Standard protein expression systems, including E. coli, frequently fail to generate folded, monodisperse, or functional eukaryotic proteins (see Small-scale Expression of Proteins in E. coli). Utilizing a eukaryotic system, such as mammalian cells, that includes the required folding and posttranslational machinery is highly advantageous for the expression of these proteins. Using polyethylenimine, we present strategies for both small- and large-scale transient expression in mammalian cells (PEI). We find this method to be more cost-effective and expedient than the conventional approach of creating stable cell lines. Initially, the best transfection conditions are identified using adherent cells on a modest scale. The conditions are then implemented in large-scale suspension cultures.
Polyethylenimine (PEI)
- Polyethylenimine (PEI) or polyaziridine is a polymer comprising repeating units of amine and two carbon aliphatic CH2CH2 spacers.
- Unlike branching polyethyleneimines, which contain primary, secondary, and tertiary amino groups, linear polyethyleneimines have only secondary amines.
- Dendrimeric forms with complete branching were also reported. PEI is produced on an industrial scale, and its polycationic nature results in several applications.
- At ambient temperature, linear PEI is a semi-crystalline solid whereas branched PEI is a totally amorphous polymer that exists as a liquid at all molecular weights. At low pH, linear polyethyleneimine is soluble in hot water, methanol, ethanol, and chloroform. It does not dissolve in cold water, benzene, ethyl ether, or acetone.
- Polyethyleneimine has a melting point of around 67 degrees Celsius. At room temperature, both linear and branched polyethyleneimine can be stored. Aqueous solutions of linear polyethyleneimine are capable of forming cryogels upon freezing and subsequent thawing.
- Aziridine can be polymerized to create branched PEI using ring opening polymerization. Depending on the reaction conditions, various degrees of branching are possible.
- Post-modification of other polymers, such as poly(2-oxazolines) or N-substituted polyaziridines, produces linear PEI. JetPEI was produced by hydrolyzing poly(2-ethyl-2-oxazoline) to produce linear PEI. As precursors, the current generation of in-vivo-jetPEI utilises poly(2-ethyl-2-oxazoline) polymers.
Principle of PEI transfection
Polyethylenimine (PEI), a stable cationic polymer, can be used for transfection to transfer DNA into a host cell. PEI compacts DNA into positively charged particles that adhere to anionic cell surfaces. Therefore, the DNA:PEI complex is endocytosed and the DNA is released into the cytoplasm. Our laboratory prefers PEI to other cell transfection reagents because of its inexpensiveness.
This procedure is suitable for CHO-S and HEK 293 GnTi- suspension cell lines. Numerous cell lines can be effectively transfected with PEI, however in our experience, these two cell lines express the most protein compared to other cells.
Equipment Required
- Laminar flow hood
- CO2 incubator
- Platform shaker
- Centrifuge
- Water bath (37 °C)
- Inverted microscope
- Hemacytometer
- Sterile 0.22 µm filters
- Sterile 250-ml polypropylene centrifuge tubes
- Sterile 50-ml polypropylene conical tubes
- Sterile 1.5-ml polypropylene tubes
- Sterile 6-well tissue culture plates micropipettors
- Sterile micropipettor tips
- Sterile disposable serological pipettes
- Sterile square polypropylene bottles
Material Required
- Plasmid DNA directing your protein of interest
- Fetal bovine serum (FBS, Invitrogen)
- Polyethylenimine ‘Max’ (linear, MW 25 000) (Polysciences, Inc.)
- l-Glutamine 100× (Invitrogen)
- Sodium hydroxide (NaOH)
- MEM α (containing Earl’s Salts and l-glutamine, but no ribonucleosides, deoxyribonucleosides, NaCO3; Invitrogen 12000)
- DMEM/F12 (with L-glutamine, but no HEPES, NaHCO3; Invitrogen 12500)
- Freestyle™ 293 medium (Invitrogen 12338-026)
- FreeStyle™ CHO-S (Invitrogen R800-07)
- Hybridoma SFM (Invitrogen 12045)
- Opti-MEM® (Invitrogen)
- HEK293S GnTI- (ATCC# CRL-3022)
- HEK293T/17 (ATCC# 11268)
Certain stock solutions include the pH indicator phenol red. This supplement has no effect on the application and may be useful if the researcher desires to monitor pH fluctuations in the solutions over time. In the event of non-CO2 incubators (such as when scaling up the production of adherent cells in roller bottles), the pH can be maintained with HEPES-buffered medium. Catalog numbers are from the Invitrogen website in the United States and may vary on other local websites.
Solutions & buffers
| PEI ‘Max’ | ||
| Dissolve 1 g PEI ‘Max’ in 900 ml distilled water. Adjust the pH to 7.0 with 1 N NaOH. Add distilled water to 1 l | ||
| Note: Stable at least 9 months at 4 °C | ||
| Make smaller volumes depending on how much is needed. PEI ‘Max’ cannot be frozen! | ||
| FreeStyle™ 293 ‘Completed’ | ||
| Component | Stock | Amount |
| FreeStyle™ 293 medium | 1 l | |
| FBS | 100% | 10 ml |
| l-Glutamine | 200 mM | 10 ml |
| DMEM:F12, 5% FBS | ||
| Add 50 ml FBS to 1 l of DMEM:F12 | ||
| Alpha MEM, 5% FBS | ||
| Add 50 ml FBS to 1 l of Alpha MEM | ||
| Hybridoma SFM, 1% FBS | ||
| Add 10 ml FBS to 1 l of Hybridoma SFM |
PEI transfection Protocol
Step 1 Small-Scale Transient Transfection (Duration 5 days)
Before scaling up a large-scale transfection, this stage will examine a range of transfection settings, including medium, cell type, ratio of PEI to DNA, and expression period, to optimise protein expression.
Antibiotics cannot be used during the process of transfection.
Step 1.1 Seed Adherent Cells For Transfection (Duration 1.5 days)
Transfer the necessary number of cells to a 6-well dish and allow them to adhere.
- Before counting the cells, they must be washed with PBS, trypsinized, mixed with 10 ml of DMEM:F12 with 5% FBS, and pipetted multiple times to ensure an equal suspension. Place the cells in a sterile 15-ml centrifuge tube. Use a hemacytometer to count the cells.
- Pour 3 x 105 cells per each transfection condition into a sterile centrifuge tube.
- Spin the cells at 200 x g for 5 minutes at room temperature. Aspirate supernatant.
- Add a final concentration of 3 x 105 cells ml-1 of DMEM:F12 plus 5% FBS to the cell pellet.
- With a serological pipette, re-suspend the cells.
- In a 6-well plate, add 2 ml of DMEM:F12+5% FBS to each well.
- Inject 1 ml of cell suspension into each well.
- Place plate in the 37 °C, 5% CO2 incubator.
- Remove the medium from each well after 24 hours.
- Add 2.7 ml of DMEM:F12 supplemented with 5% FBS to each well.
- Place the plate in the incubator.

Step 1.2 Transiently Transfect Cells (45 min active time; 4 days total)
To transfect cells, DNA and PEI ‘Max’ are added to the cells. The PEIDNA combination is made and added to the cells on the same day as the medium is changed.
- 9 µg of PEI ‘Max’ should be diluted with 150 µl of Opti-MEM. The amount of PEI is variable.
- 150 µl of Opti-MEM should be used to dilute 3 µg of DNA.
- Add the diluted PEI ‘Max’ solution to the diluted DNA solution.
- At room temperature, incubate the mixture for 30 minutes.
- Add the PEI-DNA mixture to a well of adhering cells with care. To avoid disturbing the adhering cells, carefully pipette the solution down the side of the well and not on top of them.
- Return the plate to the incubator with 5% CO2.
Tips
- Opti-MEM can be replaced with serum-free hybridization media.
- This approach utilises a 3:1 (w/w) ratio of PEI to DNA. We have determined that this ratio is ideal for the majority of genes we have expressed. However, this ratio must be evaluated for each examined gene. We typically examine ratios ranging from 1:1 to 5:1.
- In general, 1 µg of DNA per 1 ml of transfected culture is recommended. Before combining PEI and DNA, each should be diluted to 1/20 of the total culture volume.
- With suspension-adapted cells, transfections on a small scale are possible. The protocol for small-scale production is largely identical. For optimal aeration and agitation, we add only 5–12 ml of cell media to square plastic bottles designed to hold 125 ml for suspension culture.

Step 1.3 Harvest Cells And Analyze Protein Expression (1–2 days)
Collect and lyse the cells (see Lysis of mammalian and Sf9 cells). Using Western blotting, evaluate protein expression (see Western Blotting using Chemiluminescent Substrates).
- 96 hours after transfection, samples are collected for analysis. For proteins that are secreted, collect and store the media. For membrane or intracellular proteins, remove media. Lyse the cells after washing them with PBS.
- As applicable, analyse protein expression using Western blotting or ELISA.
Tips
- HEK293 GnTI-cells can be resuspended using a serological pipette and gentle up-and-down pipetting. CHO-S cells stick more strongly to the plate and must be resuspended manually using a cell scraper.
- Certain methods require trypsin digestion to separate cells from the dish. This can be avoided by scraping the cells by hand. If the protein is a membrane protein, trypsin may be able to digest it.
- Once a cell type, culture medium, and ideal ratio of PEI to DNA have been determined, this technique can be repeated and samples collected between 24 and 96 h posttransfection to maximise the length of expression.

Step 2 Large-Scale Transient Transfection Of Suspension Cells (4–8 days)
Protein production at a large scale in suspension culture. The parameters optimised in Step 1 of this technique are expanded to higher volume cultures. 400 ml of cells at a density of 2–3 x 106 cells ml-1 are required.
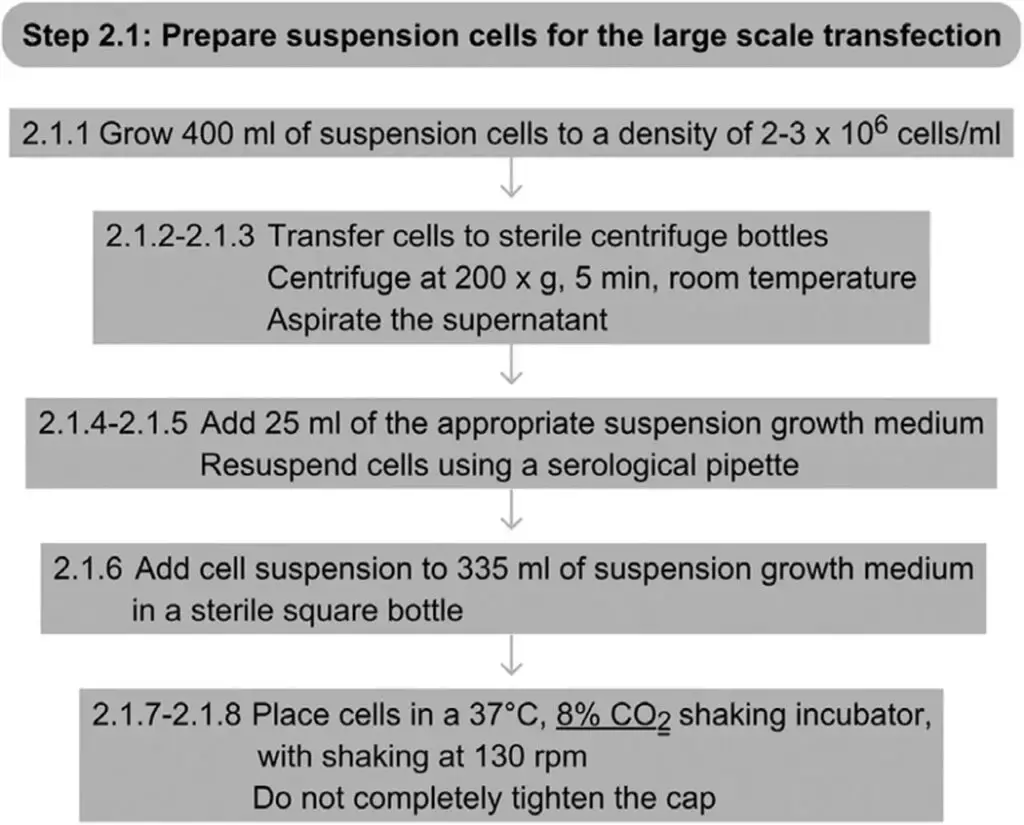
Step 2.1 Prepare The Cells To Be Transfected (30 min)
Collect and count suspension cells to ensure they have the right concentration. Resuspend the cells in a total of 360 ml of fresh suspension growth medium after centrifuging them.
- Grow 400 ml of cells to a density of between 2 and 3 x 106 cells ml-1 in the suitable suspension growth medium.
- The cell suspension should be transferred to sterile centrifuge bottles.
- Spin the cells at 200 x g for 5 minutes at room temperature. Remove the supernatant.
- Add 25 ml of the appropriate fresh growth media for suspensions.
- With a serological pipette, gently resuspend the cells.
- In a sterile square bottle, combine 335 ml of new suspension growth medium with the cells.
- Do not completely tighten the bottle cap.
- Place the cells in an incubator shaker at 37 °C with 8% CO2 and 130 rpm shaking.
11. Step 2.2 Transfect Cells (45 minutes of effective time, as defined above)
Transfect the cells using the indicated optimum ratio of PEI to DNA.
- 400 µg of DNA should be diluted in 20 cc of Hybridoma SFM (without serum).
- Using a total volume of 20 ml of Hybridoma SFM, dilute the PEI ‘Max’ concentration calculated in Step 1 to the desired level (without serum).
- Mix the diluted PEI “Max” with the diluted DNA.
- At room temperature, incubate the mixture for 30 minutes.
- Add the PEI-DNA mixture to the cells that were suspended in Step 2.1.8.
- Return the cells to the incubator shaker at 37 degrees Celsius and 130 rpm.

12. Step 2.3 Harvest Cells And Process Protein As Needed (About 1 h)
Obtain the cells (or medium for a secreted protein). Purify the protein or modify it as required for further applications (see The removal of salt from proteins via ammonium sulphate precipitation. Purification of recombinantly produced proteins by ion exchange chromatography, Gel filtration chromatography (Size exclusion chromatography) of proteins, Use and Application of Hydrophobic Interaction Chromatography for Protein Purification or Hydroxyapatite Chromatography: Purification Strategies for Recombinant Proteins, or look up the affinity purification chapters if tags have been added to the protein: Purification of His-tagged proteins, Affinity purification of a recombinant protein expressed as a fusion with the maltose-binding protein (MBP) tag, Purification of GST-tagged proteins, Protein Affinity purification.
- After determining the proper time in Step 1, spin the cells at 200 x g for 5 minutes at room temperature.
- If the protein is secreted, the medium must be collected. At room temperature, centrifuge the cells at 200 x g for 5 minutes and sterile-filter the media through a 0.22-µm filter. Add sodium azide to 0.02%. The medium can be stored at 4 °C for several months before use.
- The cell pellet should be flash-frozen in liquid nitrogen and kept at -80 °C until required for protein purification.
After determining the proper time in Step 1, spin the cells at 200 x g for 5 minutes at room temperature. If the protein is secreted, the medium must be collected. At room temperature, centrifuge the cells at 200 x g for 5 minutes and sterile-filter the media through a 0.22-µm filter. Add sodium azide to 0.02%. The medium can be stored at 4 °C for several months before use. The cell pellet should be flash-frozen in liquid nitrogen and kept at -80 °C until required for protein purification.
Tips
- Single cells are transfected more efficiently in suspension cultures than cells that have clumped together during growth. It may be necessary to optimise growing conditions for single-cell growth.
- Autoclaving square bottles requires two dry cycles (45 minutes each, 15 minutes each dry) with the lids as loose as feasible without sliding off. Before tightening the lids, they must be allowed to cool completely in the laminar flow hood. If the bottles collapse inward, cell growth will suffer.
- 24 hours after transfection, cells can be diluted between 1:2 and 1:5 in the proper medium to produce more protein. The effect of dilution and the ideal dilution ratios must be empirically evaluated.

- Text Highlighting: Select any text in the post content to highlight it
- Text Annotation: Select text and add comments with annotations
- Comment Management: Edit or delete your own comments
- Highlight Management: Remove your own highlights
How to use: Simply select any text in the post content above, and you'll see annotation options. Login here or create an account to get started.