What is Thin Layer Chromatography?
- Thin Layer Chromatography (TLC) refer as one type of adsorption chromatography, where separation of compounds is achieved by using a thin layer of adsorbent (like silica gel, alumina, or cellulose) spread on a flat surface like glass plate / plastic sheet / metal foil.
- It can define as a technique for separating non-volatile mixtures, mainly used in analytical chemistry by quick identification of substances.
- The stationary phase is made up of adsorbent solid layer, while mobile phase is liquid solvent that moves upward by capillary action.
- When mixture is applied as a small spot near bottom of plate, components get separated as the solvent moves upward.
- Movement of compounds depend on their affinity toward stationary phase and solubility in solvent phase – the one having stronger adsorption move slower.
- TLC plate after development is dried and then visualized by UV light or by spraying reagents (like iodine vapour, ninhydrin etc.).
- Each compound produce a specific Rf value (Retention factor) which used for qualitative analysis, comparing with known standards.
- Technique is very handy and fast, also it require very small sample quantity, that’s why used in organic synthesis monitoring, drug purity testing, food and plant pigment studies etc.
- Some limitations prevail like overlapping of spots, difficult quantitative estimation and solvent evaporation errors but it’s still valuable.
- In short, TLC considered as a sturdy and handy method for checking purity and identity of various substances in laboratories (educational or research type).
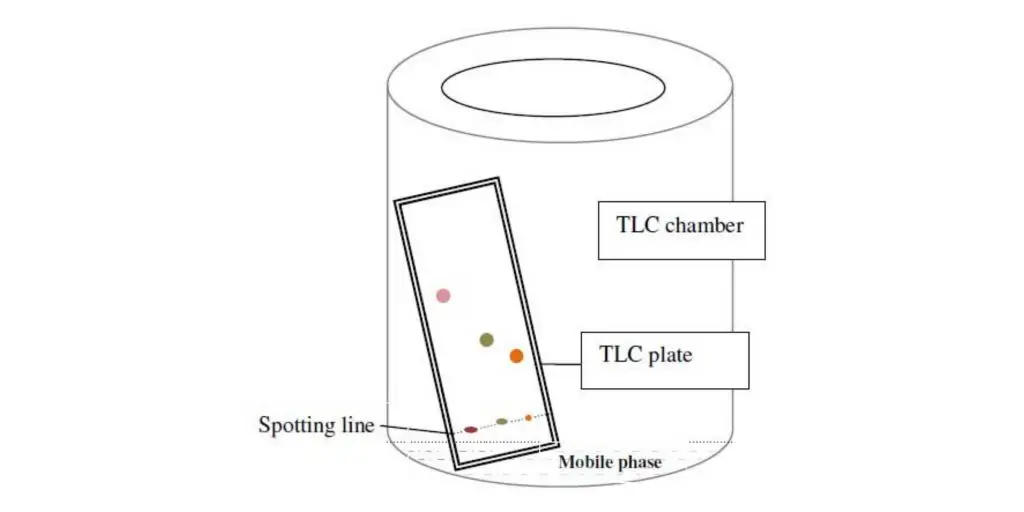
Principle of thin layer chromatography
- The principle of Thin Layer Chromatography (TLC) can define as the separation of different components in a mixture based on their differential adsorption on stationary phase and their solubility in mobile phase.
- The process is mainly governed by adsorption phenomenon, where each compound in the mixture get adsorbed on stationary phase with different strength.
- The stationary phase (like silica gel or alumina) act as adsorbent surface, while the mobile phase (solvent / solvent mixture) moves over it by capillary action.
- Compounds which have strong interaction with stationary phase move slowly, whereas those with weak interaction move faster with solvent front.
- The separation therefore depend upon relative affinity of compounds toward both stationary and mobile phase, which is influenced by polarity, molecular weight, functional groups etc.
- When the solvent moves upward, components distribute themselves between two phases according to their adsorption coefficient, so different spots are formed at different positions.
- The Rf value (Retention factor) is used as the measure of mobility of a substance, and calculated by formula –
- Rf = (Distance travelled by substance) / (Distance travelled by solvent front).
- Higher Rf indicate lower adsorption and vice versa, so substances are identified by comparing their Rf values with those of known compounds.
- In simple terms, TLC works on the principle of unequal distribution of solutes between two immiscible phases, leading to visible separation of mixture’s components.
- Hence it’s considered as an efficient, sturdy and handy technique for quick analysis of chemical substances in labs and industries etc.
Components of Thin Layer Chromatography (TLC)
1. Stationary Phase –
The stationary phase is usually made by coating a thin layer of silica gel, alumina, or cellulose on a glass plate, metal sheet, or plastic film.
The layer act as an adsorbent, where separation of mixture components take place based on their affinity difference.
Thickness of layer is usually around 0.1–0.25 mm for analytical TLC and 0.5–2 mm for preparative type.
Sometimes a binder like calcium sulfate (CaSO₄·2H₂O) is added to increase adhesion of adsorbent on plate surface.
2.Mobile Phase –
The mobile phase is a solvent or mixture of solvents which moves over stationary phase by capillary action.
Selection of solvent depend upon polarity of components to be separated, and correct solvent system ensure proper resolution of spots.
Non-polar solvents like hexane, benzene, or polar ones like methanol, acetone, chloroform etc. are commonly used.
3.Sample or Solute –
The sample mixture contain the substances which are to be separated by the technique.
It is applied as small spot near bottom edge of TLC plate using micropipette or capillary tube.
Sample amount is usually very small (few micrograms), but uniform spotting is essential for better separation.
4. Developing Chamber–
The chamber (like glass jar or tank) is used to develop the chromatogram by allowing solvent movement on plate.
Chamber must be saturated with solvent vapor before placing the plate, to maintain equilibrium and avoid evaporation errors.
5. Detection or Visualization System –
After development, the spots of separated compounds are detected by various methods.
Common visualization techniques include UV light, iodine vapors, ninhydrin reagent, sulfuric acid charring, etc.
Rf Value Measurement – Finally, distance travelled by each compound and solvent front are measured to calculate Rf (Retention factor) which helps in identification.
Therefore, all these components jointly contribute for efficient working of Thin Layer Chromatography, though minor variation in any one may affect the result accuracy.
Procedure of Thin Layer Chromatography (TLC)
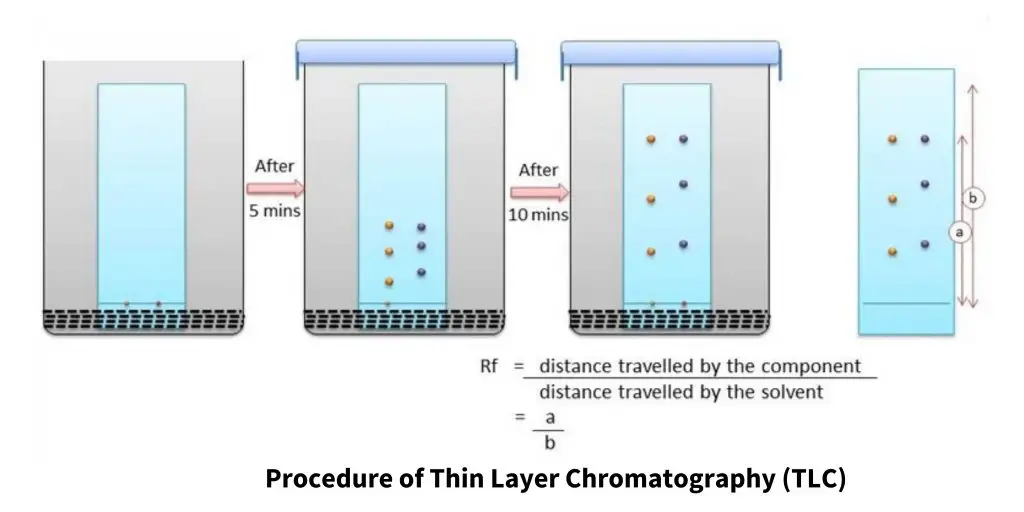
- At first, a glass plate/aluminium sheet/plastic sheet is taken on which thin layer of adsorbent material like silica gel (SiO₂) or alumina (Al₂O₃) is coated uniformly, sometimes a binder like CaSO₄ is also mixed to make the layer sturdy and hardy.
- The coated plate is then kept for drying under room temperature or sometimes it’s heated (around 100°C–110 °C) for a few minutes so that moisture can be removed completely.
- After drying, a pencil line is drawn near the bottom of plate (around 1–2 cm from edge) which marked as the origin line, and on this line small spot(s) of the sample solution are applied using capillary tube or micropipette.
- Care should be taken that the spots are small and not overlapping otherwise separation become unclear and diffuse, also plate shouldn’t be touched by hand at this region.
- The developing chamber (usually a jar or beaker) is prepared by adding a suitable mobile phase / solvent system like chloroform: methanol or hexane: ethyl acetate etc., depending on polarity of components.
- The chamber is then saturated with solvent vapor by covering it tightly for few minutes with lid or foil, this helps in achieving uniform solvent front movement later.
- The prepared TLC plate is carefully placed in the chamber so that the spotted line remains above the solvent surface (not immersed), otherwise sample will dissolve directly in the solvent.
- The solvent is allowed to rise by capillary action through the stationary phase and separation of compounds take place based on their different affinities with adsorbent and solvent system.
- When solvent front reaches almost near the top (around ¾ of the plate length) it’s immediately removed from chamber and marked quickly with pencil as solvent front line.
- The plate is then dried again in air or gentle heat to remove remaining solvent completely, sometimes under stream of air.
- The separated spots are generally invisible if the substances are colorless so they are visualized by exposing to UV light or by spraying with chemical reagents (like iodine vapour, ninhydrin for amino acids, etc.).
- The position of each spot is marked and distance travelled by solvent front and by each compound is measured (in cm or mm).
- The Rf value (Retention factor) for each compound is calculated as:
- Rf = (Distance moved by solute / Distance moved by solvent front).
- The obtained Rf values are compared with known standard compounds for identification / purity checking of sample.
- Sometimes two or more solvent systems are used sequentially when complex mixtures are analyzed, and plate can also be scanned densitometrically for quantitative analysis.
What is Retention Factor (Rf ) Value
- The Retention factor (Rf) value refers as one of the most important parameter in chromatography which describe how far a substance has travelled on the chromatogram relative to the solvent front.
- It can define as the ratio of the distance travelled by solute (sample spot) to the distance travelled by solvent front from the same origin line.
- The Rf value always remain constant for a particular substance under identical experimental condition but may change when solvent system, adsorbent type or temperature vary.
- It is expressed as a numerical value without unit, generally lies between 0 and 1 though sometimes small variation occur due to measurement errors or irregular solvent front.
- Formula of Rf is written as –
- Rf = Distance moved by solute / Distance moved by solvent front
- When a compound interacts strongly with stationary phase, it move slower and hence gives smaller Rf, while those having higher solubility in mobile phase move faster giving higher Rf value.
- The value help in identification and comparison of compounds by comparing with Rf of known standards on same plate.
- In practice, it’s observed that two compound may show similar Rf when solvent polarity not chosen properly, therefore correct solvent mixture selection is crucial for clear separation.
- Sometimes Rf values also used semi-quantitatively to estimate concentration differences when densitometric or color intensity measurements are made.
- Such as, in Thin Layer Chromatography (TLC) and Paper Chromatography, Rf is widely used for detecting purity of drugs, plant pigments, amino acids, etc.
- It’s a simple but very effective way for qualitative identification / comparison, though not very accurate for quantitative purpose.
- Thus, the Rf value gives characteristic fingerprint for a substance under given chromatographic condition and act as key for recognition.
Applications of Thin Layer Chromatography (TLC)
- TLC is widely used for qualitative analysis of compounds, where the presence of a substance in a mixture can identified by comparing Rf values with standard samples.
- It is applied for purity checking of sample materials, impurities or contaminations can easily observed by extra spots on the TLC plate.
- In pharmaceutical analysis, TLC used for the identification of drugs, alkaloids, steroids, glycosides etc., and also to determine their concentration roughly.
- Separation of mixtures like amino acids, lipids, carbohydrates is done by TLC, since different compounds travel differently on adsorbent surface.
- The technique is used for monitoring of reaction progress, in chemical synthesis, where it help to check whether reactant converted to product or not.
- In food industry, TLC applied for detection of food additives, colors, and preservatives (like benzoic acid, tartrazine etc.) that are not permitted or exceeding limits.
- TLC used by forensic laboratories for analysis of drugs, poisons, dyes, and inks; it help in crime detection and toxicological studies.
- In biochemistry, the method used for separating biomolecules (like phospholipids, amino acids, steroids), and for studying metabolic pathways.
- TLC can used as preparative tool, where separated zones are scraped off and compounds extracted for further analysis or purification.
- The method is also useful in quality control of herbal formulations, cosmetic products and pesticides to ensure product consistency.
- Sometimes TLC employed with other techniques like UV spectrophotometry or mass spectrometry (MS) for more accurate identification and quantification.
- Environmental analysis also utilizes TLC, for detection of pollutants, pesticides, hydrocarbons in soil and water samples etc.
- In clinical laboratories, TLC helps in detection of drugs of abuse and in screening of antibiotics and hormones.
- TLC plates can also used for chromatographic fingerprinting, especially in plant and herbal research for species authentication and adulteration check.
- The method is low-cost and quick, so it’s applied by small laboratories where costly instruments like HPLC not available.
Advantages of Thin Layer Chromatography (TLC)
- The method is low cost and simple equipment is used, so many labs can adopt it easily.
- Very small sample size is required (often microliters of solution or milligram quantities) which saves material and reagent.
- Rapid separation is achieved — the development time is short (often minutes rather than hours) and results can be obtained quickly.
- Versatility is provided because many kinds of substances (organic or inorganic, non-volatile compounds) can be separated by it, so it works in many different fields.
- The technique allows multiple samples or spots to be run on one plate (parallel runs) which improves throughput and comparison of standards and samples.
- Visualisation of separated components is straightforward (for instance under UV light or by staining) so identification or monitoring is easier.
- Minimal sample‐pretreatment is needed often (the sample can be applied and developed without heavy processing) which simplifies workflow.
- Good reproducibility (when done correctly) and reliable checking of sample purity or reaction progress is allowed.
Limitations of Thin Layer Chromatography (TLC)
- The method is qualitative rather than quantitative so only presence/absence or rough estimates are allowed rather than precise amounts.
- Only soluble mixture components are suitable, insoluble substances cannot effectively move on the stationary phase.
- Closely related compounds (like enantiomers or some isomers) cannot always be distinguished because it lacks chiral or very high resolution separation.
- The sample size and plate size are limited so larger scale purification or large volume separation is not efficient.
- The reproducibility of results is often problematic, because factors like humidity, temperature, plate quality, solvent front irregularities can influence outcome.
- Overloading of sample or improper spotting/solvent choice can cause streaking or poor separation, which limits reliability of the method.
- The separation length is restricted because the stationary phase layer is thin and plate dimensions are finite so resolution is lower than in techniques like HPLC or GC.
- Volatile compounds are less amenable to TLC because they may evaporate before separation is complete or interact poorly with the stationary phase.
FAQ
What is Thin Layer Chromatography (TLC)?
TLC is a chromatographic technique used for the separation and identification of compounds in a mixture based on their relative affinities to the stationary and mobile phases.
What are the components required for a TLC experiment?
The essential components include TLC plates (with a stationary phase), TLC chamber, mobile phase, sample solutions, and suitable detection techniques.
How does TLC work?
TLC relies on the differential migration of compounds between the stationary phase (adsorbent material) and the mobile phase (solvent or solvent mixture) to achieve separation based on their affinities.
What is the role of the stationary phase in TLC?
The stationary phase provides a surface for the adsorption or partition of the compounds being separated, allowing for differential movement based on their affinities.
What are the commonly used stationary phases in TLC?
Silica gel, alumina, and cellulose are frequently used as stationary phases in TLC.
What is the Rf value in TLC?
The retention factor (Rf) is a measure of the relative migration distance of a compound compared to the solvent front. It is calculated as the ratio of the distance traveled by the compound to the distance traveled by the solvent front.
How is TLC used for compound identification?
TLC can be used for the comparison of Rf values obtained from unknown compounds with those of known reference compounds to aid in identification.
What are the advantages of TLC?
Some advantages include its simplicity, short development time, easy visualization of spots, ability to isolate compounds, faster separation, higher selectivity, assessment of purity, and cost-effectiveness.
What are the limitations of TLC?
Limitations include difficulties in differentiating enantiomers and some isomers, reliance on pre-known Rf values for compound identification, limited separation length compared to other techniques, variability in reproducibility, and challenges with humidity and temperature control.
What are the applications of TLC?
TLC finds applications in various fields, such as monitoring reactions, identifying compounds, assessing substance purity, analyzing ceramides and fatty acids, detecting pesticides in food and water, studying medicinal plants, and analyzing multicomponent pharmaceutical formulations.
- Snyder, L. R., Kirkland, J. J., & Dolan, J. W. (2011). Introduction to modern liquid chromatography. John Wiley & Sons.
- Braithwaite, A., & Smith, F. (2004). Chromatographic methods. Springer Science & Business Media.
- Stahl, E. (1969). Thin-layer chromatography: A laboratory handbook. Springer Science & Business Media.
- Sherma, J., & Fried, B. (2003). Handbook of Thin-Layer Chromatography. Marcel Dekker.
Ettre, L. S. (Ed.). (1996). History of analytical chemistry. Elsevier Science.