What is Anomer of glucose?
- The anomers of glucose refer to the diastereomers that arise from the variations in configuration at the anomeric carbon of glucose’s cyclic structure. Monosaccharides, such as glucose, fructose, galactose, and xylose, can exist in different forms depending on the configuration of their cyclic structures.
- Glucose, a six-carbon sugar, can undergo a reaction known as mutarotation, which leads to the formation of its cyclic structure. In the presence of water, glucose molecules can react with the carbonyl group (C=O) of the same molecule, resulting in the formation of a hemiacetal or a cyclic acetal.
- In the cyclic structure of glucose, one carbon atom, known as the anomeric carbon, becomes a chiral center. The anomeric carbon can have two different configurations: alpha (α) and beta (β). These configurations differ in the orientation of the hydroxyl group attached to the anomeric carbon.
- In the alpha configuration, the hydroxyl group attached to the anomeric carbon is positioned below the plane of the ring structure. Conversely, in the beta configuration, the hydroxyl group is positioned above the plane of the ring structure.
- Due to the presence of these two configurations, glucose exists as two anomers: alpha-D-glucose and beta-D-glucose. The prefix “D” indicates the stereochemistry of the asymmetric carbon atom farthest from the carbonyl group. Similarly, there are also alpha-L-glucose and beta-L-glucose anomers, which differ in their spatial arrangements.
- It is important to note that the anomeric carbon in glucose is referred to as the C1 carbon because it is the first carbon in the glucose molecule to participate in the formation of the cyclic structure. The configuration at this carbon significantly affects the physical and chemical properties of glucose, as well as its interactions with other molecules in biological systems.
- The ability of glucose to exist as alpha and beta anomers is significant in various biological processes. For example, enzymes and proteins that interact with glucose often exhibit specificity towards a particular anomer. This selectivity is crucial for the proper functioning of metabolic pathways and regulatory mechanisms in living organisms.
- In conclusion, the anomers of glucose are diastereomers that differ in their configurations at the anomeric carbon of glucose’s cyclic structure. The alpha and beta configurations play a significant role in the biological activities and interactions of glucose within living systems. Understanding the different anomers of glucose is essential in comprehending the complex biochemistry of carbohydrates and their roles in various physiological processes.
Overview of Anomer Of Glucose
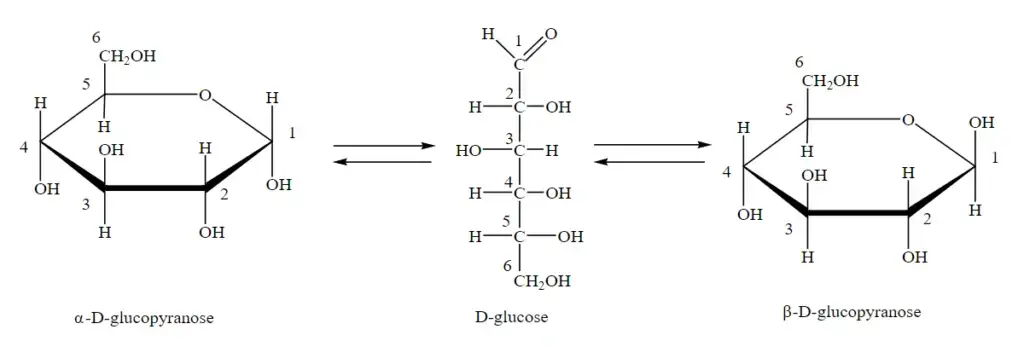
- Anomers play a significant role in the structure and properties of certain carbohydrate molecules, including glucose. Glucose, a monosaccharide with six carbon atoms, can exist in both a ring form and an open chain form. The structure of glucose includes an anomeric carbon, which is the carbon atom present in the carbonyl functional group when the molecule is in its open-chain form.
- Anomers are a type of stereoisomer that differ in configuration at a specific stereogenic center, which in this case is the anomeric carbon. The configuration at the anomeric carbon can vary, resulting in two distinct forms known as anomers. The interconversion between these anomers is referred to as anomerization.
- Glucose can exist as two anomers: the alpha (α) anomer and the beta (β) anomer. The alpha configuration refers to the orientation of the hydroxyl group attached to the anomeric carbon below the plane of the ring structure. On the other hand, the beta configuration refers to the hydroxyl group positioned above the plane of the ring structure.
- The ability of glucose to exist in both alpha and beta anomeric forms is due to the flexibility of its structure. In the ring form, glucose undergoes a reaction known as mutarotation, where the anomeric carbon can switch between the alpha and beta configurations. This dynamic interconversion is driven by the equilibrium between the open-chain and ring forms of glucose.
- The presence of these anomers has important implications for the properties and biological activities of glucose. Enzymes and other molecules that interact with glucose often exhibit selectivity towards a particular anomer. This selectivity influences various biological processes, including metabolism, signaling, and molecular recognition.
- In addition to its anomeric carbon, glucose also possesses other functional groups, such as hydroxyl groups, which contribute to its chemical reactivity and ability to form glycosidic bonds with other molecules.
- In summary, anomers of glucose are stereoisomers that differ in configuration at the anomeric carbon, which is the carbon atom involved in the carbonyl group of the open-chain form. Glucose can exist as alpha and beta anomers, and their interconversion is known as anomerization. Understanding the structure and properties of these anomers is crucial for comprehending the behavior and roles of glucose in various biological processes.
Structure of Glucose
- Glucose, a carbohydrate and monosaccharide, has a molecular formula of C6H12O6, indicating that it is composed of six carbon atoms, twelve hydrogen atoms, and six oxygen atoms. The structure of glucose can exist in two forms: an open chain structure and a cyclic structure.
- In the open chain structure, glucose is referred to as an aldohexose due to the presence of an aldehyde group. The carbon atoms in glucose are labeled from C1 to C6. The aldehyde group occupies the C1 position, while other substituents occupy positions from C2 to C5. This open chain structure of glucose is relatively rare and accounts for less than 0.02% of the total glucose in a water solution.
- The majority of glucose molecules exist in a cyclic structure, where the open chain structure undergoes a reaction known as cyclization. The cyclization can result in two different forms of the cyclic structure: a five-membered ring known as a furanose form, and a six-membered ring known as a pyranose form.
- The cyclization occurs by joining the C1 carbon with either the C4 or C5 carbon atoms through a hemiacetal linkage. The resulting structure can be a furanose form or a pyranose form, depending on the number of carbon atoms in the ring. The five-membered ring structure is referred to as furanose, while the six-membered ring structure is referred to as pyranose.
- Within the six-membered pyranose forms of glucose, the anomers of glucose exist. Anomers are stereoisomers that differ in configuration at the anomeric carbon. In the case of glucose, the anomeric carbon is the C1 carbon, which is involved in the cyclization process. The anomers of glucose include the alpha (α) and beta (β) forms, which differ in the orientation of the hydroxyl group attached to the C1 carbon.
- It is important to note that the cyclic structure of glucose is the predominant form, with only a small fraction of glucose molecules existing in the open chain form. The cyclic structure is more stable and accounts for the vast majority of glucose in biological systems.
- In summary, glucose has a structure composed of six carbon atoms, twelve hydrogen atoms, and six oxygen atoms. It can exist in both an open chain structure and a cyclic structure. The cyclic structure can be either a furanose form or a pyranose form, with the pyranose form being the most common. Within the pyranose form, the anomers of glucose, including the alpha and beta forms, exist. Understanding the structure of glucose is fundamental to comprehending its role as an essential carbohydrate in biological processes.
Anomeric Carbon of Glucose
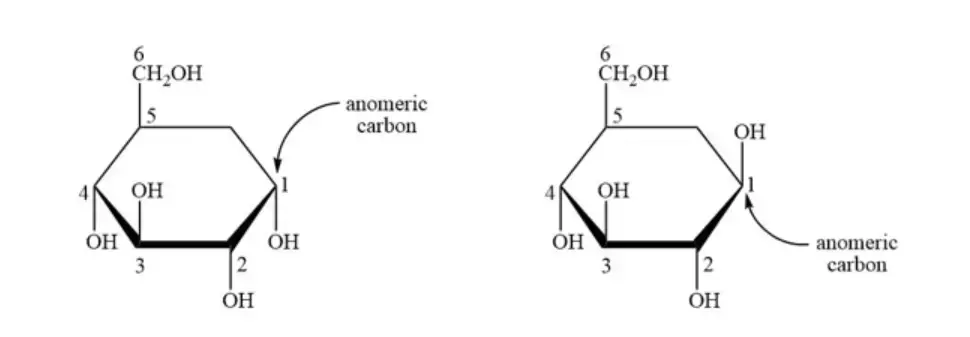
- In the structure of glucose, whether it is in its open chain or cyclic form, the carbon atom that belongs to the carbonyl functional group serves as the anomeric carbon. In other words, the carbon atom labeled as 1 in the given figure is the anomeric carbon. When the open chain structure undergoes cyclization to form the cyclic structure, this anomeric carbon becomes a chiral center.
- The anomeric carbon in the cyclic structure of glucose is the carbon atom at the first position. It is referred to as the anomeric carbon because it is the carbon involved in the formation of the cyclic structure. This carbon atom exhibits certain geometric variations or differences in configuration, leading to the existence of different anomers.
- The anomeric carbon can also be referred to as the anomeric center, as it is the central atom that determines the stereochemistry of the glucose molecule. The variations in configuration at this carbon atom give rise to the alpha (α) and beta (β) anomers of glucose.
- The alpha anomer is characterized by the hydroxyl group attached to the anomeric carbon being positioned below the plane of the ring structure. On the other hand, the beta anomer has the hydroxyl group above the plane of the ring structure.
- The configuration at the anomeric carbon is significant because it influences the physical, chemical, and biological properties of glucose. The specific configuration can affect the interactions of glucose with enzymes, proteins, and other molecules in various biochemical processes.
- In summary, the anomeric carbon of glucose is the carbon atom that belongs to the carbonyl functional group, labeled as 1 in the structure. It serves as the chiral center in the cyclic structure of glucose. Different configurations at this carbon give rise to the alpha and beta anomers, which play a crucial role in the properties and interactions of glucose in biological systems.
Alpha-D-glucopyranose and beta-D-glucopyranose
- In the pyranose form of glucose, the anomeric carbon is the C1 carbon atom. When the C1 carbon is attached to the C5 carbon through a hemiacetal linkage, two stereoisomers of glucose are obtained: alpha-D-glucopyranose and beta-D-glucopyranose. These two forms are the anomeric forms or anomers of glucose.
- The alpha-D-glucopyranose and beta-D-glucopyranose differ in their geometric orientation at the anomeric carbon (C1). Looking at the structure of both forms, it can be observed that there are variations in the orientation of the OH and H atoms at the C1 position.
- During the formation of the hemiacetal bond, the hydroxyl group (OH) of the C5 carbon attacks the C1 carbon from either side. The orientation of the OH group that attaches to the C1 carbon determines whether the glucose molecule is in the alpha or beta configuration.
- In alpha-D-glucopyranose, the hydroxyl group attached to the C1 carbon is oriented below the plane of the ring structure. This configuration is often represented as α-D-glucopyranose. On the other hand, in beta-D-glucopyranose, the hydroxyl group is oriented above the plane of the ring structure, and it is represented as β-D-glucopyranose.
- The alpha and beta anomers of glucose are diastereomers because they differ in their configuration at the C1 carbon but have the same configuration at other stereocenters. These anomers exhibit distinct chemical and biological properties and can interact differently with enzymes and other molecules in biological systems.
- In summary, in the pyranose form of glucose, the C1 carbon serves as the anomeric carbon. Through a hemiacetal linkage with the C5 carbon, two anomers are obtained: alpha-D-glucopyranose and beta-D-glucopyranose. These anomers differ in the orientation of the OH and H atoms at the C1 position, resulting in distinct geometric variations. Understanding the differences between alpha-D-glucopyranose and beta-D-glucopyranose is important in comprehending the behavior and roles of glucose in various biological processes.
Significance
The significance of the anomers of glucose lies in their impact on the chemical, physical, and biological properties of glucose. Here are some key points highlighting the significance of the anomers:
- Biological Activity: The alpha and beta anomers of glucose exhibit different interactions with enzymes, receptors, and other molecules in biological systems. These interactions can influence glucose’s biological activity, such as its recognition by transporters and enzymes involved in glucose metabolism.
- Solubility and Stability: The anomers of glucose have different solubilities and stabilities. For example, the beta anomer is often more stable than the alpha anomer. These differences can have implications for the storage, transportation, and utilization of glucose in biological systems.
- Mutarotation: The interconversion between the alpha and beta anomers through mutarotation allows glucose to dynamically adjust its configuration. This flexibility is important for glucose’s role as a readily available energy source and its participation in various metabolic pathways.
- Glycosidic Bond Formation: The anomeric carbon of glucose plays a crucial role in the formation of glycosidic bonds, which are essential for constructing larger carbohydrates like disaccharides and polysaccharides. The different orientations of the anomeric carbon in the alpha and beta anomers contribute to the diversity of carbohydrate structures and functions.
- Optical Activity: Both the alpha and beta anomers of glucose are optically active, meaning they can rotate the plane of polarized light. This property can be utilized in analytical techniques to determine the presence and concentration of glucose in biological samples.
- Sweetness Perception: The different orientations of the hydroxyl group at the anomeric carbon give rise to differences in sweetness perception. The beta anomer of glucose is generally perceived as sweeter than the alpha anomer, influencing the taste characteristics of glucose-containing substances.
- Drug Design and Biochemical Research: Understanding the specific interactions and properties of the alpha and beta anomers of glucose is important in drug design, particularly in targeting enzymes or receptors involved in glucose metabolism. Additionally, studying the behavior of glucose anomers provides insights into carbohydrate chemistry and biochemistry, contributing to advances in various fields of research.
In summary, the significance of the anomers of glucose lies in their biological activity, solubility, stability, glycosidic bond formation, mutarotation, optical activity, sweetness perception, and their relevance in drug design and biochemical research. Understanding the distinct properties and behavior of the alpha and beta anomers enhances our understanding of glucose and its role in biological systems.
FAQ
What is an anomer of glucose?
An anomer of glucose refers to the different stereoisomeric forms of glucose that vary in configuration at the anomeric carbon, which is the carbon atom involved in the carbonyl group of the molecule.
How many anomers does glucose have?
Glucose has two anomers: the alpha (α) anomer and the beta (β) anomer. These anomers differ in the orientation of the hydroxyl group attached to the anomeric carbon.
What is the anomeric carbon in glucose?
The anomeric carbon in glucose is the carbon atom that belongs to the carbonyl functional group. In the open-chain structure, it is the carbon atom labeled as 1, and in the cyclic structure, it is the carbon atom at the first position.
What is the difference between the alpha and beta anomers of glucose?
The alpha anomer has the hydroxyl group attached to the anomeric carbon positioned below the plane of the ring structure, while the beta anomer has the hydroxyl group positioned above the plane of the ring structure.
How do the alpha and beta anomers of glucose interconvert?
The interconversion between the alpha and beta anomers of glucose is known as anomerization. It occurs through a process called mutarotation, where the anomeric carbon undergoes a flip between the alpha and beta configurations.
What are the biological implications of the alpha and beta anomers of glucose?
The alpha and beta anomers of glucose can have different interactions with enzymes, proteins, and other molecules in biological systems. Their distinct configurations can influence their recognition, metabolism, and biological activities.
How do the anomers of glucose affect its chemical reactivity?
The anomeric carbon of glucose plays a significant role in its chemical reactivity. The different orientations of the hydroxyl group at the anomeric carbon can affect the ability of glucose to form glycosidic bonds and participate in various chemical reactions.
Are the alpha and beta anomers of glucose optically active?
Yes, both the alpha and beta anomers of glucose are optically active. They can rotate the plane of polarized light, although their specific optical rotation values may differ.
Can the anomers of glucose exist in equilibrium?
Yes, the alpha and beta anomers of glucose can exist in equilibrium due to the process of mutarotation. This equilibrium is influenced by factors such as temperature, pH, and solvent conditions.
Are the anomers of glucose specific to glucose alone?
No, anomers can be found in other monosaccharides as well. The concept of anomers applies to other carbohydrates that have a cyclic structure and possess an anomeric carbon, such as fructose, galactose, and ribose.