What is a tumor?
- A mass or collection of aberrant cells that develops as a tumour. A tumour might not always indicate malignancy. Several tumours are benign (not cancerous).
- Throughout the body, tumours can develop. Bone, skin, tissues, glands, and organs can all be impacted. Tumors are also referred to as neoplasms.
- A solid mass of tissue called a tumour. It could be malignant or not. While, A cyst is a tiny sac that could be filled with liquid, air, or solid matter. Cysts are typically not malignant.
- To replace ageing or damaged cells that naturally die off, your body is continuously producing new ones. The cells occasionally don’t disappear as intended. Or, new cells develop and multiply more quickly than they ought to. A tumour develops as the cells begin to accumulate.
Risk factors for tumors
- People of all ages, including children, are impacted by tumours. Gene mutations (changes), such as mutant BRCA (breast cancer) genes, are among the factors that raise the risk of developing a tumour.
- inherited diseases like Neurofibromatosis and Lynch Syndrome (NFS).
- family history of specific cancers, such as prostate or breast cancer.
- smoking, including being around those who are smoking.
- exposure to carcinogens like asbestos or benzene.
- radiation exposure in the past.
- viruses, such as HPV.
- being overweight
Types of Tumors
There is three types of Tumors such as;
1. Cancerous or Malignant Tumor
- Any location on the body might become cancerous.
- A malignant tumour is what results from the growth or lump of cancer cells. When a tumour expands into neighbouring tissues and contains cells that can separate and spread through the blood or lymphatic system to lymph nodes and distant regions of the body, it is said to be malignant.
- Tumors that are malignant or cancerous have the potential to invade adjacent tissue, glands, and other bodily regions.
- Cancerous tumours may reappear following treatment (cancer recurrence).
- These tumours may pose a risk to life.
- Metastatic cancer refers to cancer that spreads from the main tumour, where it first appeared, to a different section of the body.
- The new tumours are referred to as metastases when cancer cells spread and form them.
Types of malignant tumors
- Bone tumours are one type of malignant tumour (osteosarcoma and chordomas).
- brain malignancies like astrocytoma and glioblastoma.
- Sarcomas and malignant soft tissue tumours.
- organ cancers like pancreatic and lung cancer.
- malignancies of the ovarian germ cells.
- skin growths (such as squamous cell carcinoma).
Examples of Cancerous or Malignant Tumor
- Carcinoma: Epithelial cells, which are found in the skin and the tissue that covers or lines the body’s organs, give rise to these tumours. The stomach, prostate, pancreatic, lung, liver, colon, or breast can all develop cancer. They represent a typical variety of malignant tumour.
- Sarcoma: Sarcomas develop in connective tissue, including bone, fat, cartilage, and nerves. They come from cells that are not found in the bone marrow. Malignant sarcomas predominate.
- Germ cell tumour: These cancers form in the sperm and egg-producing cells. They typically develop in the testicles or ovaries, although they can also show up in the brain, abdomen, or chest.
- Blastoma: These tumours grow from developing cells or embryonic tissue. Children experience blastomas far more frequently than adults do. They may result in brain, ocular, or nervous system malignancies.
- Meningiomas: Meningiomas are among the most prevalent types of brain tumours, and if they are producing symptoms, they may need to be removed or treated.
2. Noncancerous or Benign
- Non-cancerous benign tumours rarely pose a hazard to human life.
- Since they are isolated, they usually don’t spread to other areas of the body or impact neighbouring tissue.
- When a doctor removes them, they often do not come back.
- Numerous benign tumours don’t require treatment. However, certain benign tumours that push on other body regions do require medical attention.
Types of benign tumors
- Benign bone tumours are among the typical non-cancerous tumours (osteomas).
- Meningiomas and schwannomas, two types of brain tumours.
- tumours of the glands, such as pituitary adenomas.
- Angiomas are a type of lymphatic tumour.
- benign tumours of soft tissues, such lipomas.
- uterine tumours.
Examples of Noncancerous or Benign
Adenomas
- Glands, organs, and other bodily parts are covered by a thin membrane called glandular epithelial tissue, where adenomas grow.
- Colon polyps, fibroadenomas, a typical type of benign breast tumour, and liver-related hepatic adenomas are a few examples.
- Cancer does not develop from adenomas. However, some of these can develop into malignant adenocarcinomas.
Fibroids
Fibroids, also known as fibromas, are benign tumours that can develop on any organ’s connective or fibrous tissue. Frequently occurring uterine fibroids may result in vaginal bleeding. pelvic discomfort or agony urination problems
The ratio of fibres to cells determines whether they are “soft” or “hard.”
AngiofibromasTrusted Source, which can show up as tiny red lumps on the face, are one type of fibroma. Dermatofibromas, which show up on the skin, frequently on the lower thighs, are another.
Some fibromas may require surgery and create symptoms. Rarely, fibroids can transform into fibrosarcomas. They are carcinogenic.
Hemangiomas
Hemangiomas are benign tumours that develop as a result of excessive blood vessel growth.
They may manifest as cutaneous lesions with a discoloured appearance or grow inside. They frequently exist at birth but vanish during infancy.
Hemangiomas often do not require treatment, but if they persist, laser surgery and other alternatives are possible.
Lipomas
Fat cells make up the soft tissue tumours known as lipomas. Although they can manifest at any age, they frequently afflict adults in the 40 to 60 age range and are not likely to progress to malignancy.
The majority of lipomas are small, painless, flexible, rubbery, and soft to the touch. They frequently show up on the tops of the legs, buttocks, shoulders, arms, and backs.
Angiolipomas, which develop beneath the skin, and fibrolipomas, which contain fat cells and fibrous connective tissue, are two different types of lipomas.
3. Premalignant or Precancerous
- If left untreated, these benign tumours may transform into malignant ones.
Types of precancerous tumors
- Precancerous tumours include the skin disease actinic keratosis.
- Cervical dysplasia.
- Polyps of the colon
- In situ ductal carcinoma is a kind of breast cancer.
Precancerous conditions
Precancerous cells are aberrant cells that, if left untreated, may grow into cancer. Some of these cells have minor alterations that may vanish without treatment. However, certain precancerous cells pass on genetic mutations and gradually become more and more aberrant as they proliferate, eventually transforming into cancerous cells. Precancerous conditions might take a long time to develop into cancer.
- Precancerous alterations vary in severity. Depending on the severity of precancerous alterations, there are various ways to describe them.
- Hyperplasia indicates that aberrant cells are dividing and multiplying more rapidly than normal cells. The cells appear normal under a microscope, however there are an abnormal number of cells. Some forms of hyperplasia are precancerous, although the majority are not.
- Atypia denotes slightly aberrant cells (atypical). Some forms of atypia may be produced by healing and inflammation, whereas others are precancerous.
- Metaplasia indicates that there has been a change in the usual cell types seen in this region of the body. Although the cells appear normal, they are not the type of cells typically present in that tissue or location. However, some kinds of metaplasia are precancerous.
- Dysplasia is characterised by aberrant cells; there are more cells than normal, they are developing quicker than normal, and they are not positioned normally. Dysplasia is a disorder that precedes cancer.
- Carcinoma in situ is the most serious precancerous transformation. The cells are extremely aberrant, yet they have not spread to neighbouring tissue. Carcinoma in situ is typically treated due to its high potential for progression to malignancy.
- People with precancerous diseases are frequently monitored so that they can receive prompt treatment if cell alterations become more severe or transform into cancer.
Examples of Premalignant
Actinic keratosis
- This development is also known as solar keratosis and is characterised by crusty, scaly, and thick skin areas.
- Fair-skinned individuals are more susceptible, and sun exposure increases the risk.
- Occasionally, actinic keratosis might evolve into squamous cell carcinoma; hence, doctors typically recommend treating it.
Cervical dysplasia
- In cervical dysplasia, the cells that line the cervix undergo a change. A physician may discover these cells during a Pap smear. Common among adolescents, the human papillomavirus (HPV) is frequently the cause of cervical dysplasia.
- The cells are not carcinogenic; however, they may become malignant 10–30 years later, leading to cervical cancer.
- A surgeon may use freezing techniques or remove a cone of tissue from the cervix to eliminate the cells.
Metaplasia of the lung
- These growths occur in the bronchi, the tubes that transport air to and from the lungs.
- The bronchial lining contains glandular cells. In certain individuals, notably smokers, these cells can transform into squamous cells or cancer.
Leukoplakia
Leukoplakia causes the development of thick white spots in the mouth.
These spots: are non-painful, have an uneven shape, and cannot be removed by scraping
If this type of patch does not disappear within a reasonable amount of time, the affected individual should consult a physician.
Additionally, they should observe the patches for changes and discontinue smoking or chewing tobacco.
If a physician suspects that the patches could develop into cancer, he or she may remove them using a laser or surgical scalpel.
Tumor Antigens
Additionally, tumour cells express unique chemicals that can be divided into two categories:
- Tumor-specific antigens
- Tumor-associated transplantation antigens
Tumor-specific antigens
- Tumor-specific antigens (TSAs), also known as antigens for tumor-specific transplantation, are unique to malignancies.
- They are not seen on any other bodily cells. Typically, they are the result of altered genes found in cancer cells.
- The aberrant proteins undergo cytosolic processing to generate unique peptides that, when presented by the proper MHC class I molecules, induce a cell-mediated immune response.
- Diverse physical and chemical carcinogens induce mutations in essential genes involved in regulating cell growth, hence causing cancer.
- TSAs include Ras proto-oncogene products such as the p21 Ras proteins and other related gene products.
- Ras proteins possess intrinsic GTPase activity and bind guanine nucleotides (GTP and GDP). The mutations linked with Ras genes in malignant cells appear to arise in the substitution of a single amino acid at certain locations (12, 13, or 61), which increases the gene product’s enzymatic activity.
- As a result, the cells acquire the ability to change. Moreover, the cellular immune response recognises these substances as foreign antigens.
- Integration with proviral genomes is an additional method by which tumour cells may express novel and distinct antigens.
- Typically, the genome of these virus-induced malignancies is integrated with the proviral genome; hence, the proteins produced and expressed are occasionally unique and recognised by the cellular immune system.
- Viruses such as Epstein–Barr virus (EBV), hepatitis B virus (HBV), and hepatitis C virus (HCV) have been linked to cancer.
Tumor-associated transplantation antigens
The other type of tumour antigens are tumor-associated transplantation antigens (TATAs). These antigens are expressed at low levels or exclusively during the differentiation process by (a) tumour cells and (b) normal cells. After the malignant transformation process, the expression of these antigens is significantly depressed or increased. TATAs are available in the following forms:
1. Tumor-associated carbohydrate antigens
- They are a variant of the mucin-associated antigen seen in breast and pancreatic tumours.
2. Differential antigens
- CD10 and prostate-specific antigens are examples (PSA). In prostate cancer, the latter is employed as a diagnostic sign.
3. Oncofetal antigens
- These antigens are present in embryonic and cancerous cells, but not in normal adult cells.
- This antigen is represented by alphafetoprotein and carcinoembryonic antigens, which are detected in hepatomas and colon malignancies, respectively.
- Cancer-associated genes that are silent in normal cells are actively translated in tumour cells.
- Tissue Specific genes or differentiation genes may be present on the surface of normal cells or be shed into the circulation, but expression levels are often quite low.
- This has practical relevance in the diagnosis of cancers, as exemplified by the PSA test for the diagnosis of prostate cancer.
PSA
It is a kallikrein-like serine protease generated solely by prostate gland epithelial cells. The antigen is detected at reasonably high concentrations in seminal plasma but at extremely low concentrations in the serum of healthy males. The measurement of serum PSA levels is a very useful indicator of prostate cancer, and is possibly the most significant serum marker for neoplasia. PSA readings in healthy men range from 0.65 to 0.66 ng/mL between ages 21 and 30 to 1.15 to 0.68 ng/mL between ages 61 and 70. Depending on the stage, 63–86% of patients with prostatic cancer exhibit significantly increased levels. Antigens of tumours capable of triggering an immune response may essentially be one of the following types:
- First, these antigens are expressed exclusively by tumour cells. In addition, there are products of genes that have undergone mutations during the transformation process, resulting in the expression of aberrant products.
- Second, certain antigens expressed by tumours are only present when normal cells are differentiating, and the immune system readily recognises these antigens as well.
- The antigens that are overexpressed by tumour cells finally induce an effective immune response.
Identification of Tumor Antigens
- Numerous antigens expressed by naturally occurring human tumours have been identified, which marks a significant development in the study of tumour immunology.
- Several biochemical and molecular genetic techniques were utilised to identify these antigens. For tumour antigens recognised by CD8+ cytotoxic T lymphocytes (CTLs), researchers have cloned lines of tumor-reactive CTLs from cancer patients and utilised them as probes to discover the relevant peptide antigens or the genes encoding the peptides.
- From patients’ T cells, for instance, several cloned CTL lines specific for melanomas have been created. Melanomas, which are malignant tumours of melanocytes, are frequently easily accessible, surgically resectable, and cultivable in tissue culture.
- T cells can be extracted from peripheral blood, lymph nodes draining the tumour, or excised tumour tissue.
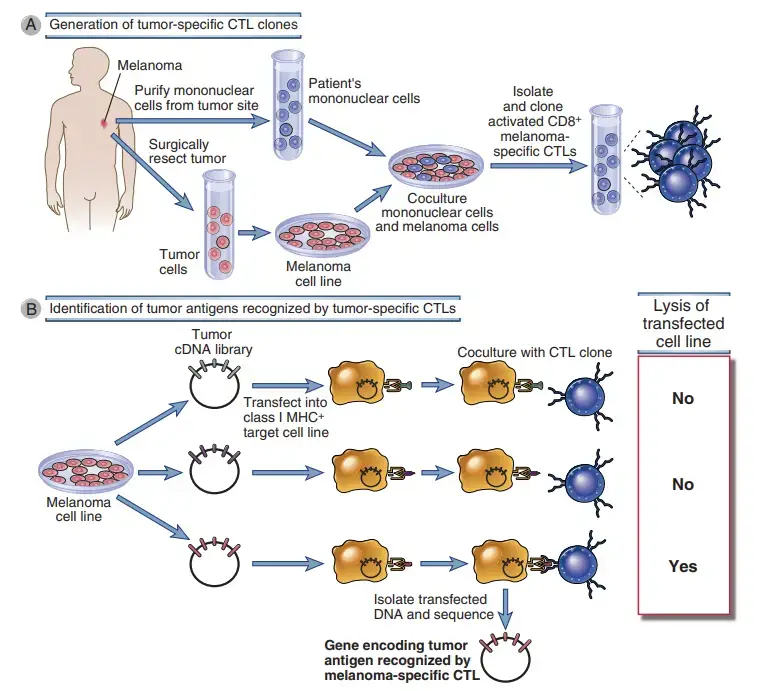
- By coculturing these T cells with tumour cells, growth can be encouraged in vitro, and specific clones can be identified. Due to the fact that the T cells and the tumour originate from the same individual, the MHC restriction of the T cells matches the MHC alleles produced by the tumour.
- These tumour antigen–specific CTL clones have been utilised to identify responses to tumor-derived peptides or proteins produced using complementary DNA (cDNA) libraries of the tumour.
- These methods were initially utilised to discover human melanoma antigens that activated CTL responses in melanoma patients. Identical approaches have been used to detect antigens recognised by CD4+ helper cells, in which case the probes are helper T cell clones produced from CD4+ T cells isolated from patients.
- The serologic study of recombinant cDNA expression is an effective technique for identifying tumour antigens that have generated humoral immune responses in cancer patients (SEREX).
- In this procedure, expression libraries of cDNA obtained from a patient’s tumour RNA are transfected into a cell line, and immunoglobulin binding to the transfected cells is detected using immunoassays.
- Thus, gene sequences for targeted proteins are retrieved, and the encoded proteins that elicited antibody responses in the patient are determined.
- We will include tumour antigens known to trigger immune responses in persons with cancer, as well as tumor-associated antigens that may not typically induce host immune responses, but are prospective immunotherapy targets or important markers for clinical diagnosis and patient monitoring.
Immune Reactions against Tumors
It has been demonstrated that the effector mechanisms of both innate and adaptive immunity destroy tumour cells. Tumor immunologists have the problem of determining which of these systems may contribute to defence against cancers and enhancing these effector mechanisms in a tumor-specific manner.
A. Innate Immune Responses to Tumors
Some of the earliest studies on the functions of effector cells of the innate immune system, such as NK cells and macrophages, focused on their capacity to kill cultivated tumour cells.
NK Cells
- Numerous types of tumour cells are eliminated by NK cells, particularly those with low class I MHC expression and ligands for NK cell activating receptors.
- In vitro, NK cells are capable of eliminating virally infected cells and certain tumour cell lines, particularly hematopoietic malignancies. NK cells also respond to the absence of class I MHC molecules, as the detection of class I MHC molecules inhibits NK cell activity.
- As we will see later, certain cancers decrease class I MHC molecule expression, possibly as a result of CTL-mediated selection against class I MHC–expressing cells. This absence of class I MHC molecules renders malignancies very attractive targets for NK cells.
- Some cancers also express the ligands for the NKG2D activating receptor on NK cells, MIC-A, MIC-B, and ULB.
- Fc receptors (FcγRIII or CD16) can additionally target NK cells to tumour cells coated with IgG antibodies. The anti-tumor effects of interferon-γ (IL-γ), interleukin-15 (IL-15), and interleukin-12 are partially attributable to the stimulation of NK cell activity.
- IL-2–activated natural killer (NK) cells, also known as lymphokine-activated killer (LAK) cells, are produced by the cultivation of peripheral blood cells or tumor-infiltrating lymphocytes from cancer patients receiving high doses of IL-2.
- These cells are more effective tumour killers than non-activated NK cells. Later, the application of LAK cells in adoptive immunotherapy for cancers will be addressed.
- It is unknown what role NK cells play in tumour immunity in vivo. In other studies, the absence of a significant prevalence of spontaneous tumours in T cell–deficient mice is related to the presence of a normal number of NK cells, which serve as immune surveillance cells.
- A small number of patients have been reported to have NK cell deficits and an elevated prevalence of EBV-associated lymphomas.
Macrophages
- Depending on their activation state, macrophages are capable of both blocking and supporting the growth and spread of malignancies.
- M1 macrophages that have been classically stimulated have many antitumor activities. These cells are more effective than normal cells at eliminating cancerous cells. It is unknown how macrophages are triggered by tumours.
- Possible methods include direct detection of certain antigens on the surface of tumour cells and activation of macrophages by IFN-γ generated by T cells that target the tumour.
- M1 macrophages can kill tumour cells by many methods, which are likely identical to the mechanisms by which macrophages eliminate pathogenic organisms. The release of lysosomal enzymes, reactive oxygen species, and nitric oxide are among these.
- M1 macrophages also produce the cytokine tumour necrosis factor (TNF), which was identified as a tumor-killing agent, as its name suggests.
- We now know that it mostly accomplishes this by causing thrombosis in tumour blood arteries. M2 macrophages, in contrast, may contribute to tumour growth.
- These cells release substances that induce tumour angiogenesis, including vascular endothelial growth factor (VEGF), transforming growth factor-β (TGF-β), and other soluble factors.
B. Adaptive Immune Responses to Tumors
Both T cell–mediated and humoral immune responses are elicited by tumours. T cells are the primary mediators of antitumor immunity, and this understanding has led to significant efforts to boost T cell responses for cancer immunotherapy.
T Lymphocytes
- The primary mechanism of adaptive tumour immunity is CD8+ CTL-mediated tumour cell lysis. The ability of CTLs to offer efficient anti-tumor immunity in vivo is particularly evident in animal research employing tumours induced by carcinogens and DNA viruses.
- CTLs may provide a surveillance function by identifying and destroying possibly malignant cells that express peptides derived from tumour antigens and presented in conjunction with class I MHC molecules, as stated previously.
- The significance of immune surveillance in the prevention of common, non-virally caused cancers remains unclear, as the prevalence of such tumours in T cell–deficient persons is not demonstrably higher than that of immunocompetent individuals.
- Nonetheless, tumor-specific CTLs can be extracted from animals and humans with established tumours, and there is evidence that the prognosis of certain types of human malignancies is improved when there are more CTLs.
- In addition, mononuclear cells produced from the inflammatory infiltrate of human solid tumours, known as tumor-infiltrating lymphocytes (TILs), contain CTLs with the ability to kill the tumour from which they originated.
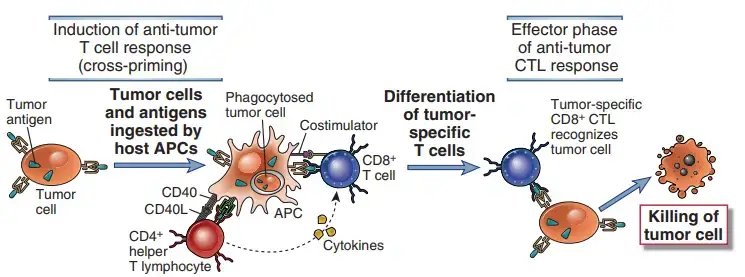
- Dendritic cell cross-presentation of tumour antigens may be required for CD8+ T cell responses to tumour antigens. The majority of tumour cells are not generated from APCs and consequently do not express the costimulators required to trigger T cell responses or the class II MHC molecules required to excite helper T cells that increase CD8+ T cell development.
- Tumor cells or their antigens are likely ingested by host APCs, particularly dendritic cells, and tumour antigens are processed within the APCs to initiate T cell responses to tumours.
- Peptides produced from these antigens are subsequently presented to CD8+ T lymphocytes coupled to class I MHC molecules. The APCs express costimulators that offer the necessary signals for the development of CD8+ T cells into anti-tumor CTLs, and they also express class II MHC molecules that may present internalised tumour antigens and activate CD4+ helper T cells.
- Once effector CTLs have been produced, they may recognise and eliminate tumour cells without the need for costimulation.
- A practical application of cross-priming is to generate dendritic cells from a cancer patient, incubate APCs with cells or antigens from that patient’s tumour, and then employ these antigen-pulsed APCs as vaccines to stimulate anti-tumor T cell responses.
- Less apparent is the significance of CD4+ helper T cells in tumour immunity. CD4+ cells may play a role in antitumor immune responses by supplying cytokines for the efficient production of CTLs.
- In addition, helper T cells specific for tumour antigens may produce cytokines, such as TNF and IFN-γ, that augment tumour cell class I MHC expression and lysis sensitivity by CTLs.
- IFN-γ may also stimulate macrophages to eliminate cancer cells. The higher occurrence of cancers in knockout mice lacking this cytokine, the IFN-γ receptor, or components of the IFN-γ receptor signalling cascade demonstrates the significance of IFN-γ in tumour immunity.
Antibodies
- It is possible for tumor-bearing hosts to generate antibodies against several tumour antigens. Patients with EBV-associated lymphomas, for instance, exhibit serum antibodies against EBV-encoded proteins expressed on the lymphoma cell surface.
- Antibodies may kill tumour cells by activating complement or by antibody-dependent cell mediated cytotoxicity, in which Fc receptor–carrying macrophages or NK cells are responsible for the killing.
- However, the ability of antibodies to destroy tumour cells has been established mostly in vitro, and there is no evidence that humoral immune responses are effective against malignancies.
- Some efficient therapeutic antitumor antibodies that are passively supplied to patients most likely function by antibody-dependent cell-mediated cytotoxicity, as will be addressed in greater detail below.
Evasion Of Immune Responses By Tumors
- Numerous cancers acquire systems that allow them to circumvent immune responses to tumours. These methods can be roughly classified as either intrinsic to tumour cells or mediated by other cells.
- Understanding the immune evasion mechanisms of tumours is a primary focus of tumour immunology, with the goal that therapies to prevent immune evasion may raise the immunogenicity of tumours and maximise the host’s immunological responses.
- Evidence from mice models suggests that immune responses to tumour cells exert selective pressures that result in the survival and development of mutant tumour cells with diminished immunogenicity, a process known as tumour editing.
- For instance, when tumours are produced by carcinogen treatment in immunodeficient or immunocompetent mice and then transplanted into new immunocompetent mice, tumours derived from immunodeficient animals are more frequently rejected by the recipient animal’s immune system.
- This result demonstrates that tumours forming in a normal immune system environment become less immunogenic over time, which is consistent with the selection of less immunogenic mutant cells.
- It is believed that tumour immunoediting explains the formation of cancers that “escape” immune monitoring. Now, we will address the intrinsic and extrinsic tumour mechanisms that may be responsible for editing and escape.
Intrinsic Mechanisms of Immune Evasion by Tumor Cells
Multiple characteristics of tumour cells allow them to evade host defences.
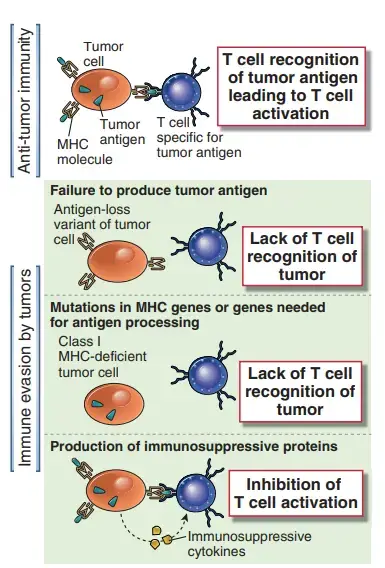
Tumors may lose expression of antigens that elicit immune responses
- Such “antigen loss variations” are prevalent in quickly growing tumours and can be easily produced in tumour cell lines by cultivating them with tumor-specific antibodies or CTLs.
- Given the high mitotic rate and genetic instability of tumour cells, mutations or deletions in genes encoding tumour antigens are prevalent.
- Antigen-negative tumour cells have a growth advantage in the host if these antigens are not necessary for tumour development or maintenance of the altered phenotype.
- The lack of antigens identified by tumor-specific CTLs corresponds with greater tumour development and metastatic potential, according to an analysis of serially transplanted tumours from one animal to another.
- In addition to tumor-specific antigens, class I MHC expression may be downregulated on tumour cells, preventing CTL recognition.
- The generation of class I MHC molecules, β2-microglobulin, and components of the antigen-processing machinery, including the transporter associated with antigen processing and several subunits of the proteasome, is reduced in a variety of malignancies.
- These processes are likely tumour modifications that evolve in response to the selective pressures of the host immune system, and they may enable tumour cells to evade T cell–mediated immune responses.
- There is no evident association between the level of MHC expression on a wide variety of experimental or human tumour cells and their in vivo growth.
Tumor antigens may be inaccessible to the immune system
- Tumor cell surface antigens may be concealed from the immune system by glycocalyx molecules, such as mucopolysaccharides containing sialic acid.
- This phenomenon is known as antigen masking, and it may be a result of the fact that tumour cells frequently express a greater quantity of these glycocalyx molecules than normal cells.
Tumors may fail to induce strong effector T cell responses because most tumor cells do not express costimulators or class II MHC molecules.
- Costimulators are essential for the start of T cell responses, whereas class II molecules are required for the activation of helper T cells, which in some cases is necessary for the differentiation of CTLs.
- Induction of tumor-specific T cell responses therefore frequently needs cross-priming by dendritic cells that express costimulators and class II molecules.
- CTLs specific for tumour cells may not form if APCs fail to effectively acquire and deliver tumour antigens and to activate T helper cells.
- Transfected tumour cells with the genes encoding the costimulators B7-1 (CD80) and B7-2 (CD86) can induce robust cell-mediated immune responses.
- CTLs produced by B7-transfected tumours are predictably efficient against the parent (B7-negative) tumour, as the effector phase of CTL-mediated death does not require costimulation.
- As we will see in the following section, these experimental discoveries are being applied to the clinical setting as immunotherapy for malignancies.
Tumors may engage molecules that inhibit immune responses
- Experimentally, there is strong evidence that T cell responses to some cancers are suppressed by the participation of CTLA-4 or PD-1, two of the most well-defined T cell inhibitory pathways.
- A possible explanation for this function of CTLA-4 is that tumour antigens are presented by APCs with low numbers of B7 costimulators in the absence of a robust innate immune response.
- These low concentrations may be sufficient to activate the high-affinity receptor CTLA-4. Numerous human cancers produce PD-L1, a B7 family protein that binds to the T cell inhibitory receptor PD-1, and animal studies indicate that PD-L1 expression compromises anticancer T cell responses.
- PD-L1 on APCs may also impede the activation of tumor-specific T cells. As we will explore later, there are ongoing clinical trials to increase tumour immunity by blocking the CTLA-4 and PD-L1/PD-1 pathways.
- Some cancers express Fas ligand (FasL), which identifies the death receptor Fas on leukocytes attempting to fight the tumour; interaction of Fas by FasL can result in apoptotic cell death.
- FasL has been discovered on a small number of spontaneous tumours, and when it is expressed in tumours via gene transfection, it is not always protective, therefore the significance of this tumour evasion mechanism has not been demonstrated.
Secreted products of tumor cells may suppress anti-tumor immune responses.
- TGF-β, which is released in high quantities by several tumours and inhibits the proliferation and effector activities of lymphocytes and macrophages, is an example of an immunosuppressive tumour product.
Extrinsic Cellular Suppression of Anti-Tumor Immunity
Several cell populations that inhibit anti-tumor immunity have been identified in tumor-bearing people and animals.
- By changing the tissue milieu and inhibiting T cell responses, macrophages associated with tumours may increase tumour growth and invasiveness. These macrophages have an M2 phenotype and release T cell-inhibiting mediators, including IL-10, prostaglandin E2, and arginase. Additionally, tumor-associated macrophages release angiogenesis-promoting proteins, such as TGF-β and VEGF, which promote tumour growth.
- Regulatory T cells may inhibit tumor-specific T cell responses. There is evidence from mice model systems and cancer patients indicating the number of regulatory T cells is elevated in tumor-bearing individuals, and that these cells are present in the cellular infiltrates of certain cancers. Depletion of regulatory T cells in mice with tumours increases anti-tumor immunity and decreases tumour progression.
- MDSCs are immature myeloid progenitors that are recruited from the bone marrow, aggregate in lymphoid tissues, blood, or tumours of tumor-bearing animals and cancer patients, and dampen anti-tumor innate and T cell responses. MDSCs are comprised of progenitors of dendritic cells, monocytes, and neutrophils. Ly6C or Ly6G and CD11b in mice and CD33, CD11b, and CD15 in humans are surface markers they share. Several proinflammatory mediators generated by malignancies stimulate the recruitment of MDSCs from the bone marrow into lymph nodes and other organs. These mediators, such as prostaglandin E2, IL-6, VEGF, and complement fragment C5a, are not tumor-specific, and MDSCs concentrate at sites of chronic inflammation unrelated to malignancies. MDSCs reduce innate immune responses by secreting IL-10, which inhibits different inflammatory macrophage functions. MDSCs inhibit T cell responses via multiple ways. They express arginase and inducible nitric oxide synthase, which generate reactive oxygen species, including peroxynitrite, that suppress T cell activation. MDSCs also generate the enzyme indolamine 2,3-dioxygenase, which catabolizes the tryptophan required for T cell growth. MDSCs inhibit antitumor T cell responses by increasing the formation of regulatory T lymphocytes (Tregs) and skewing the differentiation of helper T cells toward TH2 cells.
Symptoms of a tumor
Tumor symptoms differ based on where the tumour forms and whether it is malignant. The tumour may be palpable, similar to a breast lump. You may experience:
- Fatigue.
- Fever or chills.
- Lack of appetite or unexplained weight loss.
- Night sweats.
- Pain.
Diagnosis of tumor
- Sometimes a tumour can be seen or felt, but others are only detectable through imaging tests such as mammograms or MRIs. Additionally, colonoscopies and Pap smears can identify malignant and precancerous lesions.
- A biopsy may be required to identify the type of mass. A little tissue sample will be collected by the physician and sent to a laboratory where technicians will analyse it under a microscope.
- The sample may be collected in the doctor’s office with a needle or during surgery to remove the tumour.
- If they feel that a tumour is cancerous, or if it is pressing on a nerve or creating other complications, they may determine that the patient must undergo surgery first.
Treatment of Tumor
Numerous factors, such as the tumor’s kind (malignant or benign) and location, influence the treatment options for a tumour.
Many benign tumours do not require treatment. However, certain benign tumours may continue to expand. For instance, benign brain tumours can exert pressure on healthy tissue, impairing vision or speech. Your physician may prescribe surgical removal of the tumour.
- Among the treatments for malignant tumours is surgical removal of the tumour.
- Chemotherapy to reduce the size of the tumour before to surgery or to eliminate any remaining abnormal cells after surgery.
- Immunotherapy to stimulate the immune system in order to combat cancer.
- Radiation therapy to eliminate malignant cells.
- Targeted therapy to slow or stop cancer cell proliferation.
Prevention of Tumor
The majority of cancers have no recognised cause. However, the following measures may reduce your risk of acquiring a tumour:
- Reduce your alcohol intake and quit smoking.
- Maintain a nutritious diet and an active lifestyle.
- Reduce your exposure to poisons.
- If necessary, weight loss is recommended.
- Use condoms and receive the HPV vaccine to reduce the risk of sexually transmitted diseases such as HPV.
References
- https://my.clevelandclinic.org/health/diseases/21881-tumor
- https://www.medicalnewstoday.com/articles/249141
- https://cancer.ca/en/cancer-information/what-is-cancer/types-of-tumours
- https://sphweb.bumc.bu.edu/otlt/mph-modules/ph/ph709_cancer/PH709_Cancer9.html
- https://www.webmd.com/a-to-z-guides/benign-tumors-causes-treatments