- The SXT (sulfamethoxazole-trimethoprim) susceptibility test tells the difference between group A and B streptococci and other hemolytic streptococci.
- Group A and B streptococci can’t be killed by SXT, but other hemolytic streptococci can.
- It helps tell groups A and B streptococci apart from each other when used with bacitracin sensitivity testing.
- The normal microbiota of the pharynx can’t grow when SXT is added to the 5% sheep blood agar.
- This makes it easier to get group A streptococci, especially Streptococcus pyogenes, and group B streptococci from throat swabs.
Objective of SXT Test
- To identify the distinction between Streptococcal groups based on how well they respond to antibiotics called sulfamethoxazole/trimethoprim (SXT).
Principle of SXT Test
- Trimethoprim/sulfamethoxazole, also called co-trimoxazole, is an antibiotic made up of two antifolate agents: one part trimethoprim to five parts sulfamethoxazole.
- When given together, trimethoprim and sulfamethoxazole work better because they block different steps in the folate synthesis pathway.
- It stops bacteria from making purines, thymidine, and methionine, which they need to make DNA and proteins when they copy themselves.
- So, the overall effect of each of these drugs is to stop the replication of bacteria.
- A disc with a certain amount of Trimethoprim/sulfamethoxazole on it is put on the agar plate. This lets the antibiotic spread into the medium and stop the growth of organisms that are sensitive to it.
- After the plates have been incubated, they are checked for areas of inhibition around the discs.
- If the organism grows to the edge of the disc, it is immune to the antimicrobial compound that is in the disc.
- If the organism hasn’t grown in a zone around the edge of the disc, it can be killed by the antimicrobial in the disc.
Materials Required for SXT Test
- 5% defibrinated blood agar plate
- Inoculating loop
- Bacterial sample
- SXT disc
- Bacitracin disc
Procedure of SXT Test
This is how SXT susceptibility testing is done:
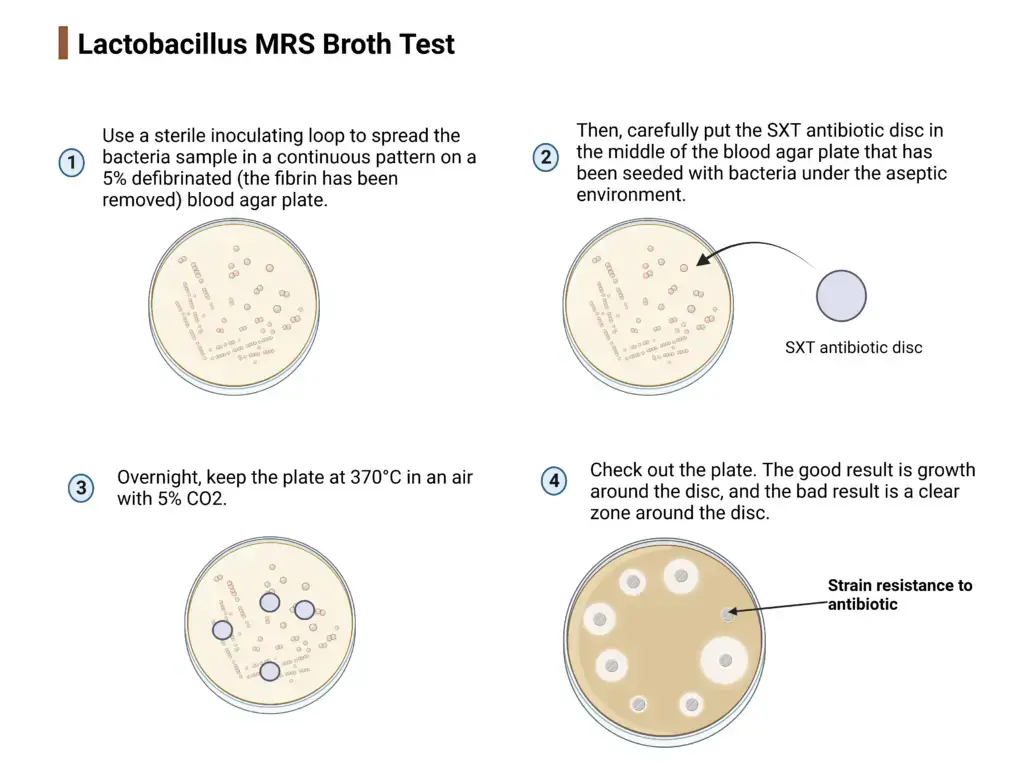
- Use a sterile inoculating loop to spread the bacteria sample in a continuous pattern on a 5% defibrinated (the fibrin has been removed) blood agar plate.
- Then, carefully put the SXT antibiotic disc in the middle of the blood agar plate that has been seeded with bacteria under the aseptic environment.
- Overnight, keep the plate at 370°C in an air with 5% CO2.
- Check out the plate.
- The good result is growth around the disc, and the bad result is a clear zone around the disc.
Alternative method using SXT blood agar
5% sheep blood agar mixed with SXT is another way to test a bacteria’s susceptibility to SXT. Here’s what to do with the alternative method:
- Use a sterile inoculation loop to spread bacteria inoculum on an agar plate with SXT blood agar in an area that is kept clean. The agar should have a smooth, wet surface that doesn’t have any water on it.
- The agar plate should be kept at 370°C with 5% CO2 for one night.
- Look at the plate to see if there are any isolated colonies with some kind of bleeding.
Note: Isolated bacteria species must be confirmed by Gram staining, biochemical tests, and bacitracin sensitivity tests.
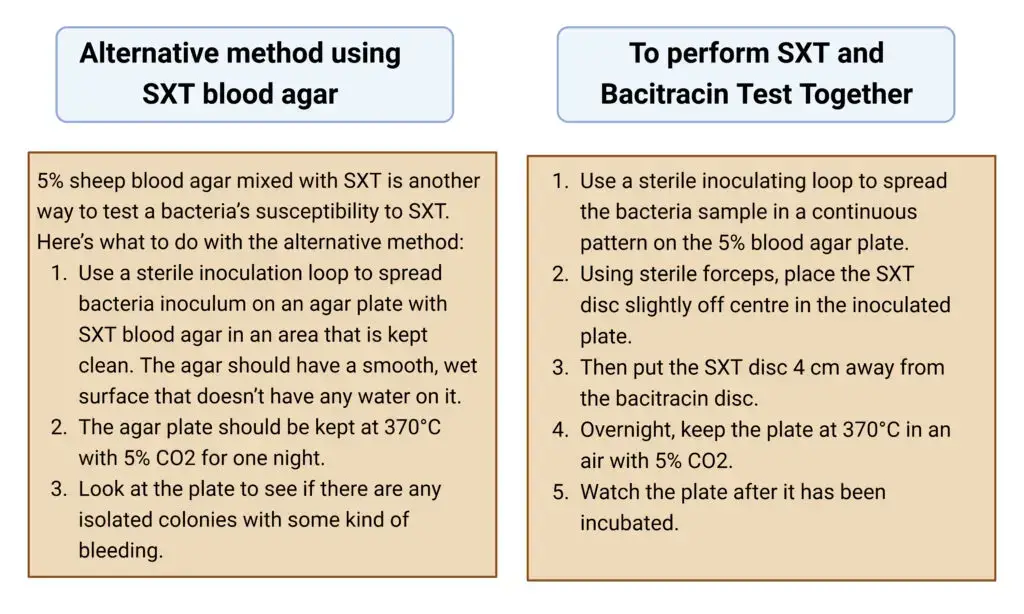
To perform SXT and Bacitracin Test Together
Here’s how to test for antibiotic resistance to SXT and bacitracin at the same time:
- Use a sterile inoculating loop to spread the bacteria sample in a continuous pattern on the 5% blood agar plate.
- Using sterile forceps, place the SXT disc slightly off centre in the inoculated plate.
- Then put the SXT disc 4 cm away from the bacitracin disc.
- Overnight, keep the plate at 370°C in an air with 5% CO2.
- Watch the plate after it has been incubated.
Result of SXT Test
The possible result of SXT and Bacitracin test together
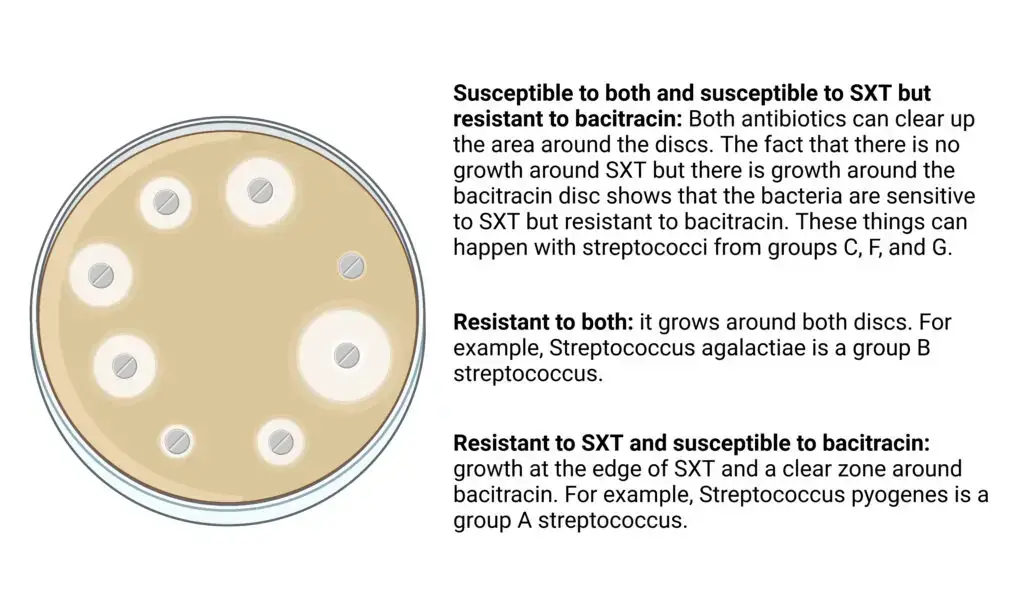
- Susceptible to both and susceptible to SXT but resistant to bacitracin: Both antibiotics can clear up the area around the discs. The fact that there is no growth around SXT but there is growth around the bacitracin disc shows that the bacteria are sensitive to SXT but resistant to bacitracin. These things can happen with streptococci from groups C, F, and G.
- Resistant to both: it grows around both discs. For example, Streptococcus agalactiae is a group B streptococcus.
- Resistant to SXT and susceptible to bacitracin: growth at the edge of SXT and a clear zone around bacitracin. For example, Streptococcus pyogenes is a group A streptococcus.
Reactions of Beta-hemolytic Streptococci to Bacitracin and SXT
| Organism | Bacitracin | SXT |
| Group A (S. pyogenes) | Susceptible | Resistant |
| Group B (S. agalactiae) | Resistant | Resistant |
| Group C, F, and G | Susceptible or Resistant | Susceptible |
Quality Control
For quality control of antibiotic susceptibility testing, the antibiotic disc must be checked with quality control strains. CLSI says that Escherichia coli ATCC 25922 and Staphylococcus aureus ATCC 25923 are used to test the quality of SXT-1.25/23.75 g.
To check the quality of SXT blood agar, you have to look at the plates to see if the product is getting worse and grow the quality control strains according to CLSI guidelines.
- Streptococcus pyogenes ATCC 19615 and ATCC 49117, Streptococcus agalactiae ATCC 13013– fair to heavy growth (positive control),
- Streptococcus mitis ATCC 13813– no growth (negative control), and
- Neisseria subflava ATCC 14799 and Neisseria sicca ATCC 9913– Partial inhibition (negative control).
Uses of SXT Test
- Beta hemolytic streptococci on blood agar are thought to be beta hemolytic streptococci when the SXT disc susceptibility test is used with bacitracin (presumptively identifying beta-hemolytic streptococci as either group A, B or not group A and B).
- The resistance to SXT is used to find groups A and B streptococci in samples that have a mixed culture. Because they are resistant, they can grow faster than contaminating bacteria that this antibiotic stops.
- Bacitracin/SXT susceptibility tests are still used when there is no way to find out a person’s serologic group.
Limitations of SXT Test
- A Bacitracin test should be done along with an SXT susceptibility test because the combined results improve the accuracy and sensitivity of the test.
- For full identification, it is suggested that biochemical, immunological, molecular, or mass spectrometry tests be done on colonies from pure culture.
References
- Gunn B. A. (1976). SXT and Taxo A disks for presumptive identification of group A and B streptococci in throat cultures. Journal of clinical microbiology, 4(2), 192-3.
- Leboffe, M., & Pierce, B. (2011). A photographic atlas for the microbiology laboratory (4th ed., pp. 94-95). Morton Publishing.
- BBL™ SXT Blood Agar. Legacy.bd.com. (2019). Retrieved 24 August 2022, from https://legacy.bd.com/ds/technicalCenter/inserts/L007413(09).pdf.
- CLSI guidelines M100, 30th edition.
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5730933/
- https://www.labce.com/spg15135_group_a_strep_a_disksxt.aspx
- https://www.coursehero.com/file/p3ess2oj/SXT-disc-susceptibility-test-used-for-the-presumptive-identification-of-beta/
- https://www.dalynn.com/dyn/ck_assets/files/tech/DB10.pdf
- https://www.researchgate.net/publication/232229031_Is_Streptococcus_pyogenes_Resistant_or_Susceptible_to_Trimethoprim-Sulfamethoxazole