A virus is a form of microscopic infectious agent that can only replicate within the cells of a living organism. Viruses are capable of infecting all forms of life, including animals, vegetation, and even bacteria. They are comprised of a minute piece of genetic material, either DNA or RNA, encased in a protein shell known as a capsid. Some viruses have a lipid-based outer envelope that helps them infect host cells.
Viruses are not considered living organisms because, outside of a host cell, they cannot perform metabolic functions or reproduce independently. Instead, they are classified as obligate intracellular parasites, meaning their reproduction and dissemination depend on a host cell.
Viruses can induce numerous diseases in humans and other animals, such as the common cold, influenza, HIV/AIDS, and COVID-19. They are also capable of infecting plants and causing diseases that can wreak havoc on agricultural production.
What is Virus Cultivation?
- Virus cultivation refers to the process of growing and propagating viruses in a laboratory setting. This process is important for studying the properties and behavior of different viruses, as well as for developing vaccines and treatments for viral infections.
- The cultivation of viruses typically involves infecting a host cell culture with the virus of interest. Host cells can include animal cells, plant cells, or bacterial cells, depending on the type of virus being studied. Once the virus infects the host cell, it begins to replicate and produce more virus particles, which can then be harvested and used for further experimentation.
- There are several different methods of virus cultivation, including tissue culture, embryonated eggs, and live animals. Tissue culture involves growing host cells in a nutrient-rich medium and infecting them with the virus. Embryonated eggs are often used for culturing influenza viruses, while live animals may be used for certain types of research or vaccine development.
- It is important to note that virus cultivation must be done under strict laboratory conditions to ensure the safety of researchers and the public. This includes using appropriate containment measures and following proper protocols for handling infectious materials.
- The majority of viruses can be grown in experimental animals, embryonated eggs, or tissue culture.
Purpose of virus cultivation
The purpose of virus cultivation is to grow and propagate viruses in a laboratory setting for scientific research and development of vaccines and treatments for viral infections. Some specific purposes of virus cultivation are:
- Understanding virus behavior: By culturing viruses in the laboratory, researchers can study their properties and behavior. This can help them understand how viruses cause diseases, how they replicate, and how they interact with host cells.
- Developing vaccines: Virus cultivation is important for the development of vaccines against viral diseases. By growing and propagating the virus, researchers can develop weakened or inactivated forms of the virus that can be used as vaccines to stimulate the immune system.
- Developing antiviral drugs: Cultivating viruses can also be useful for developing drugs to treat viral infections. Researchers can test different compounds and drugs to see if they can stop the replication of the virus or prevent it from infecting host cells.
- Studying viral evolution: Culturing viruses can also help researchers study viral evolution over time. By comparing different strains of the virus and how they evolve, scientists can gain insights into how viruses adapt and mutate to new environments.
Specimens for Culture of Virus
To culture viruses, researchers typically need a sample or specimen that contains the virus. Here are some common types of specimens that can be used for virus culture:
- Blood: Blood samples can be used to culture certain viruses, such as HIV, hepatitis B and C, and dengue virus.
- Tissue: Tissue samples, such as lung or liver tissue, can be used to culture viruses that infect those specific organs.
- Saliva: Saliva samples can be used to culture viruses that are spread through respiratory droplets, such as influenza and respiratory syncytial virus (RSV).
- Urine: Urine samples can be used to culture viruses that are shed in urine, such as the Zika virus.
- Stool: Stool samples can be used to culture viruses that are shed in the feces, such as norovirus and rotavirus.
- Swabs: Swab samples can be used to culture viruses that are present on the surface of the body, such as herpes simplex virus and varicella-zoster virus.
- Fluids: Other types of bodily fluids, such as cerebrospinal fluid (CSF) or synovial fluid, can also be used to culture certain viruses.
Methods of Cultivation of viruses
There are three methods of Virus cultivation such as;
- Animal inoculation
- Embryonated Eggs
- Tissue Culture
- Cell Culture
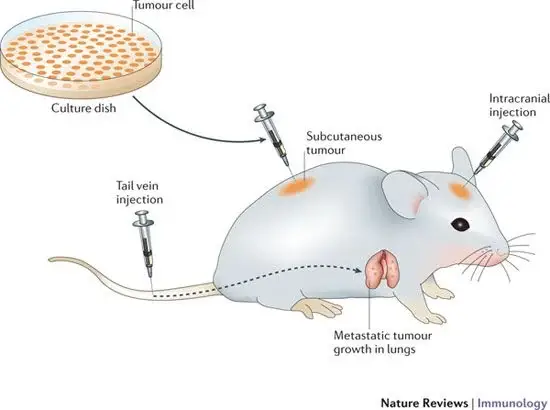
1. Animal inoculation Method of virus Cultivation
- Animal inoculation is one of the primary methods for isolating specific viruses and studying the pathogenesis of specific viral diseases.
- Lab mice (white mice), especially nursing mice, are the preferred animal for virus cultivation. Toga virus and Coxsackie virus are cultured using rodents younger than 48 hours old.
- Other animals, such as hamsters, guinea pigs, chimpanzees, etc., are occasionally used as substitutes for virus cultivation.
- The selected animals should be free of communicable diseases and in good health.
- Additionally, viruses can be administered to laboratory animals via intraperitoneal, subcutaneous, intracerebral, and intranasal routes.
- After infection, the virus multiplies within the host and causes disease. The animals are observed for disease and mortality symptoms.
- The virus is then isolated and purified from the animal tissue.
Advantages of Animal Inoculation
Animal inoculation, also known as in vivo virus cultivation, involves infecting animals with a virus to replicate and isolate the virus. Here are some potential advantages of using animal inoculation for virus cultivation:
- Mimics human infection: Animal inoculation can provide a more accurate representation of human infection than cell culture methods, as it involves the virus interacting with a living organism and its immune system.
- Produces larger quantities of virus: Animal inoculation can produce larger quantities of virus compared to cell culture, making it useful for producing viral vaccines or developing diagnostic tests.
- Enables study of disease progression: In vivo virus cultivation allows for the study of the entire disease process, including how the virus interacts with the host immune system and how the disease progresses over time.
- Facilitates the development of treatments: In vivo virus cultivation can be used to test the effectiveness of treatments and vaccines in animals before they are tested in humans.
- Helps identify new viruses: Animal inoculation can help identify new viruses that may not be detected through other methods, such as in cases where the virus cannot be cultured in cell lines.
- Provides a means of viral research: In vivo virus cultivation can also provide researchers with a tool to study the molecular biology of viruses and their interactions with host cells and the immune system.
Disadvantages of Animal Inoculation
While animal inoculation has some advantages in virus cultivation, there are also potential disadvantages to consider. Here are some of the main disadvantages of using animal inoculation for virus cultivation:
- Ethical concerns: The use of animals for experimentation raises ethical concerns, particularly if the animals are infected with a potentially deadly virus.
- Limited availability of animal models: Certain animal species may not be susceptible to certain viruses, making it difficult to find a suitable animal model for virus cultivation.
- Variation in response to infection: Animal inoculation can result in variability in the response to infection between individual animals, which can complicate experimental results.
- High costs: Animal inoculation can be a costly process, as it requires specialized equipment and expertise, as well as housing and care for the animals.
- Safety concerns: Working with live animals infected with viruses can pose safety risks to researchers, particularly if appropriate biosafety measures are not taken.
- Limited relevance to human infection: Animal inoculation may not always accurately replicate the disease in humans, which can limit the applicability of experimental results to human infection.
2. Embryonated Eggs Method of Virus Cultivation
- In 1931, Goodpasture was the first to use embryonated chicken embryos for virus cultivation techniques.
- Subsequently, Burnet’s technique was used to cultivate viruses in numerous embryonated cell locations.
- In the Cultivation of viruses- methods, viruses are typically cultured in 8–11-day-old chick embryos.
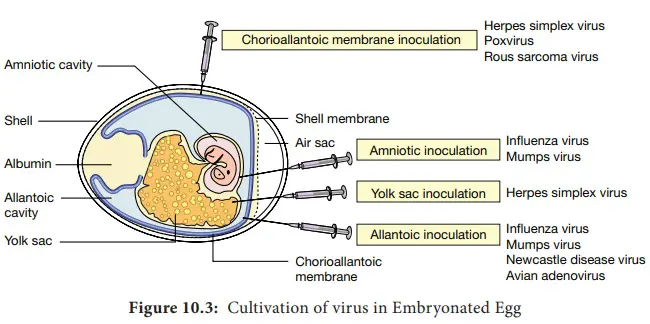
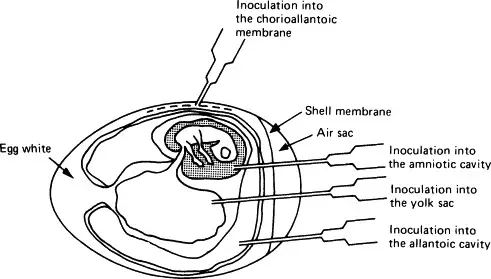
- The viruses are extracted from various egg components, such as the yolk sac, amniotic inner lining, allantoic sac, and chorioallantoic epidemic.
- Numerous of these viral infections result in distinct foci that can be used to identify, quantify, or evaluate virus-pathogenic organisms.
- The embryonated egg can also be used in laboratories to generate vaccines and cultivate elevated titre stocks of certain viruses.
- Viruses can be cultivated in various parts of the egg, including the chorioallantoic membrane, allantoic cavity, amniotic sac, and yolk sac. Each of these parts of the egg offers unique advantages and disadvantages for virus cultivation, depending on the type of virus being studied.
- Chorioallantoic membrane: The chorioallantoic membrane (CAM) is a thin, highly vascularized membrane that surrounds the developing embryo and serves as a primary site for virus replication in the egg. The CAM provides a large surface area for virus growth, and viruses can be inoculated directly onto the CAM for efficient replication. When inoculated with certain viruses, CAM developed lesions known as pocks. Each infectious virus particle generates a single pimple. The pox viruses, such as variola or vaccinia, are identified by the presence of characteristic pocks on the inoculated CAM. In a virology laboratory, inoculation of chick embryos has been supplanted by routine cell culture isolation of viruses.
- Allantoic cavity: The allantoic cavity is a fluid-filled space within the egg that contains the allantoic fluid, which can be used for virus cultivation. This fluid provides a rich source of nutrients and growth factors that can support virus replication. Inoculation in the allantoic cavity is utilised for serial passages and for obtaining significant quantities of influenza, yellow fever (17D strain), and rabies (Flury strain) viruses for vaccine preparation. Due to their larger size than chicken eggs, duck eggs were utilised in the production of rabies virus. This contributed to the production of vast quantities of rabies virus, which are used to make the inactivated non-neural rabies vaccine.
- Amniotic cavity: The amniotic sac is a protective membrane that surrounds the developing embryo and contains the amniotic fluid. This fluid can also be used for virus cultivation, and it provides a similar nutrient-rich environment as the allantoic fluid. Inoculation in the amniotic cavity is primarily used for primary influenza virus isolation.
- Yolk sac: The yolk sac is a membranous sac that surrounds the yolk of the egg and provides nutrients to the developing embryo. Viruses can also be cultivated in the yolk sac, which provides a rich source of lipids and proteins that can support virus growth. For the propagation of Japanese encephalitis, Saint Louis encephalitis, and West Nile virus, yolk sac inoculation is utilised. It is also used for chlamydia and rickettsia growth.
- Overall, the choice of which part of the egg to use for virus cultivation depends on the type of virus being studied and the research goals of the experiment. Each part of the egg offers unique advantages and disadvantages, and researchers must carefully consider these factors when designing virus cultivation experiments.
Advantages of inoculation into embryonated egg
Virus inoculation into embryonated eggs is a common method used for virus cultivation. Here are some potential advantages of using this method:
- The culture/inoculation of avian embryos is a cost-effective and practical method for virus cultivation.
- It is a popular technique for isolating viruses from clinical samples, making it a valuable diagnostic instrument.
- The embryonated egg is an optimal medium for the growth and replication of certain viruses because it contains all the nutrients and growth factors required for virus replication.
- Compared to methods such as animal inoculation, this technique is more cost-effective and easier to maintain.
- Virus inoculation of embryonated eggs requires less labour than other methods, which can result in a more efficient procedure.
- Eggs that have been fertilised are readily available from commercial hatcheries or the laboratory’s own colony of chickens.
- Eggs that have been embryonated are sterile and can provide a variety of tissues and secretions that can be used in further research.
- Eggs that have been embryonated are typically free of contaminating microbes and many latent viruses, making them a useful tool for virus isolation.
- The absence of specific and non-specific defence factors in embryonated ova simplifies the cultivation of viruses.
- Virus inoculation into embryonated eggs is a common method for growing viruses for vaccine production, as it can produce significant quantities of virus in a short period of time.
Disadvantages of inoculation into embryonated egg
- Antibodies in the yolk of eggs from vaccinated flocks may inhibit the proliferation of certain microorganisms.
- Salmonella, Mycoplasma, and other microbes can be transmitted from an infected fowl to its eggs.
3. Tissue Culture Method of Virus Cultivation
- The assay was conducted using cell culture, which is most commonly used in diagnostic virology for virus cultivation.
- In 1913, Steinhardt and his associates pioneered the use of tissue culture in diagnostic virology.
- They maintained the viability of the vaccinia virus by cultivating it in rabbit corneal tissues. Maitland (1928) then cultivated vaccine viruses on nutrient agar plates containing tissue fragments.
- Enders, Weller, and Robins (1949) were the pioneers of poliovirus manipulation in non-neuronal cultured cells. Since then, the majority of the virus has been grown in tissue culture in order to diagnose viral diseases.
- The primary limitation was bacterial contamination. Various forms of culture employed include:
a. Organ culture
- Organ culture is a technique used in virology and other fields of research to study the growth and replication of viruses in a specific organ or tissue. In this technique, small pieces of an organ or tissue are isolated from an animal or human and placed in a culture dish containing a nutrient-rich medium. The organ or tissue is kept alive and functional in the culture dish, and the virus is inoculated onto the culture.
- The advantages of organ culture include the ability to study virus replication in a more physiologically relevant environment, as the virus can interact with the specific cells and tissues of the organ or tissue being studied. This can provide insights into the mechanisms of virus-host interactions and the pathogenesis of viral diseases.
- Organ culture can be used to study a wide range of viruses, including respiratory viruses, hepatitis viruses, and herpesviruses. Examples of organs or tissues that can be used for organ culture include lung tissue, liver tissue, and skin tissue.
- Organ culture techniques can vary depending on the specific organ or tissue being studied and the virus being used. However, in general, the organ or tissue is isolated and cut into small pieces, which are then placed into a culture dish containing a nutrient-rich medium. The virus is inoculated onto the organ or tissue, and the culture is incubated under appropriate conditions to allow for virus replication and study.
- Overall, organ culture is a powerful technique that can be used to study virus replication and pathogenesis in a physiologically relevant environment. However, it is also a technically challenging and time-consuming technique, and careful attention must be paid to maintaining the viability of the organ or tissue and minimizing the risk of contamination.
Small fragments of human and animal organs are maintained in tissue culture media. This method is only used for specific purposes. To culture the Corona virus, for instance, tracheal ring culture is utilised.
Organ cultures are thin segments of in vitro-preserved organs that permit diffusion of nutritional substances or perfused organs. For several weeks, cells in organ cultures can maintain their high level of differentiation and specialised functions. This form of culture is only used in a limited number of instances due to its short shelf life and the particular difficulty of identifying viral mutations. In organ cultures, differentiated tissues include fragments of nervous tissue, ovaries, and thyroidea, as well as epithelium from the respiratory and intestinal tracts. For the cultivation of respiratory viruses (such as coronaviruses and certain rhinoviruses), organ cultures with ciliated respiratory epithelium are used.
b. Explant culture
- Explant culture is a technique used in biological research, including virology, to study the growth and replication of cells and tissues outside of the body. In this technique, a small piece of tissue or organ (called an explant) is removed from an organism and placed in a culture dish containing a nutrient-rich medium. The explant is kept alive and functional in the culture dish, and the virus is inoculated onto the explant.
- Explant culture can be used to study a wide range of viruses, including respiratory viruses, hepatitis viruses, and herpesviruses. Examples of tissues that can be used for explant culture include lung tissue, liver tissue, and skin tissue.
- The advantages of explant culture include the ability to study virus replication in a more physiologically relevant environment, as the virus can interact with the specific cells and tissues of the explant being studied. This can provide insights into the mechanisms of virus-host interactions and the pathogenesis of viral diseases.
- Explant culture techniques can vary depending on the specific tissue being studied and the virus being used. However, in general, the tissue is isolated and cut into small pieces, which are then placed into a culture dish containing a nutrient-rich medium. The virus is inoculated onto the tissue, and the culture is incubated under appropriate conditions to allow for virus replication and study.
- Overall, explant culture is a powerful technique that can be used to study virus replication and pathogenesis in a physiologically relevant environment. However, it is also a technically challenging and time-consuming technique, and careful attention must be paid to maintaining the viability of the explant and minimizing the risk of contamination.
This minute fragment of human or animal tissue is extracted and used for virus culture. This method is infrequently employed.
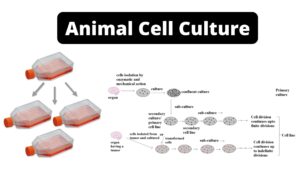
4. Cell Culture Method of Virus Cultivation
This is the most frequently employed method. Labs routinely use cell line culture for virus culture, isolation, and identification. First, in cell line culture, a balanced salt concentration, all essential amino acids, glucose, buffering agents, antibiotics, serum, etc. are included in the preparation of the growth medium. The fragment of tissue is then trypsinized to separate the cells.
The dissociated cells are rinsed, suspended in culture media in a test tube or petri dish, and incubated for the appropriate amount of time. Upon incubation, the cells divide and form a confluent monolayer on the glass surface, which is now used for virus culture. On the basis of their origin, chromosomal characteristics, and the number of generations through which they can be maintained, there are three categories of cell line culture.
- I. Primary cell line
- II. Semi-continuous (diploid) cell line
- III. Continuous cell line
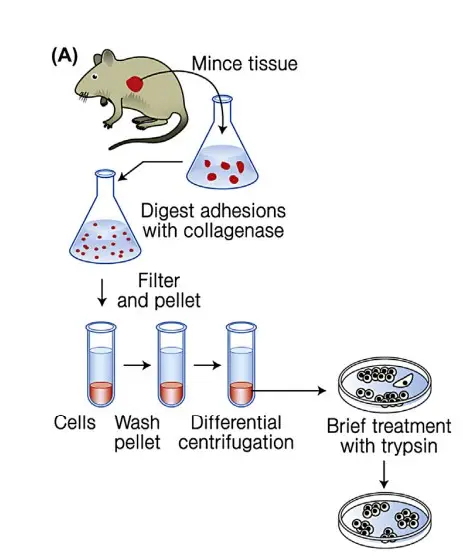
I. Primary cell line/Primary cell cultures
- Primary cell cultures are those created by cultivating cells that have been liberated from organ fragments. Tissues can be homogenised either mechanically (explant culture) or with the assistance of proteolytic enzymes.
- In the former case, groups of cells are obtained, whereas treatment with an enzyme such as trypsin results in a monodisperse cell suspension. The cells are suspended in a nutrient solution and transferred to a vessel for cultivation.
- The majority of living cells adhere spontaneously to the vessel’s bottom. Different types of cells in a primary culture have varying resiliency. However, macrophages, neurons, and muscular cells can survive for weeks to months without dividing.
- In vitro, epithelial cells can undergo approximately ten cell divisions, whereas human fibroblasts can undergo 50–60 cell divisions before the cell line is extinguished. Only cells affixed to the cultivation vessel’s bottom are capable of division.
- This division of cells covers the bottom of the vessel with a cell mosaic, and because the division of cells is dependent on anchorage, multiple layers of cells do not form, resulting in a monolayer.
- The density of the monolayer varies depending on the cell type. The phenomenon that prevents a cell from dividing when it is enveloped by other cells is known as contact inhibition.
- A monolayer in a primary culture of kidney tissue, for instance, exhibits an irregular distribution of areas where similar cells, epithelial cells or fibroblasts, are clustered.
- Due to the numerous cell types, a primary culture is susceptible to infection by a relatively broad spectrum of viruses. Renal tissue derived from embryos or amnion membranes is commonly used to establish human primary cell cultures.
- The cells from a primary culture can be multiplied by “passaging,” which involves transferring them from one cultivation vessel to another, where they are once again permitted to form a monolayer.
- The cells are suspended mechanically or via proteolytic enzymes and/or chelate-forming compounds, such as EDTA. The latter compound can bind Ca++, which is required for cell adhesion to the supporting layer.
- Following the distribution of the cell suspension into three or four new cultivation vessels, the cells attach to the bottom of the vessels and proliferate until the supporting surface is covered with a layer of cells.
- One to four weeks after the establishment of primary cell cultures, passage can be accomplished. With repetitive passage, a particular type of cell becomes dominant.
II. Semi-continuous cell line/Diploid cell lines
These cell are fibroblastic cell. They are diploid cells with the same number of chromosomes as their parents. Fibroblastic cells are obtained from embryo tissue. These cells can be sub-cultured for a limited number of generations. There is rapid cell division, and after 50 successive subcultures, the cells experience senescence. The diploid cell is susceptible to a wide variety of human virus cultures and is also utilised in the production of vaccines. Examples: Rhesus embryo cell, human embryonic lung strain, etc
- Repeated passage of cells from foetal lung tissue causes fibroblasts to dominate the culture. The cells retain their diploid nature, hence the term ‘diploid cell line’.
- This type of cell line is susceptible to a wide variety of distinct viruses. For the cultivation of viruses from patients and the production of some live virus vaccines, diploid cells are utilised.
- To enable the efficient use of diploid cell lines, the majority of cells obtained from early passages are frozen in liquid nitrogen (–196°C) and treated with dimethylsulfoxide (DMSO) to prevent cell injury caused by freezing.
- Frozen cells retain their viability for decades and are used to establish new cultures as needed.
III. Continuous cell line
- These are single-type cells that are capable of indefinite growth in vitro.
- Typically, these are cancer cells derived from malignant tissue. These cells proliferate more rapidly and are haploid.
- They are referred to as continuous cell lines because they can be subcultured indefinitely without senescence.
- HeLa cells are obtained from cervical cancer, HEP-2 (Human Epithelioma of larynx cell line), Vero (Vervet monkey) kidney cell lines, and BHK-21 (Baby Hamster Kidney cell line) are examples of other cell lines.
- Continual cell lines are maintained by serial subculture or deep freezing at -70 degrees Celsius so that the cells can be utilised as needed.
- Continuous cell line is used for virus culture, but not for vaccine preparation, as vaccines produced using continuous cell culture are deemed unsafe for human use.
Advantages of cell culture
Cell culture is a technique used in biological research, including virology, to grow and study cells outside of the body. Cells can be derived from various sources such as animal tissues or human cell lines. Cell cultures can be used to study a wide range of viruses, including respiratory viruses, hepatitis viruses, and herpesviruses. Here are some advantages of cell culture:
- Reproducibility: Cell culture is a highly reproducible technique, as the same cell line can be used for multiple experiments, ensuring consistent results.
- Convenience: Cell culture is a convenient method for growing large amounts of virus. Cultures can be easily maintained and manipulated, allowing researchers to study virus replication, host responses, and antiviral drugs.
- Control: In cell culture, researchers can control the environment in which the cells grow, including the temperature, pH, and nutrient availability. This allows for precise experimental conditions and comparisons.
- Accessibility: Cell culture can be performed using cell lines that are readily available and well-characterized, allowing researchers to easily share and compare results.
- Versatility: Cell culture can be used to study a wide range of viruses, including those that cannot be grown in animals or embryonated eggs.
- Ethical considerations: Cell culture is a more ethical alternative to animal inoculation, as it does not require the use of live animals for virus propagation.
Disadvantage of cell culture
While cell culture is a widely used technique with many advantages, there are also some disadvantages to consider:
- Cell line variability: Different cell lines may have varying susceptibilities to infection or produce different responses to viral infection. This can lead to variability in experimental results and difficulty in comparing data across studies.
- Cost: Cell culture can be a costly technique, requiring specialized equipment and reagents. Maintaining a continuous supply of cells can also be expensive.
- Contamination: Contamination of cell cultures by bacteria, fungi, or other viruses can occur, which can affect experimental results or make cultures unusable.
- Limitations of 2D culture: Most cell culture techniques use a 2D monolayer of cells, which may not fully mimic the 3D architecture of tissues and organs in the body. This can limit the usefulness of cell culture models in predicting in vivo responses.
- Species-specificity: Certain viruses may only infect certain species of cells, making it difficult to study some viruses in cell culture systems.
- Ethical concerns: While cell culture is generally considered a more ethical alternative to animal inoculation, there are still ethical concerns surrounding the use of human cell lines, particularly when those cell lines are derived from fetal tissue.
Advantages of virus cultivation
Virus cultivation has several advantages in the study of viruses and viral diseases. Here are some key advantages:
- Replication of viruses: Virus cultivation allows researchers to replicate and produce large quantities of virus particles, which can be used for further experimentation and study.
- Controlled experimental conditions: Cultivating viruses in the laboratory allows researchers to control the experimental conditions, including the type of host cell used, the timing and duration of infection, and the conditions of the culture medium.
- Standardization: Virus cultivation allows for the standardization of virus strains, which is important for developing vaccines and diagnostic tests that can be used worldwide.
- Development of vaccines and treatments: By cultivating viruses, researchers can develop vaccines and treatments for viral diseases, which can be critical for public health.
- Study of viral pathogenesis: Cultivating viruses in the laboratory allows researchers to study how viruses cause disease, how they interact with host cells, and how they evade the host immune system. This knowledge can help in the development of new treatments and vaccines for viral diseases.
- Study of viral evolution: Culturing viruses can also help researchers study viral evolution over time, which can provide insights into how viruses adapt and mutate to new environments.
Disadvantages of virus cultivation
While virus cultivation has many advantages, there are also some potential disadvantages that need to be considered. Here are some of the main disadvantages of virus cultivation:
- Safety concerns: Cultivating viruses in the laboratory can pose safety risks to researchers, as well as the general public if proper safety protocols are not followed. Some viruses can be highly infectious and can cause serious diseases, so it is essential to handle them with care and in highly specialized facilities.
- Genetic mutations: Culturing viruses in the laboratory can lead to genetic mutations in the virus, which can alter its properties and make it different from the original strain found in nature. These mutations can affect the accuracy of diagnostic tests and the effectiveness of vaccines and treatments.
- Limited host range: Many viruses can only replicate in specific host cells, which can limit the ability to cultivate them in the laboratory. This can make it difficult to study certain viruses or develop effective vaccines and treatments.
- Time-consuming and expensive: Virus cultivation can be a time-consuming and expensive process, requiring specialized equipment and highly trained personnel. This can make it difficult to conduct large-scale studies or develop vaccines and treatments for viral diseases in a timely and cost-effective manner.
- Ethical concerns: Some types of virus cultivation may raise ethical concerns, such as the use of live animals as hosts for virus propagation.
Importance of virus cultivation
The cultivation of viruses is an essential tool in the study of viruses and viral diseases, and it has many important applications in the fields of medical research, public health, and biotechnology. Here are some key reasons why virus cultivation is important:
- Understanding viral pathogenesis: By culturing viruses in the laboratory, researchers can study how they interact with host cells, how they cause disease, and how they evade the host immune system. This knowledge can help in the development of new treatments and vaccines for viral diseases.
- Developing vaccines: Virus cultivation is critical for the development of vaccines against viral diseases. By growing and propagating the virus, researchers can develop weakened or inactivated forms of the virus that can be used as vaccines to stimulate the immune system.
- Developing antiviral drugs: Cultivating viruses can also be useful for developing drugs to treat viral infections. Researchers can test different compounds and drugs to see if they can stop the replication of the virus or prevent it from infecting host cells.
- Understanding viral evolution: Culturing viruses can also help researchers study viral evolution over time. By comparing different strains of the virus and how they evolve, scientists can gain insights into how viruses adapt and mutate to new environments.
- Improving public health: Cultivating viruses is important for diagnosing and monitoring viral infections in patients. It also helps public health officials track the spread of viral diseases and develop effective strategies for controlling outbreaks.
FAQ
What is virus cultivation?
Virus cultivation is the process of growing viruses in a laboratory setting, typically using living cells or tissues as a substrate for viral growth.
Why is virus cultivation important?
Virus cultivation is important for studying the biology of viruses, developing diagnostic tests, and producing vaccines and antiviral therapies.
What are some common methods of virus cultivation?
Common methods of virus cultivation include animal inoculation, embryonated egg inoculation, organ culture, and cell culture.
How is animal inoculation performed for virus cultivation?
Animal inoculation involves injecting a virus into a live animal, which can be used to propagate and isolate the virus. This method is typically used for viruses that do not grow well in other systems.
What is embryonated egg inoculation?
Embryonated egg inoculation involves injecting a virus into an embryonated chicken egg, which provides a fertile environment for viral growth and replication.
What is organ culture?
Organ culture involves maintaining living pieces of organ tissue in a controlled laboratory environment, which can be used to study viral infection and replication.
What is cell culture?
Cell culture involves growing cells from an organism in a laboratory setting, which can be used to study viral infection and replication in a controlled environment.
What are the advantages of using cell culture for virus cultivation?
Cell culture allows for greater control over experimental conditions, including the ability to manipulate specific cell types and monitor infection in real time. It is also a more ethical and cost-effective alternative to animal inoculation.
What are the limitations of using cell culture for virus cultivation?
Cell culture may not fully mimic the complex environment of a living organism, and certain viruses may only grow in specific cell types or tissues. Contamination and variability in cell lines can also be an issue.
What are some applications of virus cultivation?
Virus cultivation is used in a wide range of applications, including basic research on virus biology, development of diagnostic tests, production of vaccines and antiviral therapies, and surveillance of emerging viral threats.
References
- FENNER F, BACHMANN PA, GIBBS EPJ, MURPHY FA, STUDDERT MJ, WHITE DO. Cultivation and Assay of Viruses. Veterinary Virology. 1987:39–53. doi: 10.1016/B978-0-12-253055-5.50007-4. Epub 2014 Jun 27. PMCID: PMC7173454.
- Ryu, W.-S. (2017). Diagnosis and Methods. Molecular Virology of Human Pathogenic Viruses, 47–62. doi:10.1016/b978-0-12-800838-6.00004-7
- Wadell G. Cultivation of viruses. Textbook of Medical Virology. 1983:38–44. doi: 10.1016/B978-0-407-00253-1.50010-4. Epub 2014 Jun 27. PMCID: PMC7173560.
- Isolation, Culture, and Identification of Viruses. (2022, March 5). https://bio.libretexts.org/@go/page/5303
- https://www.sigmaaldrich.com/IN/en/technical-documents/technical-article/cell-culture-and-cell-culture-analysis/microbial-cell-culture/virus-cultivation
- https://www.brainkart.com/article/Cultivation-of-Viruses_41043/
- https://www.shivajicollege.ac.in/sPanel/uploads/econtent/998bb45fddbd73e7486a00e5b22c4250.pdf
- https://www.simplepharmanotes.com/2020/10/viruses-cultivation-of-viruses.html
- https://unacademy.com/content/kerala-psc/study-material/general-microbiology/cultivation-of-viruses-methods/
- https://www.pharmacy180.com/article/cultivation-of-human-viruses-416/
- https://www.sigmaaldrich.com/IN/en/products/pharma-and-biopharma-manufacturing/viral-clearance/virus-inactivation?gclid=CjwKCAjw6IiiBhAOEiwALNqncVAo-uWi7zvSWmTf5alSX6P5yCELOWdjC1noJgD2YSqds8LUZJ2XlhoC5OQQAvD_BwE
- https://www.ndvsu.org/images/StudyMaterials/Micro/Techniques-of-Virus-cultivation.pdf
- https://decs.bvsalud.org/en/ths/resource/?id=15179&filter=ths_exact_term&q=CULTIVO%20DE%20VIRUS
- https://www.onlinebiologynotes.com/cultivation-of-virus/
- https://microbiologyinfo.com/techniques-of-virus-cultivation/
- http://www.janatamhvcha.org/uploaded_files/METHODS_OF_CULTIVATION_OF_VIRUSES-converted_Dr_Wankar.pdf
- https://www.slideshare.net/vivekaiden/cultivation-of-virus-120166509