishment of the electrolyte within the electrodes.
- Details: Proper maintenance of the electrolyte levels is essential for the accurate performance and longevity of the pH meter.
Each part of the pH meter contributes to its overall function, ensuring accurate and reliable measurements of a solution’s pH.
| Parts of a pH meter | Description |
|---|---|
| High Input Impedance Meter | Central unit with a microprocessor for processing electrode voltages and displaying pH measurements. |
| Combined Electrode | Fusion of reference electrode and pH glass electrode. |
| pH Glass Electrode | Glass bulb sensitive to hydrogen ions, producing a millivolt output corresponding to the solution’s pH. |
| Reference Electrode | Provides stable potential, with a reference material submerged in an electrolyte and a porous ceramic junction. |
| Amplifier | Enhances pH reading accuracy, keeping voltage within the pH range of 0–14. |
| Thermometer Probe | Measures solution temperature for Automatic Temperature Compensation (ATC) adjustments. |
| Electrometer/Voltmeter | Detects minute electrical potential differences in the circuit for accurate pH measurements. |
| Sample Chamber | Holds the solution for electrode submersion and pH measurement. |
| Calibration Solution | Used for periodic calibration to ensure pH meter accuracy. |
| Power Source | Provides power, can be batteries or electrical outlet connection. |
| Case | Protects the pH meter’s delicate components from damage. |
| Probe | Multifunctional, including a glass electrode, reference electrode, and temperature probe for pH measurement. |
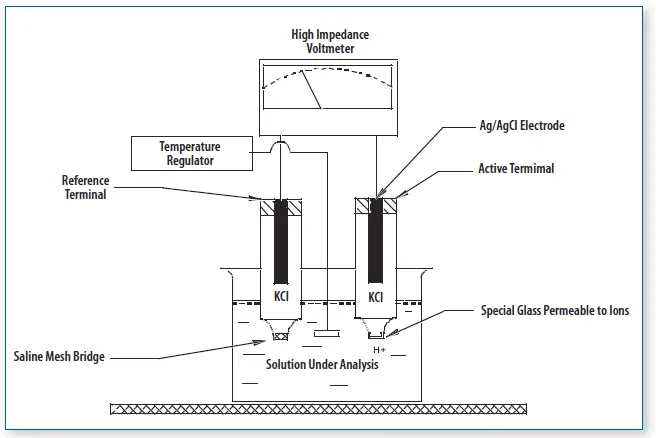
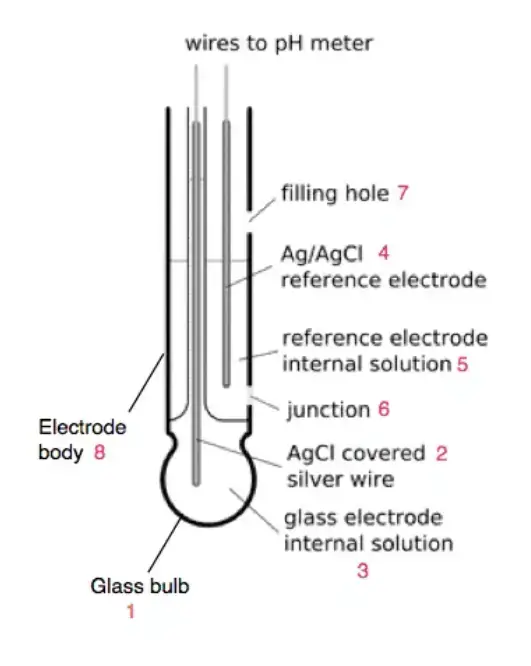
pH Meter Operating Procedure
The accurate measurement of pH requires careful preparation and proper handling of the pH meter and its components. The following steps outline the procedure for operating a pH meter:
- Preparation
- Temperature Stabilization: Ensure that all samples are at the same temperature since pH readings can be affected by temperature variations. The ideal temperature for measurements is 25°C. Use a thermometer to check the temperature, and either manually input this into the pH meter or use an Automatic Temperature Compensation (ATC) probe for automatic adjustment.
- Sample Preparation: Uncover the sample beakers and prepare the samples to ensure they are ready for measurement.
- Electrode Preparation
- Initial Cleaning: Remove the pH electrode from its storage solution. Rinse the electrode with deionized water to remove any contaminants. This step is crucial to prevent cross-contamination between samples.
- Drying: Gently blot the electrode with non-abrasive materials, such as Kimwipes or Shurwipes, to remove excess water without damaging the electrode.
- Measurement
- Submersion: Insert the pH electrode into the sample beaker, ensuring that the electrode tip and junction are fully submerged in the sample. Avoid using the same beaker for rinsing and measuring to prevent contamination.
- Stirring: Stir the sample gently and uniformly to ensure an accurate measurement.
- Setting the Meter: Activate the meter and set it to begin taking a reading. Allow the meter to stabilize; this usually takes 1 to 2 minutes.
- Recording Data
- Stabilization: Wait until the pH reading stabilizes. Once stable, record the pH value and the temperature of the sample.
- Repeat Measurements: For additional samples, repeat steps 2 through 4. Ensure that the electrode is submerged to the same depth in each sample for consistency.
- Post-Measurement Care
- Cleaning the Electrode: After completing the measurements, rinse the electrode with deionized water to remove any residual sample. Blot dry with Kimwipes.
- Storage: Place the electrode in a pH electrode storage solution, typically a 3M potassium chloride solution, to maintain its condition for future use.
Types of pH Meter
pH meters are critical instruments in various fields, and their design and functionality can vary significantly depending on their intended use. The following are the main types of pH meters, categorized based on portability, usage, level of advancement, and reading method:
Based on Portability
- Pen Testers
- Description: Compact and portable, pen testers are designed for ease of transport and use. They integrate the pH meter, display, and electrode into a single, pocket-sized unit.
- Applications: Ideal for quick checks in fields such as hydroponics, food production, and pool maintenance.
- Handheld Meters
- Description: Handheld meters are slightly larger than pen testers and feature a separate electrode from the meter. This design allows for interchangeable electrodes suited to different measurement needs.
- Applications: Commonly used in environmental research, agriculture, and water treatment.
- Benchtop Meters
- Description: These meters are larger and designed to be used on a desk or mounted on a wall. They offer high accuracy and are often used in laboratory settings.
- Applications: Suitable for precise measurements in laboratories, environmental monitoring, and food processing.
Based on Usage
- Laboratory pH Meters
- Description: These meters are characterized by their wide measurement range and high accuracy. They are versatile and suited for detailed scientific analysis.
- Applications: Used in academic research, quality control in manufacturing, and detailed chemical analysis.
- Industrial (Online) pH Meters
- Description: These meters are designed for continuous monitoring in industrial settings. They feature digital intelligence and can handle boundary alarms and control functions.
- Applications: Ideal for real-time process control in industries such as chemical manufacturing and water treatment.
Based on Advanced Level
- Economic pH Meters
- Description: These meters offer basic functionality at a lower cost. They are suitable for general use where high precision is not critical.
- Intelligent pH Meters
- Description: These advanced meters come with additional features for various applications, including automatic temperature compensation and data logging.
- Applications: Used in diverse fields such as water conditioning, aquariums, food processing, and laboratories.
- Precision pH Meters
- Description: Known for their high accuracy, precision pH meters are categorized into pointer and digital types.
- Pointer pH Meters: Utilize a needle to indicate pH levels on a dial. Accuracy depends on the user’s ability to interpret the needle’s position.
- Digital pH Meters: Display the pH value numerically, offering easier and more precise readings.
- Description: Known for their high accuracy, precision pH meters are categorized into pointer and digital types.
Based on Reading Method
- Analog pH Meters
- Description: These meters use a needle to indicate the pH level on a scale. While they provide reliable readings, they require careful interpretation of the needle’s position.
- Applications: Suitable for basic fieldwork where digital precision is not essential.
- Digital pH Meters
- Description: Digital meters display the pH value directly on a screen, making them easier to read and more accurate than analog meters.
- Applications: Preferred in both laboratory and field settings for their ease of use and precision.
What is pH Electrode?
- A pH electrode is a device used to measure the acidity or alkalinity of a solution, which is expressed as its pH value. It consists of two main components: a glass electrode and a reference electrode.
- The glass electrode is the sensing element of the pH electrode. It is typically a thin, bulbous-shaped glass membrane that is selectively permeable to hydrogen ions (H+). The glass membrane interacts with the H+ ions in the solution being tested, causing an exchange of ions and generating an electrical potential. The potential is directly related to the pH of the solution, with acidic solutions generating a higher potential and alkaline solutions generating a lower potential.
- The reference electrode provides a stable reference point for the pH measurement. It is typically a silver/silver chloride (Ag/AgCl) electrode immersed in a potassium chloride (KCl) electrolyte solution. The reference electrode maintains a constant electrical potential, serving as a reference against which the potential of the glass electrode is measured. This allows for accurate and consistent pH measurements.
- To measure pH, the glass electrode and the reference electrode are connected to a pH meter. The pH meter measures the potential difference between the two electrodes and converts it into a pH value. The pH meter may also incorporate temperature compensation to account for the effect of temperature on pH measurements.
- Different types of pH electrodes are available for specific applications. Combination electrodes combine both the glass and reference electrodes into a single unit, making them convenient and versatile. There are also specialized electrodes such as flat surface electrodes for measuring pH on solid surfaces or microelectrodes for small volume samples.
- pH electrodes require proper handling and maintenance to ensure accurate and reliable measurements. Regular calibration, storage in appropriate solutions, and cleaning are essential for maintaining electrode performance and prolonging its lifespan.
- In summary, a pH electrode is a crucial tool for measuring pH in various industries and applications. Its design, incorporating a glass electrode and a reference electrode, allows for the accurate determination of the acidity or alkalinity of a solution, enabling precise control and analysis in scientific, industrial, and environmental settings.
How pH Electrodes Works?
A pH electrode operates by utilizing the principle of electrochemical potential to measure the pH of a solution. Here is how it works:
- Glass Electrode: The pH electrode consists of a glass electrode and a reference electrode. The glass electrode has a thin membrane made of pH-sensitive glass. This glass is selective and reacts with hydrogen ions (H+) present in the solution being measured. The glass electrode is filled with an internal reference solution of known pH, creating a stable reference point.
- Reference Electrode: The reference electrode is designed to maintain a constant electrical potential. It typically consists of a metal wire or rod coated with a reference electrolyte. This electrolyte helps maintain a stable electrical potential at the reference electrode.
- Interaction with Solution: When the pH electrode is immersed in a solution, hydrogen ions from the solution interact with the pH-sensitive glass membrane of the glass electrode. The hydrogen ions pass through the glass membrane, causing a change in the electrical potential of the glass electrode.
- Potential Difference: The interaction of hydrogen ions with the glass electrode creates a potential difference between the glass electrode and the reference electrode. This potential difference, known as the pH voltage, is directly proportional to the pH of the solution. The greater the concentration of hydrogen ions, the higher the acidity and lower the pH value.
- Measurement with pH Meter: The pH meter is connected to the pH electrode and measures the potential difference between the glass electrode and the reference electrode. It converts this electrical signal into a numerical pH value, which is displayed on the pH meter.
- Calibration and Compensation: To ensure accurate measurements, pH electrodes often require calibration and compensation. Calibration involves adjusting the pH meter based on known pH standards to account for any deviations or drift in the electrode’s response. Additionally, temperature compensation may be applied to account for the effect of temperature on pH measurements.
Types of pH Electrodes
There are several types of pH electrodes available, including:
- Glass electrodes: Glass electrodes are the most common type of pH electrode, and are used in a wide range of applications. They are made of a thin glass membrane that is coated with a specific type of glass called pH glass. The pH glass is designed to react with hydrogen ions in the solution, which allows it to measure the pH of the solution.
- Combination electrodes: Combination electrodes are a type of pH electrode that combines a glass electrode with a reference electrode in a single unit. They are often used in portable or handheld pH meters, as they are more compact and convenient to use than separate glass and reference electrodes.
- Flat surface electrodes: Flat surface electrodes are a type of pH electrode that is designed to measure the pH of flat surfaces or thin films. They are often used in applications such as coating or film thickness measurements, where a traditional glass electrode may not be suitable.
- Specialized electrodes: There are also many specialized types of pH electrodes available for specific applications, such as low temperature electrodes for use in freezing environments, high temperature electrodes for use in hot environments, and electrodes for use in highly viscous or abrasive solutions.
Overall, the type of pH electrode that is best suited for a particular application depends on the specific requirements of the measurement, such as accuracy, sensitivity, durability, and cost.
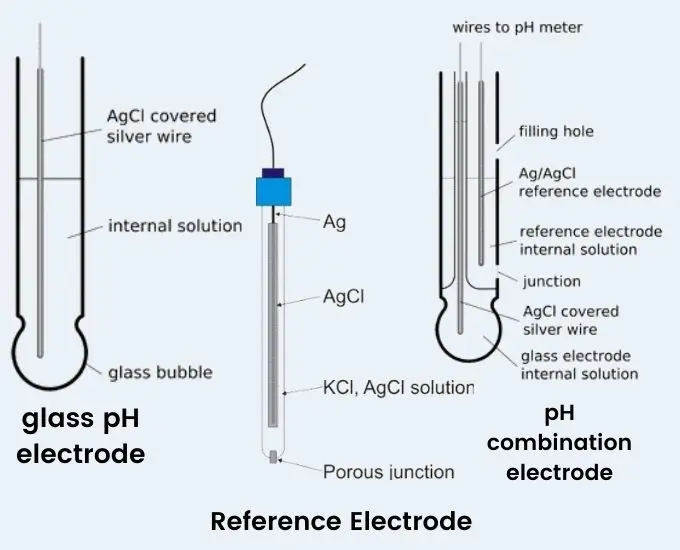
What is Effect of temperature on pH measurement?
Temperature plays a pivotal role in determining the accuracy of pH measurements. The relationship between temperature and pH is intricate, and understanding this interplay is crucial for precise pH determinations. Here’s an in-depth examination of how temperature impacts pH measurements:
- Electrode Sensitivity: The core component of a pH meter is its glass electrode, which possesses a pH-sensitive glass membrane. This membrane’s responsiveness to hydrogen ions (H+) varies with temperature. Specifically, as the temperature escalates, the electrode’s sensitivity diminishes.
- Variability of the pH Scale: The pH scale, which categorizes solutions based on their acidity or alkalinity, is not static across different temperatures. The dissociation constants of water and other substances, which are fundamental to the pH scale, shift with temperature changes. Notably, the standard pH scale is calibrated at 25°C, and any deviation from this benchmark can introduce measurement inaccuracies.
- Water Ionization Dynamics: Water’s propensity to ionize into hydrogen ions (H+) and hydroxide ions (OH-) is not constant but fluctuates with temperature. Elevated temperatures augment the concentration of hydrogen ions, thereby lowering the pH. In contrast, cooler temperatures elevate hydroxide ion concentrations, resulting in a pH increase.
- Compensatory Mechanisms: Recognizing the profound effect of temperature on pH, modern pH meters incorporate automatic temperature compensation (ATC) mechanisms. These systems adjust the pH reading in accordance with the detected temperature, leveraging algorithms and predefined temperature coefficients. This ensures that the readings account for changes in both electrode sensitivity and the pH scale due to temperature variations.
- Electrode Integrity and Performance: Extreme temperatures, whether excessively high or low, can compromise the functionality and longevity of the pH electrode. Such conditions can induce electrode drift, diminish its sensitivity, or even inflict physical harm. Hence, it’s imperative to operate pH meters within their designated temperature confines.
For optimal pH measurement accuracy, it’s essential to calibrate the pH meter using standard buffer solutions that match the intended operational temperature. Furthermore, harnessing the ATC features and ensuring a stable measurement environment temperature-wise can significantly mitigate potential temperature-induced errors in pH assessments.
pH Meter Calibration method
Calibration of the pH metre is a crucial duty that must be performed daily before to doing any tests with the pH metre.
Utilize the pH metre and electrode system in accordance with the manufacturer’s instructions or the pertinent SOPs. All measurements should be conducted between 20 and 25 degrees Celsius. The device is calibrated with the potassium hydrogen phthalate buffer solution (primary standard) (buffer pH 4.0) and another buffer solution with a different pH, preferably buffer pH 9.2. The pH measurement of a third buffer with a pH of 7.0 must not vary by more than 0.05 units.
Preparation of Standard Buffer
- Buffer Solution pH 4.00 (200C): Transfer the contents of the pH 4.00 buffer capsule or tablet to a 100 ml volumetric flask. Dissolve in approximately 80 ml of pure water, top off to 100 ml with purified water, and stir.
- Buffer Solution pH 7.00 (200C): Transfer the contents of the pH 7.00 buffer pill or capsule to a 100 ml volumetric flask. Dissolve in approximately 80 ml of pure water, top off to 100 ml with purified water, and stir.
- Buffer Solution pH 9.20 (200C): Transfer the contents of the pH 9.2 buffer pill or capsule to a 100 ml volumetric flask. Dissolve in approximately 80 ml of pure water, top off to 100 ml with purified water, and stir.
pH Meter Calibration Procedure
To calibrate a pH meter and ensure accurate measurements, follow this calibration procedure:
- Select Calibration Buffer: Choose the appropriate pH buffer solution for calibration. pH meters are commonly calibrated using pH 4, pH 7, and pH 9.2 buffer solutions. It is recommended to start with pH 7 calibration.
- Immerse the Electrode: Immerse the pH electrode in the pH 7 buffer solution. Ensure that the electrode is fully submerged in the solution.
- Temperature Adjustment: Determine the temperature of the buffer solution using a thermometer, if necessary. Adjust the temperature knob on the pH meter to match the solution’s temperature. Temperature affects pH readings, so accurate temperature compensation is important.
- pH Mode Selection: Set the Function Switch on the pH meter to the pH Mode. This mode allows calibration and pH measurement.
- Calibration Knob Adjustment: Adjust the “Calibrate” knob on the pH meter until the display reads 7.00, matching the pH value of the calibration buffer solution.
- Function Switch Reset: Turn the Function Switch back to the Standby position after calibration. This prepares the pH meter for the next step in the calibration procedure.
- Rinse the Electrode: Remove the pH electrode from the pH 7 buffer solution and rinse it thoroughly with distilled water. Ensure that the electrode is free from any residual buffer solution.
- Multiple Buffer Solutions: Place the pH electrode in the first of the remaining buffer solutions. These solutions typically include pH 4 and pH 9.2. Ensure the electrode is fully immersed in the solution.
- Slope Adjustment: Adjust the “Slope%” control on the right side of the pH meter until the display reads 4,000. This adjustment ensures the pH meter is correctly calibrated to accurately measure the pH values of acidic and alkaline solutions.
- Rinse the Electrode: After calibration with the pH 4 buffer solution, remove the electrode and rinse it thoroughly with distilled water to remove any residual solution.
- Repeat Calibration: If necessary, repeat steps 7 to 10 for the remaining buffer solutions.
- Standby Mode: Always keep the Function Switch in the Standby position when not in use after pH measurement or calibration.
Why we need to Calibrate a pH Meter? what will happen if we don’t calibrate?
Calibrating a pH meter is essential for several reasons:
- Accuracy: pH meters are designed to provide precise and reliable measurements of pH. However, over time, factors such as electrode aging, contamination, and drift can affect their accuracy. Calibration compensates for these factors and ensures that the pH meter is providing accurate readings.
- Standardization: pH calibration involves comparing the pH meter’s readings to known pH values of calibration buffer solutions. This process establishes a reference point and standardizes the pH meter’s response. It allows for consistent and comparable measurements across different instruments and laboratories.
- Sensitivity: pH meters are sensitive instruments, and even minor changes in electrode performance or conditions can impact the accuracy of pH readings. Calibration optimizes the sensitivity of the pH meter, ensuring it can detect and measure small pH changes effectively.
- Quality Control: In various industries, such as food and beverage, pharmaceuticals, environmental monitoring, and research, accurate pH measurements are critical for quality control. Calibration ensures that pH measurements adhere to established standards and regulatory requirements, helping to maintain product quality, safety, and compliance.
- Process Optimization: In some applications, pH levels need to be maintained within specific ranges for optimal performance. Calibration ensures that the pH meter accurately reflects the true pH of the solution, enabling appropriate adjustments and interventions to maintain desired pH levels.
Failure to calibrate a pH meter can have several consequences:
- Inaccurate Measurements: Without calibration, the pH meter may provide readings that deviate from the actual pH value of the solution. This can lead to incorrect decisions, improper adjustments, or an inability to identify critical pH changes that could impact processes, product quality, or safety.
- Misinterpretation of Results: Uncalibrated pH meters may produce inconsistent or unreliable readings, leading to misinterpretation of experimental or process data. This can result in erroneous conclusions, wasted resources, and compromised research or production outcomes.
- Compliance Issues: In regulated industries, failure to calibrate pH meters can lead to non-compliance with quality standards and regulatory requirements. This may result in legal and financial implications, product recalls, or compromised safety and efficacy of products.
- Process Failures: In applications where pH levels directly influence chemical reactions, enzymatic processes, or microbial growth, uncalibrated pH meters can lead to process failures. For example, in wastewater treatment, an inaccurate pH reading may result in ineffective treatment or environmental harm.
To ensure reliable and accurate pH measurements, regular calibration of pH meters is crucial. It enables confidence in the results obtained, supports quality control efforts, and helps to prevent adverse consequences in various scientific, industrial, and environmental applications.
How to clean ph meter?
To clean a pH meter, you will need to follow the specific instructions for your pH meter. Here are some general steps that you may need to follow:
- Disconnect the pH meter: If your pH meter is plugged into an electrical outlet, unplug it. If your pH meter is battery-powered, remove the battery.
- Rinse the probe: Rinse the probe with distilled water to remove any dirt or debris. Avoid using tap water, as it may contain minerals that can interfere with the accuracy of the pH meter.
- Wipe the probe: Wipe the probe with a soft, dry cloth to remove any remaining dirt or debris. Avoid using abrasive materials or harsh chemicals, as these may damage the probe.
- Dry the probe: Allow the probe to air dry or gently blot it with a soft, dry cloth. Do not use heat or a hair dryer to dry the probe, as this may damage it.
- Clean the meter: Wipe the exterior of the pH meter with a soft, dry cloth. Avoid using abrasive materials or harsh chemicals, as these may damage the meter.
- Store the pH meter: After cleaning, store the pH meter in a dry, dust-free location. If the pH meter will not be used for an extended period of time, you may want to store it in a storage solution to help preserve the probe.
Application of pH Meter
pH meters have a wide range of applications across various industries and sectors. Here are some common applications of pH meters:
- Agriculture: pH meters are used in agriculture to assess soil pH levels. This information helps farmers determine the acidity or alkalinity of the soil, enabling them to make informed decisions about soil amendments and fertilizer application. Maintaining the optimal pH range is crucial for crop growth and maximizing yields.
- Water Treatment: pH meters play a vital role in water treatment facilities. They are used to monitor and control the pH levels of water to ensure it meets regulatory standards and is safe for consumption. pH adjustment is necessary to optimize the efficiency of water treatment processes and prevent issues such as corrosion or scaling.
- Industrial Processes: pH meters are utilized in various industrial processes, including chemical industries. They are used to measure and control the pH of wastewater generated by industries such as steel, pulp and paper, pharmaceuticals, biotechnology, and petrochemicals. Proper pH control is essential for minimizing environmental impact and complying with regulations.
- Quality Control in Manufacturing: pH meters are employed in the quality control of chemical compounds and food products. They help ensure that products meet specific pH requirements for safety, effectiveness, and shelf life. For example, in the food industry, pH meters are used to monitor acidity levels in dairy products, beverages, sauces, and other food items.
- Medical and Biological Applications: pH meters are used in medical and biological research to measure the pH of biological fluids such as blood, urine, gastric acid, and cell culture media. Monitoring pH levels in these fluids provides valuable insights into the physiological and metabolic conditions of organisms and aids in disease diagnosis and treatment.
- Detergent Manufacturing: pH meters find application in the detergent manufacturing industry. They are used to monitor and control the pH of detergent formulations to ensure optimal cleaning performance and stability of the products.
Advantages of pH Meter
pH meters offer several benefits across a range of applications due to their accuracy, versatility, and ease of use. The following points outline the key advantages of pH meters:
- Accuracy and Precision
- High Accuracy: pH meters provide highly accurate pH measurements, essential for precise control in various industrial and scientific applications.
- Consistent Precision: The device offers reproducible results, ensuring that measurements are reliable and consistent across different tests.
- Versatility in Measurement
- Wide Range of Solutions: pH meters can measure pH levels in both oxidizing and reducing solutions, making them suitable for diverse chemical environments.
- Applicable to Various Solutions: They are effective in measuring the pH of colloidal, turbid, and colored solutions without interference, which is crucial for complex samples.
- Ease of Use and Portability
- User-Friendly Operation: The device is simple to operate, making it accessible for users with varying levels of expertise. Clear instructions and straightforward controls enhance usability.
- Portability: pH meters are portable and can be used conveniently in various locations, including fieldwork and laboratory settings, without the need for extensive setup.
- Cost-Effectiveness
- Low Installation Costs: pH meters do not require high installation expenses, making them a cost-effective solution for pH measurement.
- Long-Term Durability: When properly maintained, pH meters have a long service life, providing sustained value over time.
- Non-Destructive Testing
- Solution Integrity: pH meters do not alter or affect the solution being tested. This non-destructive feature ensures that samples remain intact for further analysis or use.
- Rapid Measurement Capability
- Quick Results: pH meters deliver rapid measurements, facilitating efficient data collection and process monitoring in both laboratory and industrial settings.
- Adaptability
- Automatic Recording and Control: pH meters are well-suited for continuous automatic recording and control in industrial and commercial processes, enhancing operational efficiency.
- Comparative Advantage
- Superior to pH Strips: Compared to pH strips, pH meters provide more precise and accurate readings, making them preferable for applications requiring high precision.
Limitations of pH Meter
While pH meters offer many advantages, they also have certain limitations that users should be aware of. Here are some common limitations of pH meters:
- Cleaning and Contamination: pH meters require regular cleaning to prevent contamination of samples. If the electrode or probe is not cleaned properly, residue from previous measurements can affect the accuracy of subsequent readings. Contaminants can interfere with the response of the pH meter and lead to incorrect pH measurements.
- Fragility of Glass Electrode: The glass tip of the pH meter’s probe is delicate and susceptible to damage. Exposure to corrosive chemicals or rough handling can cause the glass electrode to break or get damaged, rendering the pH meter unusable or inaccurate. Care must be taken to handle the pH meter with caution and avoid exposing it to harsh substances.
- Temperature Effects: External factors, particularly temperature, can impact the output readings of pH meters. pH measurements are temperature-dependent, and variations in temperature can affect the accuracy of the readings. It is essential to calibrate the pH meter at the operating temperature or use temperature compensation techniques to obtain accurate results.
- Deposits and Build-up: Deposits on the electrode membranes can interfere with the measurement process. Substances present in the sample solution may accumulate on the electrode over time, leading to reduced sensitivity or slower response times. Regular maintenance and cleaning are necessary to prevent deposits and ensure optimal performance.
- Calibration Requirements: pH meters require regular calibration using buffer solutions to maintain accuracy. Calibration adjusts the pH meter to provide correct readings within a specified range. Failure to calibrate the pH meter before use or inadequate calibration can result in inaccurate pH measurements and distorted results.
- Dependence on Buffer Solutions: pH meters rely on specific buffer solutions for calibration. These solutions need to be prepared correctly and stored properly to ensure their accuracy. Without proper buffer solutions, it becomes challenging to calibrate the pH meter accurately and obtain reliable pH measurements.
Precautions
When working with a pH meter, it is important to take certain precautions to ensure accurate and reliable measurements. Here are some key precautions to consider:
- Handle pH Electrodes with Care: pH electrodes are sensitive and fragile, so it is crucial to handle them gently. Avoid using them as a stirring rod in the solution, as this can lead to damage or breakage. Treat the electrode with care to maintain its integrity.
- Calibrate Regularly: pH meters should be calibrated daily or as recommended by the manufacturer using standard buffer solutions. Calibration ensures that the pH meter is providing accurate readings. Regular calibration is essential for obtaining reliable results.
- Protect from Sunlight: pH readings can be influenced by temperature, and direct exposure to sunlight can affect the temperature of the pH meter. To prevent temperature variations, avoid exposing the pH meter to direct sunlight during measurements.
- Clean Glassware and Apparatus: Before conducting pH measurements, ensure that all test tubes, glassware, and other apparatus are properly cleaned with distilled water. Residual substances or contaminants on the glassware can interfere with pH readings and affect the accuracy of the measurement.
- Use Fresh Droppers or Glass Rods: To prevent cross-contamination between samples, use a new dropper or glass rod for each new sample. Alternatively, if reusing the dropper or rod, ensure thorough washing with water between uses to eliminate any residue that could affect subsequent measurements.
- Prepare Fresh Solutions: To ensure accurate pH measurements, it is important to use freshly prepared solutions. Solutions that have been sitting for an extended period may undergo changes in pH, compromising the accuracy of the readings. Prepare fresh solutions for each measurement to obtain reliable results.
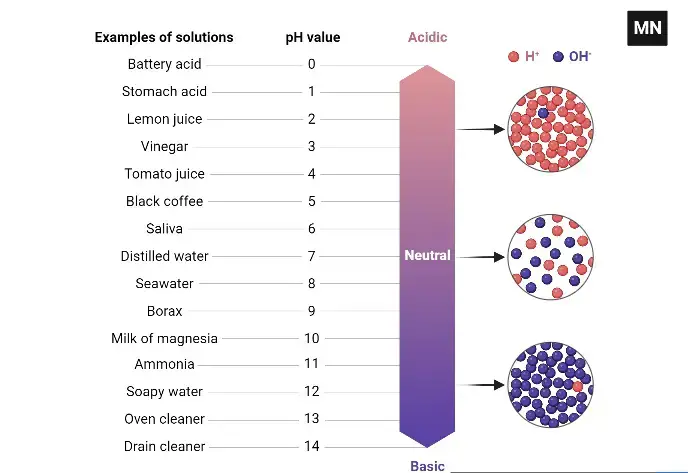
Why Range of pH (1 to14)?
The pH scale is a measure of the acidity or basicity of a solution. It ranges from 0 to 14, with a pH of 7 being neutral. Solutions with a pH less than 7 are acidic, while solutions with a pH greater than 7 are basic or alkaline.
The range of pH from 1 to 14 was chosen because it covers the full range of acidity and basicity that can be found in natural and man-made solutions. At the lower end of the scale, solutions with a pH of 1 are extremely acidic, while at the upper end of the scale, solutions with a pH of 14 are extremely basic.
The pH scale is logarithmic, which means that each increment on the scale represents a tenfold difference in acidity or basicity. For example, a solution with a pH of 4 is ten times more acidic than a solution with a pH of 5, and a solution with a pH of 9 is ten times more basic than a solution with a pH of 8.
Overall, the range of pH from 1 to 14 is used to measure the acidity or basicity of solutions because it covers the full range of possible pH values and allows for precise measurements of the acidity or basicity of a solution.
How to use a ph meter?
Using a pH meter generally involves the following steps:
- Calibrate the pH meter: Before using the pH meter, it is important to calibrate it to ensure accurate readings. This typically involves immersing the pH meter’s probe in a solution of known pH (such as a buffer solution) and adjusting the meter’s calibration settings to match the known pH of the solution.
- Prepare the sample: Depending on the type of sample you are testing, you may need to prepare it in a specific way. For example, if you are testing the pH of a liquid, you may need to dilute it or filter it to remove any particulates. If you are testing the pH of soil, you may need to mix a small amount of soil with water to create a slurry.
- Immerse the probe in the sample: Carefully insert the pH meter’s probe into the sample. Make sure that the probe is fully immersed in the sample to ensure an accurate reading.
- Wait for the reading: Some pH meters will give a reading almost immediately, while others may take a few seconds or minutes to stabilize. Be sure to follow the specific instructions for your pH meter and wait for the reading to stabilize before recording the result.
- Record the result: Once you have a stable reading, record the pH of the sample. Make sure to also note any other relevant information, such as the temperature of the sample and the time of the measurement.
- Clean and store the pH meter: After use, it is important to clean and store the pH meter properly to ensure its accuracy and longevity. Follow the manufacturer’s instructions for cleaning and storing the pH meter to ensure that it is properly cared for.
It’s also a good idea to regularly check the accuracy of your pH meter and recalibrate it as needed to ensure that it is giving accurate readings.
pH Meter Examples
Here are 10 pH meters that you might consider buying:
- Milwaukee MW102 pH Meter: This meter is portable and has a large, easy-to-read LCD display. It also has automatic temperature compensation, automatic buffer recognition, and automatic calibration.
- HANNA HI 98129 pHep 4 pH Tester: This meter is compact and portable, making it easy to take with you wherever you go. It also has automatic temperature compensation, automatic calibration, and a waterproof design.
- Oakton EcoTestr pH 2 Waterproof pH Tester: This meter is waterproof and has automatic temperature compensation, making it ideal for use in aquatic environments. It also has a backlit LCD display for easy reading in low light conditions.
- Extech pH200 pH/Temperature Pen Meter: This pen-style pH meter is portable and easy to use. It has automatic temperature compensation and a large, easy-to-read LCD display.
- Apera Instruments AI209 pH Pocket Tester: This compact and portable pH meter has automatic temperature compensation and automatic calibration. It also has a durable design, making it suitable for use in a variety of settings.
- Bluelab pH Pen: This pen-style pH meter is portable and easy to use. It has a backlit LCD display and automatic temperature compensation, making it ideal for use in a variety of settings.
- Thermo Scientific Orion Star A211 pH Benchtop Meter: This benchtop pH meter has automatic temperature compensation and automatic calibration. It also has a large, easy-to-read LCD display and a durable design.
- Oakton Waterproof pH 10 pH Tester: This waterproof pH meter has automatic temperature compensation, making it ideal for use in aquatic environments. It also has a backlit LCD display for easy reading in low light conditions.
- Fisher Scientific Traceable pH Meter: This pH meter has automatic temperature compensation and automatic calibration, as well as a large, easy-to-read LCD display. It is suitable for use in a variety of settings.
- HANNA HI 98127 Checker pH Tester: This compact and portable pH meter has automatic temperature compensation and automatic calibration. It also has a durable design and a backlit LCD display for easy reading in low light conditions.
When choosing a pH meter, it’s important to consider factors such as accuracy, precision, and the intended use of the meter. It’s also a good idea to read reviews and compare features to find the best option for your needs.
What is soil ph meter?
A soil pH meter is a device that measures the pH of soil, which is a measure of the acidity or basicity of the soil. The pH scale ranges from 0 to 14, with 7 being neutral. Soil pH is an important factor in plant growth because it affects the availability of nutrients to plants. Acidic soil has a pH below 7, while alkaline soil has a pH above 7. Most plants have optimal growth in soil that is slightly acidic to slightly alkaline, with a pH between 6.0 and 7.5.
Soil pH meters typically use a probe that is inserted into the soil to measure the pH. The probe contains a sensor that measures the electrical resistance of the soil, which is directly related to the pH of the soil. Some soil pH meters are handheld and portable, while others are more stationary and are meant to be used in a laboratory or greenhouse setting.
It is important to regularly test the pH of your soil to ensure that it is within the optimal range for your plants. If the soil pH is too low or too high, you can adjust it by adding lime or sulfur, respectively, to the soil. Adjusting the soil pH can help improve the growth and health of your plants.
Best ph meter for soil
Here are 10 pH meters that you might consider for soil testing:
- Milwaukee MW102 pH Meter: This meter is portable and has a large, easy-to-read LCD display. It also has automatic temperature compensation, automatic buffer recognition, and automatic calibration.
- Oakton EcoTestr pH 2 Waterproof pH Tester: This meter is waterproof and has automatic temperature compensation, making it ideal for use in outdoor environments. It also has a backlit LCD display for easy reading in low light conditions.
- HANNA HI 98129 pHep 4 pH Tester: This meter is compact and portable, making it easy to take with you wherever you go. It also has automatic temperature compensation, automatic calibration, and a waterproof design.
- Thermo Scientific Orion Star A211 pH Benchtop Meter: This benchtop pH meter has automatic temperature compensation and automatic calibration. It also has a large, easy-to-read LCD display and a durable design.
- Fisher Scientific Traceable pH Meter: This pH meter has automatic temperature compensation and automatic calibration, as well as a large, easy-to-read LCD display. It is suitable for use in a variety of settings.
- Oakton Waterproof pH 10 pH Tester: This waterproof pH meter has automatic temperature compensation, making it ideal for use in outdoor environments. It also has a backlit LCD display for easy reading in low light conditions.
- Bluelab pH Pen: This pen-style pH meter is portable and easy to use. It has a backlit LCD display and automatic temperature compensation, making it ideal for use in a variety of settings.
- Apera Instruments AI209 pH Pocket Tester: This compact and portable pH meter has automatic temperature compensation and automatic calibration. It also has a durable design, making it suitable for use in a variety of settings.
- Extech pH200 pH/Temperature Pen Meter: This pen-style pH meter is portable and easy to use. It has automatic temperature compensation and a large, easy-to-read LCD display.
- HANNA HI 98127 Checker pH Tester: This compact and portable pH meter has automatic temperature compensation and automatic calibration. It also has a durable design and a backlit LCD display for easy reading in low light conditions.
When choosing a pH meter for soil, it’s important to consider factors such as accuracy, precision, and the intended use of the meter. It’s also a good idea to read reviews and compare features to find the best option for your needs.
How to test ph of soil with ph meter? – How to use a ph meter for soil?
To test the pH of soil with a pH meter, you will need a pH meter with a soil probe and a small sample of soil. Here are the steps to follow:
- Calibrate the pH meter: Before testing the soil, it is important to calibrate the pH meter to ensure accurate readings. This typically involves immersing the pH meter’s probe in a solution of known pH (such as a buffer solution) and adjusting the meter’s calibration settings to match the known pH of the solution.
- Prepare the soil sample: Take a small sample of soil (about the size of a golf ball) and mix it with an equal amount of water to create a slurry. This will help to evenly distribute the soil particles and make it easier to measure the pH of the soil.
- Immerse the probe in the soil sample: Carefully insert the pH meter’s probe into the soil sample. Make sure that the probe is fully immersed in the soil sample to ensure an accurate reading.
- Wait for the reading: Some pH meters will give a reading almost immediately, while others may take a few seconds or minutes to stabilize. Be sure to follow the specific instructions for your pH meter and wait for the reading to stabilize before recording the result.
- Record the result: Once you have a stable reading, record the pH of the soil sample. Make sure to also note any other relevant information, such as the temperature of the soil sample and the time of the measurement.
- Clean and store the pH meter: After use, it is important to clean and store the pH meter properly to ensure its accuracy and longevity. Follow the manufacturer’s instructions for cleaning and storing the pH meter to ensure that it is properly cared for.
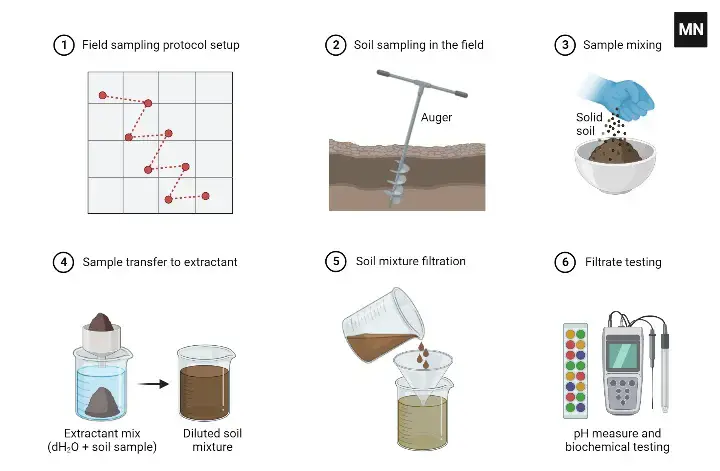
It’s a good idea to take multiple soil samples from different areas of your garden or field and test the pH of each sample to get a more accurate overall picture of the soil pH. You can then use this information to determine whether the soil pH is optimal for the plants you are growing and make any necessary adjustments to the soil pH as needed.
ph meter for water
A pH meter is a device that measures the pH of a water sample. pH is a measure of the acidity or basicity of a solution and is measured on a scale from 0 to 14, with 7 being neutral. Water pH is an important factor to consider in a variety of applications, including agriculture, aquaculture, and water treatment.
There are several types of pH meters that are specifically designed for measuring the pH of water. These meters typically use a probe that is placed in the water sample and measures the electrical resistance of the water, which is directly related to the pH of the water. Some pH meters for water are portable and handheld, while others are more stationary and are meant to be used in a laboratory or industrial setting.
When choosing a pH meter for water, it’s important to consider factors such as accuracy, precision, and the intended use of the meter. It’s also a good idea to read reviews and compare features to find the best option for your needs.
How to reset ph meter?
To reset a pH meter, you will need to follow the specific instructions for your pH meter. Here are some general steps that you may need to follow:
- Turn off the pH meter: Locate the power switch or button on the pH meter and turn it off.
- Remove the battery: If your pH meter uses a battery, remove the battery from the pH meter.
- Reinsert the battery: After a few seconds, reinsert the battery into the pH meter.
- Turn on the pH meter: Locate the power switch or button on the pH meter and turn it on.
- Calibrate the pH meter: Once the pH meter is turned on, it will need to be calibrated before it can be used. This typically involves immersing the pH meter’s probe in a solution of known pH (such as a buffer solution) and adjusting the meter’s calibration settings to match the known pH of the solution.
- Follow the specific instructions for your pH meter to complete the reset process. If you are not sure how to reset your specific pH meter, you may want to consult the manufacturer’s instructions or contact the manufacturer for assistance.
It is important to follow the specific instructions for your pH meter when resetting the meter to ensure that it is properly reset and calibrated. It is also a good idea to regularly check the accuracy of your pH meter and recalibrate it as needed to ensure that it is giving accurate readings.
What is the best ph meter for soil?
There are many different pH meters available for soil, and the best one for you will depend on your specific needs and budget. Here are a few things to consider when looking for a pH meter for soil:
- Accuracy: Look for a pH meter that is accurate to within +/- 0.1 pH, as this will give you the most accurate readings.
- Resolution: A pH meter with a higher resolution (e.g., 0.01 pH) will be able to measure small changes in pH more accurately than a meter with a lower resolution (e.g., 0.1 pH).
- Range: Make sure that the pH meter has a range that is appropriate for your needs. For example, if you are measuring the pH of soil, you will need a pH meter with a range of at least 3.5 to 10 pH.
- Probe type: There are two main types of probes for pH meters: glass and solid-state. Glass probes are more fragile but are generally more accurate, while solid-state probes are more durable but may not be as accurate.
- Ease of use: Look for a pH meter that is easy to use, with clear instructions and a user-friendly interface.
- Price: Consider your budget when shopping for a pH meter. There are many options available at different price points, so you should be able to find a pH meter that fits your needs and budget.
It is a good idea to read reviews and compare features to find the best pH meter for your needs. You may also want to consult with a scientific supply store or the manufacturer of the pH meter for recommendations.
What are 2 methods of testing pH?
There are several methods for testing the pH of a solution. Here are two common methods:
- pH strips: pH strips are strips of paper or plastic that are coated with a pH-sensitive dye. To use pH strips, you will need to dip the strip in the solution that you want to test and compare the color of the strip to a color chart to determine the pH of the solution. pH strips are convenient and easy to use, but they are not as accurate as other methods.
- pH meter: A pH meter is a device that is used to measure the pH of a solution. It consists of a probe that is immersed in the solution being tested, as well as a device that measures and displays the pH of the solution. To use a pH meter, you will need to calibrate it first to ensure accurate readings. This typically involves immersing the pH meter’s probe in a solution of known pH (such as a buffer solution) and adjusting the meter’s calibration settings to match the known pH of the solution. Once the pH meter is calibrated, you can immerse the probe in the solution that you want to test and read the pH from the meter’s display. pH meters are more accurate than pH strips, but they can be more expensive and require more maintenance.
It is important to choose the appropriate method for testing the pH of a solution based on your specific needs and the accuracy required. In some cases, it may be necessary to use multiple methods to ensure the accuracy of the pH measurement.
What are the 3 pH indicators?
pH indicators are substances that change color in response to the acidity or basicity of a solution. They are used to determine the pH of a solution by comparing the color of the indicator to a color chart. Here are three common pH indicators:
- Phenolphthalein: Phenolphthalein is a pH indicator that is commonly used in laboratories. It is colorless in neutral and acidic solutions but turns pink or red in basic solutions.
- Methyl orange: Methyl orange is a pH indicator that is commonly used in laboratories. It is orange in acidic solutions and yellow in basic solutions.
- Universal indicator: Universal indicator is a pH indicator that is composed of a mixture of several different indicators. It is available in liquid or paper form and can be used to determine the pH of a wide range of solutions. Universal indicator is color-coded, with different colors corresponding to different pH values.
There are many other pH indicators available, each with its own specific range of pH values and colors. It is important to choose the appropriate pH indicator for your specific needs and to follow the manufacturer’s instructions for using the indicator.
What is the best pH indicator?
A pH indicator is a substance that changes color in response to the pH of a solution. pH indicators are used to measure the acidity or basicity of a solution and are commonly used in laboratories and other scientific settings. There are many different pH indicators available, each with a specific range of pH values in which it will change color.
Some common pH indicators include:
- Litmus paper: This is a simple and inexpensive pH indicator that is often used in classroom settings. Litmus paper is a strip of paper that has been impregnated with a pH-sensitive dye. It changes color in the presence of an acid or base, with red indicating an acid and blue indicating a base.
- Phenolphthalein: This is a colorless pH indicator that turns pink in the presence of a base. It has a wide range of pH values in which it changes color, making it useful for measuring basic solutions over a wide range of pH values.
- Methyl orange: This pH indicator turns orange in the presence of an acid and yellow in the presence of a base. It is useful for measuring pH values in the range of 3.1 to 4.4.
- Bromothymol blue: This pH indicator turns yellow in the presence of an acid and blue in the presence of a base. It is useful for measuring pH values in the range of 6.0 to 7.6.
There are many other pH indicators available, each with its own specific range of pH values in which it will change color. The choice of pH indicator will depend on the specific needs of the application, such as the range of pH values to be measured and the sensitivity required.
pH Meter Working Animation Video
Quiz
What is the primary function of a pH meter?
a) Measure temperature
b) Measure electrical conductivity
c) Measure the acidity or alkalinity of a solution
d) Measure the density of a solution
Which component of a pH meter is sensitive to hydrogen ions and helps in measuring pH?
a) Reference electrode
b) Glass bulb
c) Junction
d) Filling hole
At what temperature is the standard pH scale defined?
a) 0°C
b) 25°C
c) 37°C
d) 100°C
Which solution is typically used to store pH electrodes when not in use?
a) Distilled water
b) Sodium chloride
c) Potassium chloride
d) Hydrochloric acid
What does ATC stand for in the context of pH meters?
a) Automatic Temperature Control
b) Automatic Temperature Compensation
c) Advanced Technical Calibration
d) Automated Test Control
Which of the following is NOT a common error in pH measurement?
a) Electrode contamination
b) Temperature fluctuations
c) Using a fresh electrode
d) Inadequate rinsing of the electrode
What is the pH value of a neutral solution at 25°C?
a) 0
b) 5
c) 7
d) 14
Which part of the pH electrode allows ion exchange while maintaining electrical separation between the electrodes?
a) Glass bulb
b) Electrode body
c) Junction
d) Filling hole
What is the purpose of calibrating a pH meter?
a) To increase the lifespan of the electrode
b) To ensure accurate and consistent readings
c) To change the measurement units
d) To adjust the temperature settings
Which of the following solutions is commonly used for calibrating pH meters?
a) Distilled water
b) Hydrochloric acid
c) Buffer solution
d) Sodium chloride
FAQ
How does a ph meter work?
A pH meter is a device that measures the pH of a solution, which is a measure of the acidity or basicity of the solution. The pH scale ranges from 0 to 14, with 7 being neutral.
Most pH meters work by measuring the electrical resistance of the solution. The pH of a solution is directly related to the concentration of hydrogen ions (H+) in the solution. The concentration of hydrogen ions determines the electrical charge of the solution, which in turn determines the electrical resistance of the solution.
A pH meter consists of a probe that is immersed in the solution being tested. The probe contains a sensor that measures the electrical resistance of the solution. The pH meter also contains a reference electrode, which is a separate electrode that is used to measure the electrical potential of the solution.
The reference electrode is connected to the pH meter’s circuitry, which is calibrated to determine the pH of the solution based on the measured electrical resistance and electrical potential. The pH meter then displays the pH of the solution on an LCD display or other readout.
Some pH meters also have additional features, such as automatic temperature compensation, which allows the pH meter to accurately measure the pH of the solution over a range of temperatures. Other features may include automatic calibration, automatic buffer recognition, and the ability to store and retrieve data.
What is ph meter?
A pH meter is a device that measures the pH of a solution, which is a measure of the acidity or basicity of the solution. The pH scale ranges from 0 to 14, with 7 being neutral. A pH meter consists of a probe that is immersed in the solution being tested and a device that measures and displays the pH of the solution.
pH meters are commonly used in a variety of applications, including water treatment, agriculture, aquaculture, and laboratory research. In water treatment, pH meters are used to measure the pH of drinking water and wastewater to ensure that they meet regulatory standards. In agriculture and aquaculture, pH meters are used to measure the pH of soil and water to optimize the growth and health of plants and animals. In the laboratory, pH meters are used to measure the pH of various chemical solutions in research and quality control applications.
There are several types of pH meters, including handheld and portable meters, benchtop meters, and online meters. Handheld and portable meters are convenient for field use, while benchtop meters are more suited for laboratory use. Online pH meters are permanently installed and are used to continuously monitor the pH of a solution in real-time.
How to calibrate ph meter without solution?
Calibrating a pH meter typically involves immersing the pH meter’s probe in a solution of known pH (such as a buffer solution) and adjusting the meter’s calibration settings to match the known pH of the solution. Without a solution of known pH, it is not possible to calibrate a pH meter accurately.
If you do not have a solution of known pH available, you can try calibrating the pH meter using a solution of distilled water. Distilled water has a neutral pH of 7.0, so you can set the pH meter’s calibration settings to 7.0 and see if the meter gives accurate readings when tested with distilled water. However, keep in mind that this method may not be as accurate as calibrating with a buffer solution and may not work for all pH meters.
It is important to regularly calibrate your pH meter to ensure that it is giving accurate readings. If you do not have a solution of known pH available, you may want to consider purchasing a buffer solution or contacting the manufacturer of the pH meter for assistance with calibration.
Where to buy ph meter?
There are many places where you can buy a pH meter, including scientific supply stores, online retailers, and through the manufacturer of the pH meter. Here are a few options to consider:
Scientific supply stores: Many cities have stores that sell scientific equipment, including pH meters. These stores may have a wide selection of pH meters to choose from and may also be able to answer questions about specific models.
Online retailers: There are many online retailers that sell pH meters, including Amazon, eBay, and scientific equipment websites such as LabX.com. Shopping online allows you to easily compare different models and prices, and you may be able to find good deals on used equipment.
Manufacturer’s website: You can also purchase a pH meter directly from the manufacturer’s website. This can be a good option if you have specific requirements or questions about the pH meter.
When shopping for a pH meter, it’s important to consider factors such as accuracy, precision, and the intended use of the meter. It’s also a good idea to read reviews and compare features to find the best option for your needs.
What does the ph meter measure?
A pH meter measures the pH of a solution, which is a measure of the acidity or basicity of the solution. The pH scale ranges from 0 to 14, with 7 being neutral. A solution with a pH below 7 is considered acidic, while a solution with a pH above 7 is considered basic.
pH is a measure of the concentration of hydrogen ions (H+) in a solution. The concentration of hydrogen ions determines the electrical charge of the solution, which in turn determines the electrical resistance of the solution.
A pH meter measures the electrical resistance of a solution and uses this information to determine the pH of the solution. The pH meter consists of a probe that is immersed in the solution being tested, as well as a device that measures and displays the pH of the solution.
pH meters are commonly used in a variety of applications, including water treatment, agriculture, aquaculture, and laboratory research. In water treatment, pH meters are used to measure the pH of drinking water and wastewater to ensure that they meet regulatory standards. In agriculture and aquaculture, pH meters are used to measure the pH of soil and water to optimize the growth and health of plants and animals. In the laboratory, pH meters are used to measure the pH of various chemical solutions in research and quality control applications.
How often to calibrate ph meter?
It is recommended to calibrate a pH meter before each use to ensure accurate readings. However, the frequency of calibration may vary depending on the specific pH meter, the stability of the readings, and the intended use of the meter.
Some pH meters have automatic calibration, which allows the meter to calibrate itself with the push of a button. Other pH meters may require manual calibration, which involves immersing the pH meter’s probe in a solution of known pH (such as a buffer solution) and adjusting the meter’s calibration settings to match the known pH of the solution.
If you are using a pH meter for critical applications or for long-term monitoring, it is a good idea to calibrate the meter more frequently to ensure accuracy. It is also a good idea to regularly check the accuracy of the pH meter and recalibrate it as needed to ensure that it is giving accurate readings.
If you are not sure how often to calibrate your pH meter, you may want to consult the manufacturer’s recommendations or contact the manufacturer for guidance.
How to read a ph meter?
Once you have a stable reading, you can read the pH of the sample from the pH meter’s display. The pH meter may display the pH as a number (e.g., 7.0) or as a color-coded scale (e.g., green for neutral, red for acidic, and blue for basic).
What is the purpose of calibrating a ph meter?
Calibrating a pH meter is the process of adjusting the meter’s calibration settings to match the known pH of a solution. The purpose of calibrating a pH meter is to ensure that it is giving accurate readings.
pH meters work by measuring the electrical resistance of a solution and using this information to determine the pH of the solution. However, the electrical resistance of a solution can be affected by factors such as temperature, humidity, and the presence of contaminants. As a result, pH meters may drift over time and may not give accurate readings unless they are calibrated regularly.
Calibrating a pH meter involves immersing the pH meter’s probe in a solution of known pH (such as a buffer solution) and adjusting the meter’s calibration settings to match the known pH of the solution. This helps to correct for any drift or error in the pH meter’s readings and ensures that it is giving accurate readings.
It is important to regularly calibrate your pH meter to ensure that it is giving accurate readings. If you are using a pH meter for critical applications or for long-term monitoring, it is a good idea to calibrate the meter more frequently to ensure accuracy. It is also a good idea to regularly check the accuracy of the pH meter and recalibrate it as needed to ensure that it is giving accurate readings.
How to calibrate ph meter with baking soda?
Calibrating a pH meter with baking soda is not a recommended method for calibrating a pH meter. Baking soda (sodium bicarbonate) has a pH of around 8.3, which is slightly basic, but it is not a pure substance and may not provide a reliable reference for calibration.
In order to calibrate a pH meter accurately, it is recommended to use a buffer solution, which is a solution of known pH that is designed specifically for calibrating pH meters. Buffer solutions are available in a range of pH values, such as 4.0, 7.0, and 10.0, and are more reliable than other substances for calibrating pH meters.
If you do not have a buffer solution available, you can try calibrating the pH meter using a solution of distilled water. Distilled water has a neutral pH of 7.0, so you can set the pH meter’s calibration settings to 7.0 and see if the meter gives accurate readings when tested with distilled water. However, keep in mind that this method may not be as accurate as calibrating with a buffer solution and may not work for all pH meters.
To calibrate a pH meter with a buffer solution, follow these steps:
Rinse the probe: Rinse the probe with distilled water to remove any dirt or debris.
Fill the calibration cup: Fill the calibration cup with the appropriate pH buffer solution. The buffer solution should be at room temperature.
Immerse the probe in the buffer solution: Carefully insert the pH meter’s probe into the buffer solution. Make sure that the probe is fully immersed in the solution to ensure an accurate reading.
Calibrate the meter: Follow the instructions for your specific pH meter to calibrate
Why are buffer solutions used to calibrate the ph meter?
Buffer solutions are used to calibrate pH meters because they provide a stable, known pH value that can be used to adjust the meter’s calibration settings.
A buffer solution is a solution of known pH that is designed to resist changes in pH when small amounts of acid or base are added to it. This makes buffer solutions ideal for calibrating pH meters, as they provide a stable reference point that can be used to adjust the meter’s calibration settings.
Buffer solutions are available in a range of pH values, such as 4.0, 7.0, and 10.0, and are more reliable than other substances for calibrating pH meters. This is because they are pure substances that do not contain contaminants or impurities that could affect the pH of the solution.
To calibrate a pH meter with a buffer solution, you will need to immerse the pH meter’s probe in the buffer solution and adjust the meter’s calibration settings to match the known pH of the buffer solution. This helps to correct for any drift or error in the pH meter’s readings and ensures that it is giving accurate readings.
It is important to regularly calibrate your pH meter to ensure that it is giving accurate readings. If you are using a pH meter for critical applications or for long-term monitoring, it is a good idea to calibrate the meter more frequently to ensure accuracy. It is also a good idea to regularly check the accuracy of the pH meter and recalibrate it as needed to ensure that it is giving accurate readings.
What is pH and pH meter?
pH is a measure of the acidity or basicity of a solution. It is defined as the negative logarithm of the concentration of hydrogen ions (H+) in a solution. The pH scale ranges from 0 to 14, with 7 being neutral. A solution with a pH below 7 is considered acidic, while a solution with a pH above 7 is considered basic.
A pH meter is a device that is used to measure the pH of a solution. It consists of a probe that is immersed in the solution being tested, as well as a device that measures and displays the pH of the solution.
To use a pH meter, you will need to calibrate it first to ensure accurate readings. This typically involves immersing the pH meter’s probe in a solution of known pH (such as a buffer solution) and adjusting the meter’s calibration settings to match the known pH of the solution. Once the pH meter is calibrated, you can immerse the probe in the solution that you want to test and read the pH from the meter’s display.
pH meters are commonly used in a variety of applications, including water treatment, agriculture, aquaculture, and laboratory research. In water treatment, pH meters are used to measure the pH of drinking water and wastewater to ensure that they meet regulatory standards. In agriculture and aquaculture, pH meters are used to measure the pH of soil and water to optimize the growth and health of plants and animals. In the laboratory, pH meters are used to measure the pH of various chemical solutions in research and quality control applications.
What is pH meter full form?
The full form of pH meter is “potentiometric hydrogen ion meter.” A pH meter is a device that is used to measure the pH of a solution. It consists of a probe that is immersed in the solution being tested, as well as a device that measures and displays the pH of the solution.
The term “potentiometric” refers to the fact that pH meters work by measuring the electrical potential of a solution. The pH meter’s probe contains a reference electrode and a measuring electrode, which are connected to a voltmeter. The voltmeter measures the difference in electrical potential between the two electrodes, which is proportional to the concentration of hydrogen ions (H+) in the solution.
The term “hydrogen ion” refers to the fact that pH is a measure of the acidity or basicity of a solution, which is determined by the concentration of hydrogen ions in the solution. The pH scale ranges from 0 to 14, with 7 being neutral. A solution with a pH below 7 is considered acidic, while a solution with a pH above 7 is considered basic.
pH meters are commonly used in a variety of applications, including water treatment, agriculture, aquaculture, and laboratory research. They are an important tool for monitoring and controlling the pH of solutions in order to optimize various processes and ensure the health and safety of people and the environment.
What colour is high pH?
The color of a solution at a high pH (basic) will depend on the pH indicator that is used. Different pH indicators have different color ranges and will change color at different pH values.
For example, with phenolphthalein as the pH indicator, a high pH solution will be pink or red. With methyl orange as the pH indicator, a high pH solution will be yellow. With universal indicator, a high pH solution will be blue or purple.
It is important to choose the appropriate pH indicator for your specific needs and to follow the manufacturer’s instructions for using the indicator. This will ensure that you get accurate and reliable results when testing the pH of a solution.
What is the pH of water?
The pH of pure water is 7. This means that it is neutral, with equal amounts of hydrogen ions (H+) and hydroxide ions (OH-). The pH scale ranges from 0 to 14, with values below 7 being acidic, values above 7 being basic, and a value of 7 being neutral. The concentration of hydrogen ions in a solution is what determines its pH.
The pH of water can be affected by various factors, such as the presence of dissolved minerals or pollutants. The pH of natural water sources can vary significantly depending on the source, but most are close to neutral. The pH of water can be measured using a pH meter or pH test strips.
What is the pH of milk?
The pH of milk is around 6.5 to 6.7, which is slightly acidic. The pH of milk is affected by several factors, including the type of milk (such as cow’s milk, goat’s milk, or plant-based milk), the presence of bacteria and other microorganisms, and the presence of dissolved minerals. The pH of milk is generally lower than that of pure water due to the presence of lactic acid, which is produced by bacteria during the fermentation of lactose (a sugar found in milk).
In general, the pH of milk is not as critical as the pH of some other products, as milk is generally consumed within a few days of production and does not undergo significant pH changes over time. However, the pH of milk can be important in some contexts, such as cheese making or the production of fermented milk products like yogurt.
What is the strongest pH?
The pH scale ranges from 0 to 14, with 0 being the most acidic, 14 being the most basic, and 7 being neutral. The strength of an acid or base is determined by the concentration of hydrogen ions (H+) or hydroxide ions (OH-), respectively, in the solution. A solution with a high concentration of H+ ions is more acidic, while a solution with a high concentration of OH- ions is more basic.
The pH of a solution can be affected by a variety of factors, including the concentration and type of ions present in the solution, the temperature, and the presence of other substances that may react with the acid or base. It is important to note that the pH scale is logarithmic, meaning that each increment on the scale represents a tenfold difference in acidity or basicity. For example, a solution with a pH of 4 is ten times more acidic than a solution with a pH of 5, and a solution with a pH of 9 is ten times more basic than a solution with a pH of 8.
What is basic on pH scale?
On the pH scale, which ranges from 0 to 14, a substance is considered basic if it has a pH greater than 7. A basic substance has a higher concentration of hydroxide ions (OH-) and a lower concentration of hydrogen ions (H+) compared to a neutral substance, such as pure water.
The pH scale is logarithmic, meaning that each increment on the scale represents a tenfold difference in acidity or basicity. For example, a substance with a pH of 8 is ten times more basic than a substance with a pH of 7, and a substance with a pH of 9 is 100 times more basic than a substance with a pH of 7.
There are many substances that are basic, including household cleaning products like ammonia and bleach, and personal care products like soap and shampoo. The pH of a substance can be measured using a pH meter or pH test strips. It is important to note that the pH of a substance can be affected by various factors, such as the presence of dissolved minerals or other substances, and can change over time.
Is there a unit for pH?
The pH of a substance is a measure of its acidity or basicity, and is expressed on a scale that ranges from 0 to 14. The pH scale is logarithmic, meaning that each increment on the scale represents a tenfold difference in acidity or basicity. For example, a substance with a pH of 4 is ten times more acidic than a substance with a pH of 5, and a substance with a pH of 9 is ten times more basic than a substance with a pH of 8.
The unit of measurement for pH is the pH unit, which is a unitless quantity. The pH of a substance is calculated using the concentration of hydrogen ions (H+) in the solution. The pH of a solution is defined as the negative logarithm (base 10) of the concentration of H+ ions in the solution:
pH = -log[H+]
For example, a solution with a concentration of H+ ions of 1 x 10^-4 moles per liter (mol/L) would have a pH of 4. A solution with a concentration of H+ ions of 1 x 10^-8 mol/L would have a pH of 8.
It is important to note that the pH scale is a measure of the acidity or basicity of a substance and does not directly reflect the concentration of H+ ions in the solution. The concentration of H+ ions in a solution can be calculated using the pH of the solution and the equation above.
Why pH is 0 to 14?
The pH scale is a measure of the acidity or basicity of a substance, and it ranges from 0 to 14. The pH scale was developed as a way to quantify the acidity or basicity of a substance based on the concentration of hydrogen ions (H+) in the solution.
The pH scale is logarithmic, meaning that each increment on the scale represents a tenfold difference in acidity or basicity. A substance with a pH of 4 is ten times more acidic than a substance with a pH of 5, and a substance with a pH of 9 is ten times more basic than a substance with a pH of 8.
The pH scale was designed to cover a wide range of acidity and basicity, from highly acidic substances with a pH of 0 to highly basic substances with a pH of 14. The pH scale is useful for measuring the acidity or basicity of substances in a wide range of applications, including food, medicine, and industrial processes.
It is important to note that the pH scale is a measure of the acidity or basicity of a substance and does not directly reflect the concentration of H+ ions in the solution. The concentration of H+ ions in a solution can be calculated using the pH of the solution and the equation:
pH = -log[H+]
This equation can be used to convert between the pH of a solution and the concentration of H+ ions in the solution.
Can pH be negative?
No, the pH of a substance cannot be negative. The pH scale is a measure of the acidity or basicity of a substance, and it ranges from 0 to 14. The pH scale is logarithmic, meaning that each increment on the scale represents a tenfold difference in acidity or basicity. A substance with a pH of 4 is ten times more acidic than a substance with a pH of 5, and a substance with a pH of 9 is ten times more basic than a substance with a pH of 8.
The pH scale was developed as a way to quantify the acidity or basicity of a substance based on the concentration of hydrogen ions (H+) in the solution. The pH of a substance is calculated using the concentration of H+ ions in the solution:
pH = -log[H+]
The concentration of H+ ions in a solution cannot be negative, as it is a measure of the number of ions present in the solution. Therefore, the pH of a substance cannot be negative.
It is important to note that the pH scale is a measure of the acidity or basicity of a substance and does not directly reflect the concentration of H+ ions in the solution. The concentration of H+ ions in a solution can be calculated using the pH of the solution and the equation above.
Does a 0 pH exist?
A substance with a pH of 0 is extremely acidic and is considered to be at the lower end of the pH scale, which ranges from 0 to 14. While it is theoretically possible for a substance to have a pH of 0, it is extremely unlikely to occur in nature or in laboratory settings.
The pH scale is a measure of the acidity or basicity of a substance based on the concentration of hydrogen ions (H+) in the solution. The pH of a substance is calculated using the concentration of H+ ions in the solution:
pH = -log[H+]
The concentration of H+ ions in a solution cannot be negative, as it is a measure of the number of ions present in the solution. Therefore, the pH of a substance cannot be negative.
The pH scale is logarithmic, meaning that each increment on the scale represents a tenfold difference in acidity or basicity. A substance with a pH of 4 is ten times more acidic than a substance with a pH of 5, and a substance with a pH of 9 is ten times more basic than a substance with a pH of 8.
It is important to note that the pH scale is a measure of the acidity or basicity of a substance and does not directly reflect the concentration of H+ ions in the solution. The concentration of H+ ions in a solution can be calculated using the pH of the solution and the equation above.
What is the weakest pH level?
The pH scale is a measure of the acidity or basicity of a solution. It ranges from 0 to 14, with 0 being the most acidic, 7 being neutral, and 14 being the most basic. Therefore, the weakest pH level is 0, which is the most acidic.
It’s important to note that the pH scale is logarithmic, meaning that each increment on the scale represents a tenfold difference in acidity or basicity. For example, a solution with a pH of 4 is ten times more acidic than a solution with a pH of 5, and a solution with a pH of 3 is 100 times more acidic than a solution with a pH of 5.
Is pH 0 strong or weak?
pH 0 is considered to be a strong acid. The pH scale is a measure of the acidity or basicity of a solution, and it ranges from 0 to 14. A solution with a pH of 0 is highly acidic, while a solution with a pH of 14 is highly basic. A solution with a pH of 7 is neutral, meaning it is neither acidic nor basic.
It’s important to note that the pH scale is logarithmic, meaning that each increment on the scale represents a tenfold difference in acidity or basicity. For example, a solution with a pH of 4 is ten times more acidic than a solution with a pH of 5, and a solution with a pH of 3 is 100 times more acidic than a solution with a pH of 5.
In general, a solution with a pH less than 7 is considered to be an acid, while a solution with a pH greater than 7 is considered to be a base. Solutions with a pH of 7 or less are considered to be strong acids, while solutions with a pH greater than 7 but less than 14 are considered to be weak acids. Similarly, solutions with a pH of 14 or greater are considered to be strong bases, while solutions with a pH greater than 7 but less than 14 are considered to be weak bases.
Is pH 7 always neutral?
pH 7 is considered to be neutral, meaning it is neither acidic nor basic. The pH scale is a measure of the acidity or basicity of a solution, and it ranges from 0 to 14. A solution with a pH of 0 is highly acidic, while a solution with a pH of 14 is highly basic. A solution with a pH of 7 is neutral, meaning it is neither acidic nor basic.
It’s important to note that the pH scale is logarithmic, meaning that each increment on the scale represents a tenfold difference in acidity or basicity. For example, a solution with a pH of 4 is ten times more acidic than a solution with a pH of 5, and a solution with a pH of 3 is 100 times more acidic than a solution with a pH of 5.
In general, a solution with a pH less than 7 is considered to be an acid, while a solution with a pH greater than 7 is considered to be a base. Solutions with a pH of 7 or less are considered to be strong acids, while solutions with a pH greater than 7 but less than 14 are considered to be weak acids. Similarly, solutions with a pH of 14 or greater are considered to be strong bases, while solutions with a pH greater than 7 but less than 14 are considered to be weak bases.
It is possible for a solution to have a pH that is very close to 7, but not exactly 7. In these cases, the solution would be considered to be slightly acidic or slightly basic, but it would not be considered to be neutral.
Is Milk an acid or a base?
Milk is a slightly acidic substance, with a pH that ranges from 6.5 to 6.7. The pH scale is a measure of the acidity or basicity of a solution, and it ranges from 0 to 14. A solution with a pH of 0 is highly acidic, while a solution with a pH of 14 is highly basic. A solution with a pH of 7 is neutral, meaning it is neither acidic nor basic.
The pH of milk is influenced by a variety of factors, including the type of milk (cow’s milk, goat’s milk, etc.), the diet of the animal producing the milk, and the processing methods used to produce the milk. In general, milk is considered to be a slightly acidic substance because it contains lactic acid, which is produced by the action of bacteria on the lactose in the milk.
It’s important to note that the pH scale is logarithmic, meaning that each increment on the scale represents a tenfold difference in acidity or basicity. For example, a solution with a pH of 4 is ten times more acidic than a solution with a pH of 5, and a solution with a pH of 3 is 100 times more acidic than a solution with a pH of 5.
In general, a solution with a pH less than 7 is considered to be an acid, while a solution with a pH greater than 7 is considered to be a base. Solutions with a pH of 7 or less are considered to be strong acids, while solutions with a pH greater than 7 but less than 14 are considered to be weak acids. Similarly, solutions with a pH of 14 or greater are considered to be strong bases, while solutions with a pH greater than 7 but less than 14 are considered to be weak bases.
What has a pH of 1?
There are many substances that have a pH of 1. Some examples of substances that have a pH of 1 include:
Hydrochloric acid: Hydrochloric acid is a strong acid that is commonly used in a variety of industrial and laboratory settings. It is also found naturally in the stomach, where it helps to digest food. Hydrochloric acid has a pH of 1.
Lemon juice: Lemon juice is a common ingredient in cooking and is known for its sour taste. It is made up primarily of citric acid, which gives it a pH of around 2.
Battery acid: Battery acid is a strong acid that is used in lead-acid batteries, such as those found in cars. It has a pH of around 1.
Tomato juice: Tomato juice is a common beverage that is made from tomatoes and has a pH of around 4.5. However, some types of tomato juice, such as those that are highly concentrated or have been subjected to certain processing methods, may have a pH of 1 or lower.
It’s important to note that the pH scale is logarithmic, meaning that each increment on the scale represents a tenfold difference in acidity or basicity. For example, a solution with a pH of 4 is ten times more acidic than a solution with a pH of 5, and a solution with a pH of 3 is 100 times more acidic than a solution with a pH of 5.
What is the pH of sugar?
Sugar, or sucrose, is a sweet, crystalline substance that is composed of glucose and fructose. It has a pH of around 6.5 to 7.0, which means it is slightly basic.
The pH scale is a measure of the acidity or basicity of a solution, and it ranges from 0 to 14. A solution with a pH of 0 is highly acidic, while a solution with a pH of 14 is highly basic. A solution with a pH of 7 is neutral, meaning it is neither acidic nor basic.
It’s important to note that the pH scale is logarithmic, meaning that each increment on the scale represents a tenfold difference in acidity or basicity. For example, a solution with a pH of 4 is ten times more acidic than a solution with a pH of 5, and a solution with a pH of 3 is 100 times more acidic than a solution with a pH of 5.
In general, a solution with a pH less than 7 is considered to be an acid, while a solution with a pH greater than 7 is considered to be a base. Solutions with a pH of 7 or less are considered to be strong acids, while solutions with a pH greater than 7 but less than 14 are considered to be weak acids. Similarly, solutions with a pH of 14 or greater are considered to be strong bases, while solutions with a pH greater than 7 but less than 14 are considered to be weak bases.
It’s worth noting that the pH of sugar can vary depending on the concentration of the solution and the presence of other substances that may affect the pH. For example, a solution of sugar in water may have a different pH than a solution of sugar in milk, or a solution of sugar in a different type of liquid.
What is the pH of soap?
Soap is a basic substance, with a pH that ranges from 9 to 10. The pH scale is a measure of the acidity or basicity of a solution, and it ranges from 0 to 14. A solution with a pH of 0 is highly acidic, while a solution with a pH of 14 is highly basic. A solution with a pH of 7 is neutral, meaning it is neither acidic nor basic.
Soap is basic because it contains alkaline substances, such as sodium hydroxide or potassium hydroxide, which are responsible for its cleaning properties. These alkaline substances help to neutralize acidic substances, such as grease and oil, making them easier to wash away.
It’s important to note that the pH scale is logarithmic, meaning that each increment on the scale represents a tenfold difference in acidity or basicity. For example, a solution with a pH of 4 is ten times more acidic than a solution with a pH of 5, and a solution with a pH of 3 is 100 times more acidic than a solution with a pH of 5.
In general, a solution with a pH less than 7 is considered to be an acid, while a solution with a pH greater than 7 is considered to be a base. Solutions with a pH of 7 or less are considered to be strong acids, while solutions with a pH greater than 7 but less than 14 are considered to be weak acids. Similarly, solutions with a pH of 14 or greater are considered to be strong bases, while solutions with a pH greater than 7 but less than 14 are considered to be weak bases.
It’s worth noting that the pH of soap can vary depending on the specific type of soap and the ingredients used to make it. Some soaps may have a slightly lower or higher pH than others, depending on the ingredients and manufacturing process.
What is the pH of coffee?
Coffee is a slightly acidic substance, with a pH that ranges from around 5.0 to 6.5. The pH scale is a measure of the acidity or basicity of a solution, and it ranges from 0 to 14. A solution with a pH of 0 is highly acidic, while a solution with a pH of 14 is highly basic. A solution with a pH of 7 is neutral, meaning it is neither acidic nor basic.
The pH of coffee is influenced by a variety of factors, including the type of beans used to make the coffee, the roasting process, the brewing method, and the ratio of coffee to water used to make the coffee. In general, coffee is considered to be a slightly acidic substance because it contains a number of acids, including chlorogenic acid and quinic acid, which give it its characteristic flavor and aroma.
It’s important to note that the pH scale is logarithmic, meaning that each increment on the scale represents a tenfold difference in acidity or basicity. For example, a solution with a pH of 4 is ten times more acidic than a solution with a pH of 5, and a solution with a pH of 3 is 100 times more acidic than a solution with a pH of 5.
In general, a solution with a pH less than 7 is considered to be an acid, while a solution with a pH greater than 7 is considered to be a base. Solutions with a pH of 7 or less are considered to be strong acids, while solutions with a pH greater than 7 but less than 14 are considered to be weak acids. Similarly, solutions with a pH of 14 or greater are considered to be strong bases, while solutions with a pH greater than 7 but less than 14 are considered to be weak bases.
References
- http://m.lohandbio.com/info/ph-meter-classification-24135460.html
- https://www.pharmaguideline.com/2015/08/principle-and-working-of-pH-probes.html
- http://www.ph-meter.info/pH-electrodehttps://en.wikipedia.org/wiki/PH_meter
- https://www.omega.co.uk/prodinfo/pH-meter.html
- https://www.slideshare.net/Haddies/ph-meter-56234681
- https://electricalfundablog.com/ph-measurement-working-principle-applications/
- http://www.seafriends.org.nz/dda/ph.htm
- https://www.explainthatstuff.com/how-ph-meters-work.html
- https://www.grainger.com/know-how/equipment-information/kh-ph-electrode-types-uses