Hopkin’s Cole/Adamkiewicz–Hopkins Test Definition
Hopkins Cole Test also referred to as glyoxylic Acid Reaction is among the qualitative testing methods for determining the differences between different types of amino acids and proteins. The test is used to determine the presence of tryptophan, an amino acid, in proteins.
- It was first described in the work of Frederick Gowland Hopkins and Sydney W. Cole in the year 1901 as part of their work on the initial isolation of tryptophan.
- Tryptophan is also the only amino acid with an indole ring.
- In this experiment, a solution of protein was blended in with Hopkins Cole reagent, which is composed of glyoxylic acid. A concentrated sulfuric acid will be gradually added to create two layers. A purple ring forms within the layers when the test is positive for tryptophan.
- Tryptophan is part of the group of amino acids that are essential.
- Tryptophan can be a precursor for Niacin vitamin, and is introduces Serotonin’s nervous system.
- Tryptophan is a component that helps to increase the effectiveness of the vitamin B complex, it also increase nervous system health, improve emotional stability and improve feelings of calm, decrease sleepiness and enhance the production of the growth hormone. A portion of the amino acids is present in egg whites.
Objectives of Hopkin’s Cole Test
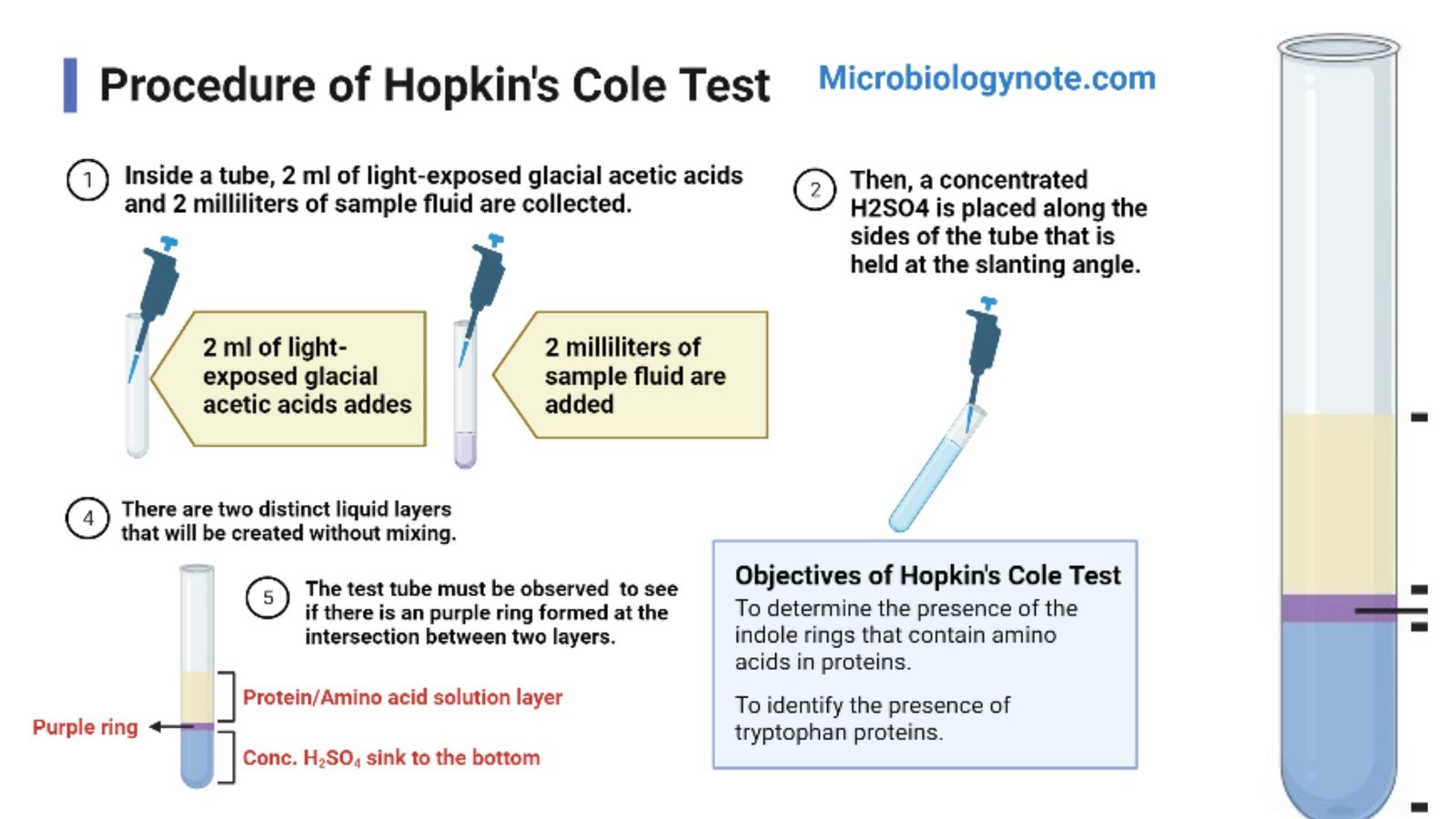
- To determine the presence of the indole rings that contain amino acids in proteins.
- To identify the presence of tryptophan proteins.
Principle of Hopkin’s Cole Test
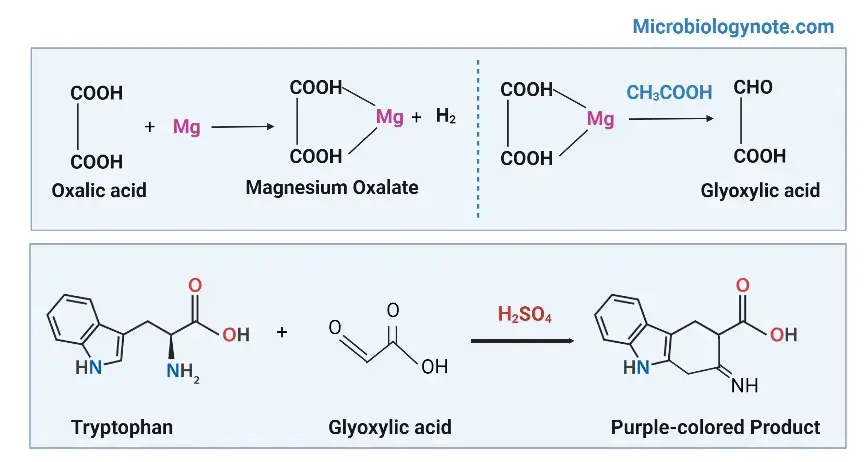
- In this test, Hopkins Cole Reagent, which is a source of the glyoxylic acid (C2H2O3) reacts with sulfuric acid (H2SO4).
- The tryptophan contained in the protein solution will condense by the aldehyde group (Indole groups) in the glyoxylic acids with the aid of a powerful oxidizing sulphuric acid (H2SO4).
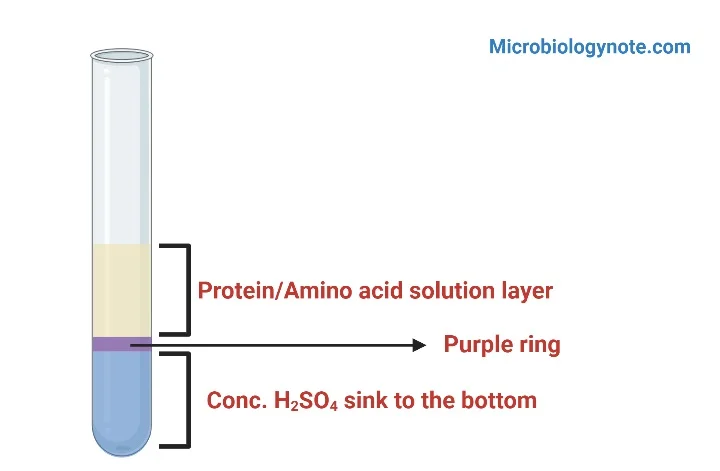
- The positive result can be seen in the appearance of purple rings in between two distinct layers.
- Glyoxylic acid is made by reducing the oxalic acid with sodium amalgam or magnesium.
- Glacial acetic, which was exposed to sunlight, also contains glyoxylic acids and can therefore be used in this test.
- This test uses sulphuric acid in order to help stabilize the glyoxylic acid to stop its breakdown in carbon dioxide and its release.
- It is also crucial to keep in mind that the presence of chlorates, nitrates as well as excess chlorides can prevent the reaction from taking place.
Reaction
Requirements
Reagent
- Hopkin’s Cole reagent: Glyoxylic acid is made by exposure of glacial acetic acid to the sun for a few days.
- Concentrated H2SO4
- Sample:0.1% amino acid solution
Material Required: Test tubes, Test tube stand, Pipettes
Procedure of Hopkin’s Cole Test
- Inside a tube, 2 ml of light-exposed glacial acetic acids and 2 milliliters of sample fluid are collected.
- Then, a concentrated H2SO4 is placed along the sides of the tube that is held at the slanting angle. There are two distinct liquid layers that will be created without mixing.
- The test tube must be watched to see if there is an purple ring formed at the intersection between two layers.
Result and Interpretation of Hopkin’s Cole Test
- Positive result: A positive result can be observed by the appearance of a purple-colored ring near the intersection between two layers. This indicates the presence of tryptophan-containing proteins.
- Negative result: Negative results can be observed by the absence of an orange-colored ring within the test tube. This indicates the absence of tryptophan-containing proteins.
Precautions
Below are some guidelines that must be observed when conducting the tests.
- Clean the apparatus prior to or after each experiment thoroughly.
- Test tube must have no impurities.
- Take care to measure the solution prior to placing it in the tube test.
- Make sure you put the acids in the test tube in a sideways manner. If not, it will yield a false reading.
Uses of Hopkin’s Cole Test
- The test can be used to detect amino acids and proteins within the sample.
- It is a straightforward and simple test that can be performed easily. It helps to distinguish tryptophan from various amino acids.
Limitations
- Compounds like nitritesand chlorates, nitrates, as well as excessive chlorides block the production of condensation products.
- Gelatin and Other proteins that don’t contain tryptophan, do not trigger this reaction.
FAQ
Which amino acid gives a positive hopkins-cole test?
Tryptophan is an α-amino acid, which gives a positive hopkins-cole test?
Which is the positive indicator for Hopkins Cole test?
A positive result is indicated by formation of a purple-color ring at the junction of two layers.
Is casein positive for Hopkins Cole test?
Yes, casein gave positive Hopkins Cole test.
What is the role of sulfuric acid in Hopkins Cole test?
Sulphuric acid is added to the reagent to stabilize the glyoxylic acid and prevent its decomposition and release of carbon dioxide.
References
- https://medicalstudyzone.com/hopkins-cole-test/
- https://biocheminsider.com/hopkins-cole-test-principle-reaction-reagents-and-result-interpretation/
- http://biocheminfo.com/2020/04/05/hopkins-cole-test-adamkiewicz-hopkins-test-principle-reaction-reagents-procedure-and-result-interpretation/
- https://www.onlinebiologynotes.com/adamkiewicz-reaction-hopkins-cole-test-objective-principle-reagents-procedure-and-result/
- http://www.biosciencenotes.com/adamkiewicz-hopkins-test/
- https://en.wikipedia.org/wiki/Hopkins%E2%80%93Cole_reaction
- https://noteshippo.com/hopkins-cole-test/
- https://microbenotes.com/hopkins-cole-test/
- Text Highlighting: Select any text in the post content to highlight it
- Text Annotation: Select text and add comments with annotations
- Comment Management: Edit or delete your own comments
- Highlight Management: Remove your own highlights
How to use: Simply select any text in the post content above, and you'll see annotation options. Login here or create an account to get started.