What are Disaccharides?
- Disaccharides, also known as double sugars or biose, are a type of sugar molecule formed when two monosaccharides are joined together through a glycosidic linkage. Similar to monosaccharides, disaccharides are soluble in water. Some commonly known examples of disaccharides include sucrose, lactose, and maltose.
- Within the realm of carbohydrates, disaccharides belong to one of the four chemical groupings, which include monosaccharides, disaccharides, oligosaccharides, and polysaccharides. The most frequently encountered types of disaccharides, namely sucrose, lactose, and maltose, consist of 12 carbon atoms and share the general formula C12H22O11. The distinguishing characteristics among these disaccharides arise from the atomic arrangements within their respective molecules.
- The process of joining two monosaccharides to form a double sugar occurs through a condensation reaction. This reaction involves the elimination of a water molecule from the functional groups involved in the linkage. Conversely, the separation of a disaccharide into its constituent monosaccharides is accomplished through hydrolysis, with the aid of specialized enzymes called disaccharidases. As the synthesis of a larger sugar molecule releases a water molecule, the breakdown process requires the consumption of a water molecule. These reactions play a vital role in metabolic processes. Each disaccharide is broken down by a specific disaccharidase enzyme (such as sucrase, lactase, or maltase).
- In summary, disaccharides are sugars formed by the combination of two monosaccharides through a glycosidic linkage. They are soluble in water and are an important class of carbohydrates. Hydrolysis of disaccharides yields two molecules of monosaccharides, which can be identical or different. The glycosidic linkage is established after the elimination of a water molecule, connecting the two monosaccharide units through an oxygen atom.
Definition of Disaccharides
Disaccharides are sugars formed by the joining of two monosaccharide molecules through a glycosidic linkage. They are soluble in water and include examples like sucrose, lactose, and maltose.
Properties of Disaccharides
Disaccharides possess several properties that are influenced by their structural characteristics and composition:
- Bond Formation: The glycosidic bond in disaccharides can be formed between any hydroxy group of the component monosaccharides. Different combinations of bond positions (regiochemistry) and configurations (alpha or beta) result in diastereoisomers of disaccharides, each having distinct chemical and physical properties.
- Crystallinity: Disaccharides can exhibit varying degrees of crystallinity. Some disaccharides, such as lactose, can form crystalline structures under certain conditions.
- Solubility: The solubility of disaccharides in water varies. While some disaccharides, like lactose and maltose, are soluble in water, others, such as cellobiose, may have limited solubility.
- Taste and Texture: Certain disaccharides, including sucrose, are known for their sweet taste. Disaccharides can also have a sticky or syrupy texture, as observed in substances like maltose.
- Glycoside Formation: Disaccharides can function as functional groups by forming glycosidic bonds with other organic compounds, leading to the formation of glycosides. This property is significant in various biological processes.
- Chemical Composition: Disaccharides, like all carbohydrates, consist of hydrogen, carbon, and oxygen atoms. The ratio of hydrogen atoms to oxygen atoms in disaccharides is often 2:1, earning them the name “hydrates of carbon.” The general chemical formula for disaccharides is C12H22O11. Due to the presence of carbon and covalent bonds, disaccharides are classified as organic compounds.
- Relationship to Other Carbohydrates: Disaccharides differ from oligosaccharides and polysaccharides in terms of the number of monosaccharide units they contain. Disaccharides consist of two monosaccharides, whereas oligosaccharides have three to ten units, and polysaccharides contain multiple monosaccharide units.
These properties contribute to the diverse nature and functionality of disaccharides, making them essential components in various biological processes and food chemistry.
Classification of Disaccharides
Disaccharides can be classified into two distinct categories based on their functional properties:
- Reducing disaccharides: This class of disaccharides includes lactose, maltose, and cellobiose. In reducing disaccharides, one of the monosaccharides retains a free hemiacetal unit, which can act as a reducing aldehyde group. The other monosaccharide is connected through a glycosidic bond, preventing it from functioning as a reducing agent. Reducing disaccharides can be identified through tests such as the Woehlk test or Fearon’s test using methylamine.
- Non-reducing disaccharides: Sucrose and trehalose are examples of non-reducing disaccharides. In this category, the monosaccharides are bonded through an acetal linkage between their anomeric centers. As a result, neither of the monosaccharides possesses a free hemiacetal unit capable of acting as a reducing agent. The absence of a free hemiacetal group contributes to the reduced chemical reactivity of non-reducing disaccharides compared to reducing sugars. This lower reactivity can be advantageous for storage stability purposes.
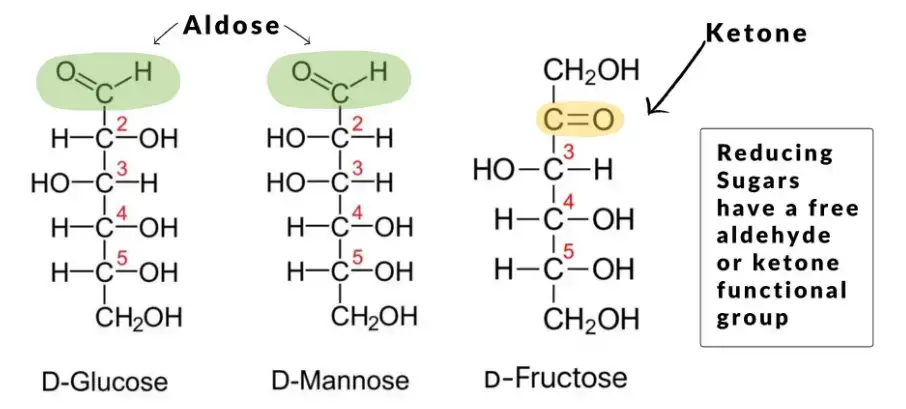
By distinguishing between reducing and non-reducing disaccharides, we can better understand their chemical behavior and functional properties.
Formation of Disaccharides
- The formation of disaccharides involves the joining of two monosaccharide molecules through a dehydration reaction, also known as a condensation reaction or dehydration synthesis. In this process, a hydroxy group (-OH) from one monosaccharide combines with a hydrogen nucleus (proton) from the other monosaccharide, resulting in the formation of a disaccharide molecule. This reaction leads to the displacement of a water molecule.
- The removal of a water molecule during the formation of a disaccharide is why this process is referred to as a dehydration reaction. It can also be called a condensation reaction or dehydration synthesis. This reaction occurs when the vacant bonds on the monosaccharides come together to link the two monomers, resulting in the formation of a glycosidic bond.
- For instance, lactose, which is the disaccharide found in milk, is formed by the condensation of one molecule of glucose and one molecule of galactose. Similarly, sucrose, found in sugar cane and sugar beet, is a condensation product of glucose and fructose. Maltose, another common disaccharide, is formed by the condensation of two glucose molecules.
- The glycosidic bonds formed during the dehydration reaction are crucial in the structure and function of disaccharides, as well as in the formation of more complex polysaccharides. These bonds play a significant role in carbohydrate chemistry and are responsible for the diverse properties and functions exhibited by disaccharides.
Breakdown of Disaccharides
- The breakdown of disaccharides into their monosaccharide components occurs through a process called hydrolysis. Hydrolysis involves the addition of a water molecule to break the glycosidic bond between the two monosaccharides. Enzymes called disaccharidases facilitate this hydrolysis reaction.
- During hydrolysis, a water molecule is split into its components: an -OH (hydroxyl) group and an H (hydrogen) atom. The -OH group attaches to one monosaccharide, and the H atom attaches to the other monosaccharide, effectively breaking the glycosidic bond. The resulting monosaccharides are then free to participate in metabolic processes or be utilized as a source of energy.
- It is important to note that the hydrolysis of disaccharides is different from the process of dissolution. Dissolution refers to the dissolving of solid sugar in water, where the sugar molecules remain intact and do not undergo any structural changes. In contrast, hydrolysis involves the enzymatic breakdown of disaccharides into their individual monosaccharide units through the addition of a water molecule.
- Hydrolysis reactions play a crucial role in digestion, as various disaccharides need to be broken down into their respective monosaccharides for efficient absorption in the digestive system. Specific enzymes, such as sucrase, lactase, and maltase, are responsible for catalyzing the hydrolysis of sucrose, lactose, and maltose, respectively, into their constituent monosaccharides.
- Overall, the breakdown of disaccharides through hydrolysis allows for the release and utilization of individual monosaccharides, which are essential for energy production and various metabolic processes in the body.
Examples of Disaccharides
Disaccharides are a class of sugars formed by the combination of two monosaccharide units. Here are some examples of disaccharides:
- Sucrose: Sucrose is a common disaccharide found in plants, particularly in sugar cane and sugar beets. It consists of one molecule of glucose bonded to one molecule of fructose. On hydrolysis, it yields dextrorotatory glucose and laevorotatory fructose. The overall mixture is laevorotatory due to the higher laevorotation of fructose compared to the dextrorotation of glucose.
- Maltose: Maltose is a disaccharide composed of two α-D-glucose units. The glucose molecules are linked by the first carbon of one glucose unit and the fourth carbon of the other glucose unit. Maltose is a reducing sugar as it can produce a free aldehyde at the first carbon of the second glucose unit, exhibiting reducing properties.
- Lactose: Lactose, often referred to as milk sugar, is found in milk and dairy products. It consists of one molecule of β-D-galactose bonded to one molecule of β-D-glucose. The bond is formed between the first carbon of galactose and the fourth carbon of glucose. Lactose is also a reducing sugar.
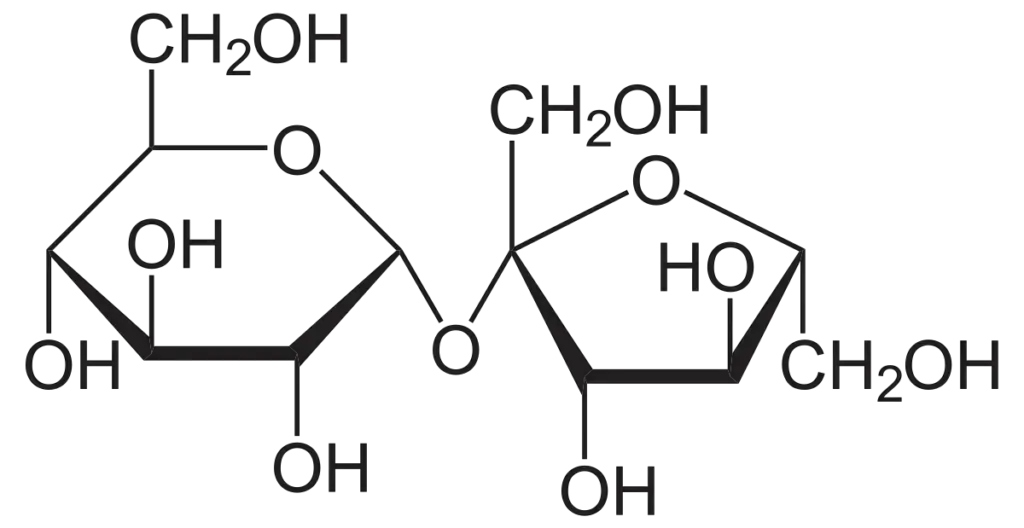
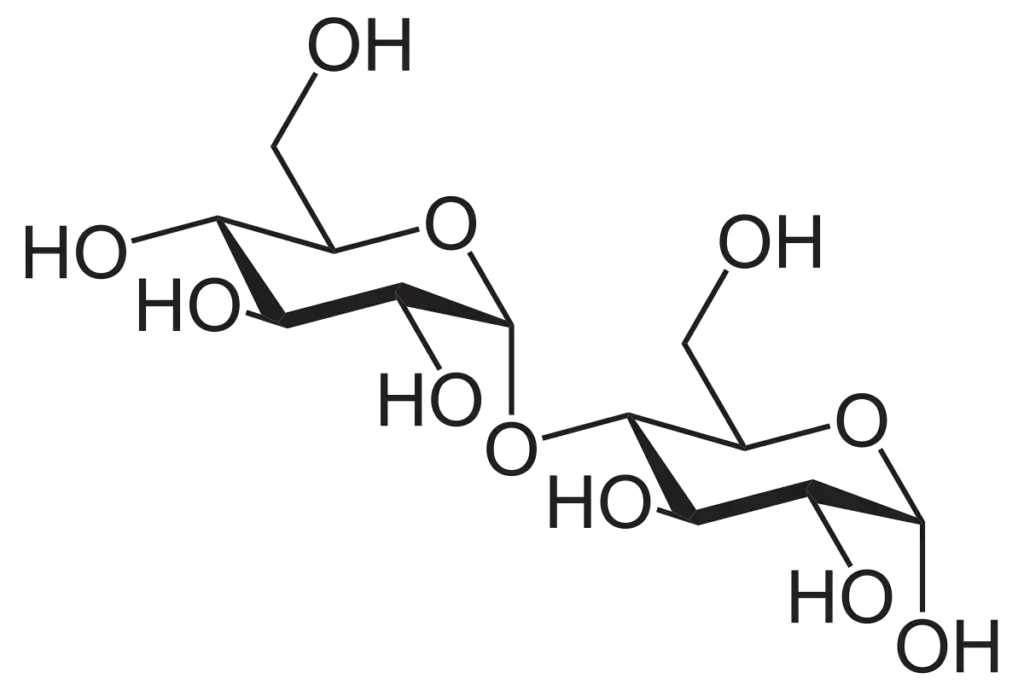
Additional types of disaccharides include:
- Trehalose: Trehalose is composed of two glucose molecules linked in a different arrangement. It can be found in fungi, plants, and insects.
- Lactulose: Lactulose is formed from galactose and fructose. It is used in the treatment of constipation and liver diseases.
- Cellobiose: Cellobiose is made up of two glucose molecules arranged differently. It is commonly observed in bacteriology as a form of chemical analysis.
- Chitobiose: Chitobiose consists of two glucosamine molecules linked together. It is found in certain bacteria, the exoskeletons of insects, and in marine organisms such as fish, octopus, and squid.
These examples demonstrate the structural and compositional diversity of disaccharides, each with their own unique properties and biological significance.
Biological Importance of Disaccharides
Disaccharides play a significant biological role, both as a source of energy and as essential components in various biological processes. Here are the key biological importance of disaccharides:
- Energy Source: Disaccharides, along with other carbohydrates, serve as a source of energy. Upon consumption and digestion, disaccharides are broken down into monosaccharides (such as glucose and fructose) which are then utilized as metabolites for ATP synthesis. ATP (adenosine triphosphate) is the primary energy currency of cells, and the breakdown of glucose through processes like glycolysis and oxidative phosphorylation generates ATP molecules.
- Metabolic Function: Glucose, derived from disaccharides, is a crucial monosaccharide utilized by cells for various metabolic functions. It serves as a substrate for ATP production through processes like glycolysis and oxidative phosphorylation. Glucose also plays a key role in the regulation of blood sugar levels and acts as a precursor for the synthesis of other important molecules like glycogen and fatty acids.
- Dietary Importance: Disaccharides, such as sucrose, lactose, and maltose, are commonly found in the human diet. They provide a source of sweetness and are used as sweeteners in various food and beverage products. Sucrose is widely used as table sugar, while lactose is naturally present in milk. Maltose, although less sweet than sucrose, can be used as a sweetener as well. These disaccharides are broken down into monosaccharides during digestion to provide a source of energy and essential nutrients.
- Nutrient Source for Infants: Lactose, the disaccharide found in breast milk, serves as a vital nutrient source for infants. It provides energy and contributes to the healthy growth and development of the baby. The enzyme lactase breaks down lactose into glucose and galactose, which can be easily absorbed and utilized by the infant’s body.
- Industrial Applications: Disaccharides like lactose and maltose find applications in the food industry. Lactic acid bacteria can ferment lactose to produce lactic acid, which is used in the production of various dairy products such as yogurt and cheese. Maltose may also be used in food processing and brewing.
- Nutrient Transport in Plants: Vascular plants, particularly sucrose, form disaccharides as a nutrient source for transportation within the plant. Sucrose is synthesized in photosynthetic tissues and transported through phloem tissues to provide energy and nutrients to different parts of the plant.
While disaccharides are important for energy production and various biological processes, it is crucial to maintain a balanced intake of carbohydrates and be mindful of excessive sugar consumption to avoid health issues such as diabetes, obesity, tooth decay, and cardiovascular diseases.
FAQ
What are disaccharides?
Disaccharides are a type of carbohydrate made up of two monosaccharide units joined together by a glycosidic bond.
What are some examples of disaccharides?
Common examples of disaccharides include sucrose (table sugar), lactose (found in milk), and maltose (found in germinating grains).
How are disaccharides formed?
Disaccharides are formed through a dehydration reaction or condensation reaction, where a hydroxyl group from one monosaccharide combines with a hydrogen atom from another, resulting in the formation of a glycosidic bond and the release of a water molecule.
What is the difference between reducing and non-reducing disaccharides?
Reducing disaccharides have one monosaccharide unit that retains a free hemiacetal group capable of acting as a reducing agent. Non-reducing disaccharides lack a free hemiacetal group and cannot act as reducing agents.
Are disaccharides soluble in water?
Yes, disaccharides are generally soluble in water due to their hydrophilic nature, which allows them to form hydrogen bonds with water molecules.
Do all disaccharides have a sweet taste?
Many disaccharides, such as sucrose and lactose, have a sweet taste. However, the intensity of sweetness may vary depending on the specific disaccharide.
How are disaccharides broken down in the body?
Disaccharides are broken down into their component monosaccharides through a process called hydrolysis, which involves the addition of a water molecule. Enzymes called disaccharidases catalyze this hydrolysis reaction.
What is the biological importance of disaccharides?
Disaccharides serve as a source of energy in the diet, as they are broken down into monosaccharides that can be used for ATP synthesis. They also play roles in cellular communication and serve as building blocks for more complex carbohydrates.
Can disaccharides cause health issues?
Excessive consumption of certain disaccharides, such as sucrose, can contribute to health issues like obesity, diabetes, tooth decay, and cardiovascular diseases. Some individuals may also experience digestive discomfort or intolerance to specific disaccharides, such as lactose intolerance.
Where can disaccharides be found in nature?
Disaccharides can be found in various natural sources. For example, sucrose is abundant in sugar cane and sugar beet, lactose is present in milk and dairy products, and maltose is produced during the germination of grains.