Silver staining is the process used for detecting and identifying proteins and nucleic acids in different gels, and it is highly sensitive in nature. It is the method where silver ions (Ag⁺) binds to different chemical groups present in proteins like carboxyl and sulfhydryl groups, and these ions is then reduced into metallic silver (Ag⁰). It produces a visible dark-brown to black image on the gel surface. It is the formation of tiny silver particles on the nucleation sites of the protein surface, and it is these particles which help in visualizing the protein bands. It is used in polyacrylamide gel electrophoresis for several decades and the image formed usually shows low background.
It is the process that occurs through different steps like fixation, sensitization, impregnation of silver and the development of the image. These steps help in binding the silver ions firmly to the protein structure so that the reduction process is clear and stable. Some of the main features of this method is that proteins in the low nanogram range can be detected, and the final stained gel can remain stable for some weeks. This is referred to as one of the most sensitive staining methods used in modern biochemical studies.
Principle of silver staining
The principle of silver staining is based on the ability of silver ions (Ag⁺) to bind with different chemical groups present in proteins and other biological structures, and then these ions is reduced into metallic silver (Ag⁰) which forms a visible dark deposit on the gel. It is the localized redox reaction where the soluble silver ions attach to the biomolecules first, and then during development these ions are converted into insoluble metallic silver which produces a clear contrast image. It is the process which is highly sensitive, and the initial silver deposition is helping in further reduction, so careful control of chemicals is needed to reduce background formation.
In gels, the silver binds to the functional groups like carboxyl groups, imidazole groups, sulfhydryl groups and amine groups of different amino acids. After this binding a colorless complex is formed which is then reduced by a developing agent such as formaldehyde or reducing sugars, and the metallic silver appears as dark brown to black bands. It is the method which detects very small amount of proteins and this sensitivity is much higher than general stains.
It is also the process used in histological staining where argentaffin and argyrophil reactions are observed. Argentaffin structures can bind and reduce silver ions without external reducer, while argyrophil structures only bind silver and need an external reducing agent for the metallic silver to appear. These are important distinctions in tissue staining.
In many protocols oxidation, sensitization and toning steps are used to improve specificity. Oxidation helps in forming aldehyde groups, sensitizers help nucleation, and toning with gold chloride is used to stabilize the image by replacing silver with gold. It is these combined reactions which form the complete principle behind silver staining.
Properties of silver stain
- It is highly sensitive and detects very low quantity of proteins in gels, usually in the low nanogram range.
- It is the method that shows almost 50 times more sensitivity than general stains used for protein detection.
- It binds to different functional groups of proteins like sulfhydryl, imidazole, carboxyl and amine groups, showing specific chemical affinity.
- It produces dark brown to black coloured bands, and sometimes the colour varies due to different sizes of silver grains.
- It forms high contrast images with clear background which helps in easy visualization.
- It is the process that can show argentaffin or argyrophil reactions in histological staining depending on the reducing capacity of the target.
- It sometimes uses oxidation steps for forming aldehyde groups which help in reducing silver.
- It has limited linear range, so the intensity of the stain does not always match the protein amount.
- It is less reproducible because the reaction is temperature sensitive and self-catalytic in nature.
- It uses aldehyde developers which may cause cross-linking of proteins, making the sample less suitable for mass spectrometry analysis.
- It can produce artifacts like hollow spots especially when staining negatively charged proteins.
Reagents and solutions Required for Silver staining
Reagents for Protein Gel Electrophoresis (SDS–PAGE)
It is the process which uses different solutions in a sequence like fixation, sensitization, silver impregnation, development and stopping. Clean glassware and pure water is required for preparing all these solutions.
Fixation Solutions
These solutions contain methanol or ethanol together with acetic acid and water. It is used for fixing and precipitating proteins inside the gel and also removing interfering chemicals like SDS. Formaldehyde or glutaraldehyde is not used here when the sample is required for mass spectrometry.
Sensitizing Solutions
It mainly includes dilute sodium thiosulfate solution. In some methods DTT is used and in few cases potassium ferricyanide may be used in pretreatment steps. These helps in increasing the sensitivity of silver binding.
Silver Impregnation Solution
It is prepared with silver nitrate (AgNO₃) in water. This solution provides silver ions for binding with the protein groups. Sometimes a small quantity of formalin is added to increase the staining sensitivity.
Developing Solution
It contains sodium carbonate or potassium carbonate to provide alkaline condition. Fresh formaldehyde is added just before use, and it reduces silver ions into metallic silver which forms the visible image. A sugar-based alkaline solution is used when aldehyde-free protocols are required.
Stop Solution
It is prepared with acetic acid, EDTA or citric acid. It stops the reduction process and prevents background staining.
Reagents for Histopathology (Tissue Sections)
These reagents are specialized depending on the staining method used for fungi, reticulin fibres, melanin or nerve structures.
Silver Solutions
Ammoniacal silver is used in stains like Fontana–Masson and Gordon & Sweet. Methenamine silver is used in GMS stain for fungi. Alkaline silver iodide is used in Gallyas method for neurofibrillary tangles.
Oxidizing Agents
Chromic acid, potassium permanganate or periodic acid are used for forming aldehyde groups in tissues. These aldehydes help the binding and reduction of silver.
Sensitizers and Bleaching Agents
Ferric ammonium sulfate is used for sensitizing reticulin fibres. Oxalic acid is used for removing discoloration produced after oxidation.
Reducing Solutions (Developers)
Formalin is used to reduce silver into metallic form in reticulin staining. Hydroquinone with sodium sulfite is used in some argyrophil cell staining methods.
Toning and Fixing Agents
Gold chloride is used for toning which improves contrast and stabilizes the stain. Sodium thiosulfate is used for removing unreduced silver and preventing background darkening.
Counterstains
Nuclear fast red is used for staining nuclei. Light green is used as background contrast in GMS fungal staining.
Destaining Reagents
It is used when the gel requires removal of excess silver stain.
Ferricyanide–Thiosulfate Solution
It is a combination of potassium ferricyanide and sodium thiosulfate and is widely used for removing silver deposits.
Copper-Based Solution
It contains cupric sulfate, sodium chloride and ammonia and can be used as an alternative destaining method.
Procedure for silver staining
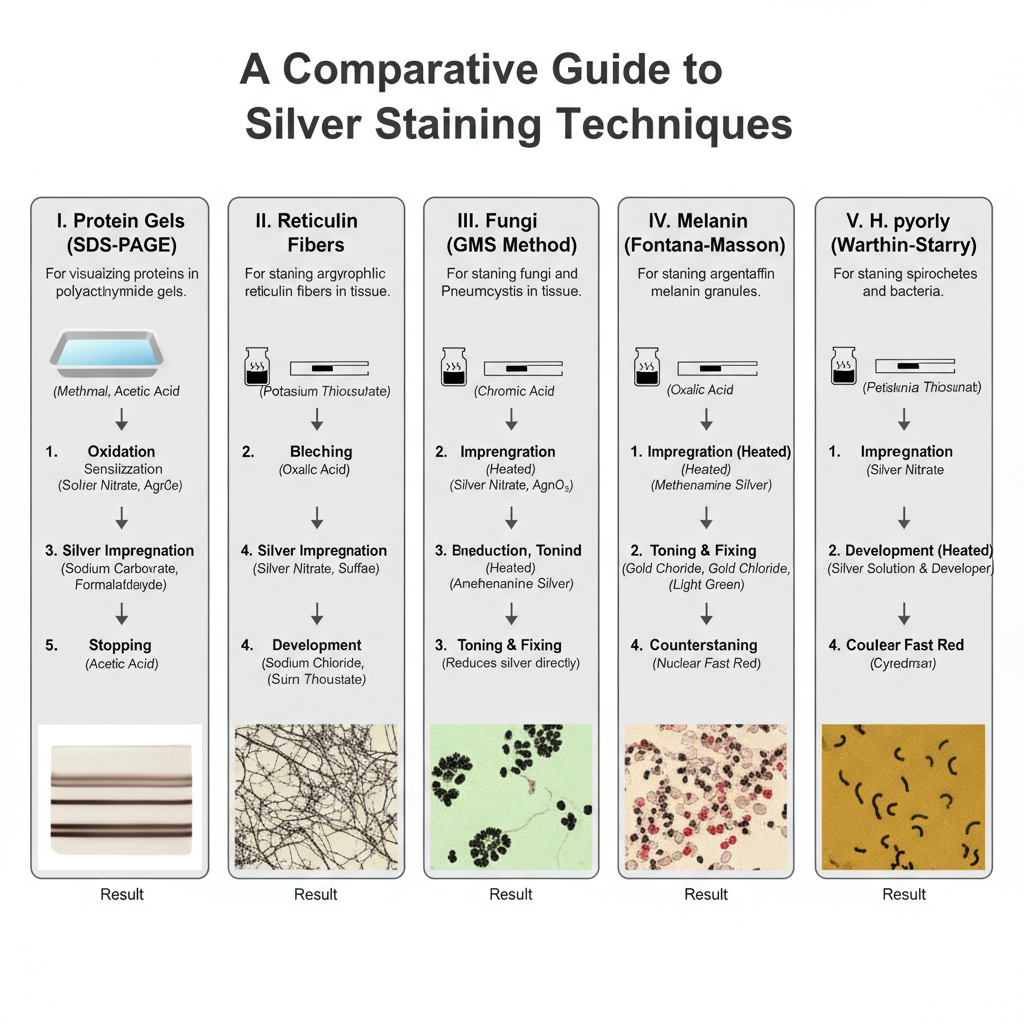
I. Silver Staining for Protein Gels (SDS–PAGE)
It is the procedure used to visualize very small quantities of proteins separated on polyacrylamide gels. The solutions should be prepared fresh and the trays must be clean because any impurity will produce background staining. The process generally follows the steps mentioned below.
1. Fixation
The gel is placed in the fixation solution containing methanol and acetic acid. It is the step where proteins are precipitated and fixed in the gel matrix, and interfering substances like SDS is removed. The gel is kept for 20 minutes or more depending on its thickness, and after fixation it is washed with water or ethanol.
2. Sensitization
The gel is then treated with dilute sodium thiosulfate solution. It is the process which increases the sensitivity of the staining. The gel is kept for 1–2 minutes and then rinsed properly with clean water.
3. Silver Impregnation
The gel is submerged in silver nitrate solution. It is the step where silver ions bind to the protein functional groups like carboxyl, imidazole, sulfhydryl and amine groups. The process takes around 20–30 minutes.
4. Development
The gel is placed in a developing solution containing sodium carbonate and formaldehyde. It is the process where bound silver ions are reduced into metallic silver which appears as dark bands. The step is fast, usually 2–5 minutes, and needs visual monitoring.
5. Stopping (Quenching)
A stop solution like acetic acid or EDTA is added to stop further development. It prevents background staining and fixes the image formed on the gel.
II. Silver Staining for Histopathology
These procedures are used for staining tissue sections and depend on whether the structure is argentaffin or argyrophil.
A. Procedure for Reticulin Fibers
This process follows oxidation, sensitization, impregnation and reduction steps.
1. Oxidation
Tissue sections are treated with oxidizing agents like potassium permanganate or chromic acid which form aldehyde groups.
2. Bleaching
Oxalic acid is used to remove excess colour formed after oxidation.
3. Sensitization
Ferric ammonium sulfate (iron alum) is applied which sensitizes the fibers.
4. Silver Impregnation
Ammoniacal silver solution is used and it binds to the sensitized fibers.
5. Reduction
Since reticulin is argyrophilic, formalin is used as reducing agent to form metallic silver.
6. Toning
Gold chloride solution is used which replaces silver with gold and stabilizes the colour.
7. Fixing
Sodium thiosulfate removes unreduced silver and prevents darkening.
B. Procedure for Fungal Staining (GMS Method)
This method is used for fungi and Pneumocystis species.
1. Oxidation
Chromic acid converts fungal polysaccharides into aldehydes.
2. Impregnation
Methenamine silver solution is applied at higher temperature. Aldehydes reduce the silver and make the fungi visible.
3. Toning and Fixing
Gold chloride and sodium thiosulfate are used to improve contrast and remove excess silver.
4. Counterstaining
Light green stain is used to colour the background.
C. Procedure for Melanin Staining (Fontana–Masson)
Melanin is argentaffin, so it reduces silver directly.
1. Impregnation
Ammoniacal silver solution is applied at 58–60°C. Melanin granules reduce silver without a separate developer.
2. Toning and Fixing
Gold chloride and sodium thiosulfate stabilize the dark precipitate.
3. Counterstaining
Nuclear fast red is applied for background contrast.
D. Procedure for H. pylori Detection (Warthin–Starry Method)
This method is used for spirochetes and bacteria.
1. Impregnation
Slides are treated with silver nitrate solution.
2. Development
Both the silver solution and the developer are heated to 65°C which helps in reducing silver on the bacterial surface.
3. Result
The bacteria appear black against a golden–yellow background.
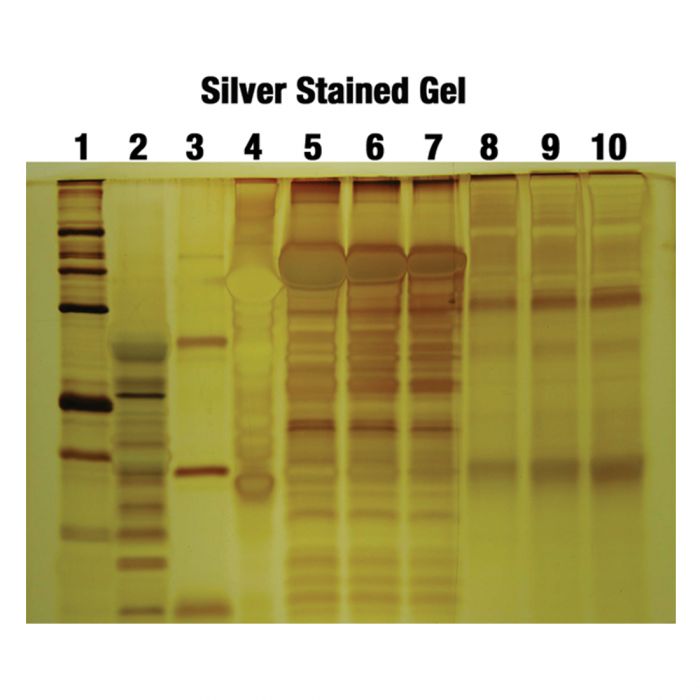
Result of silver staining
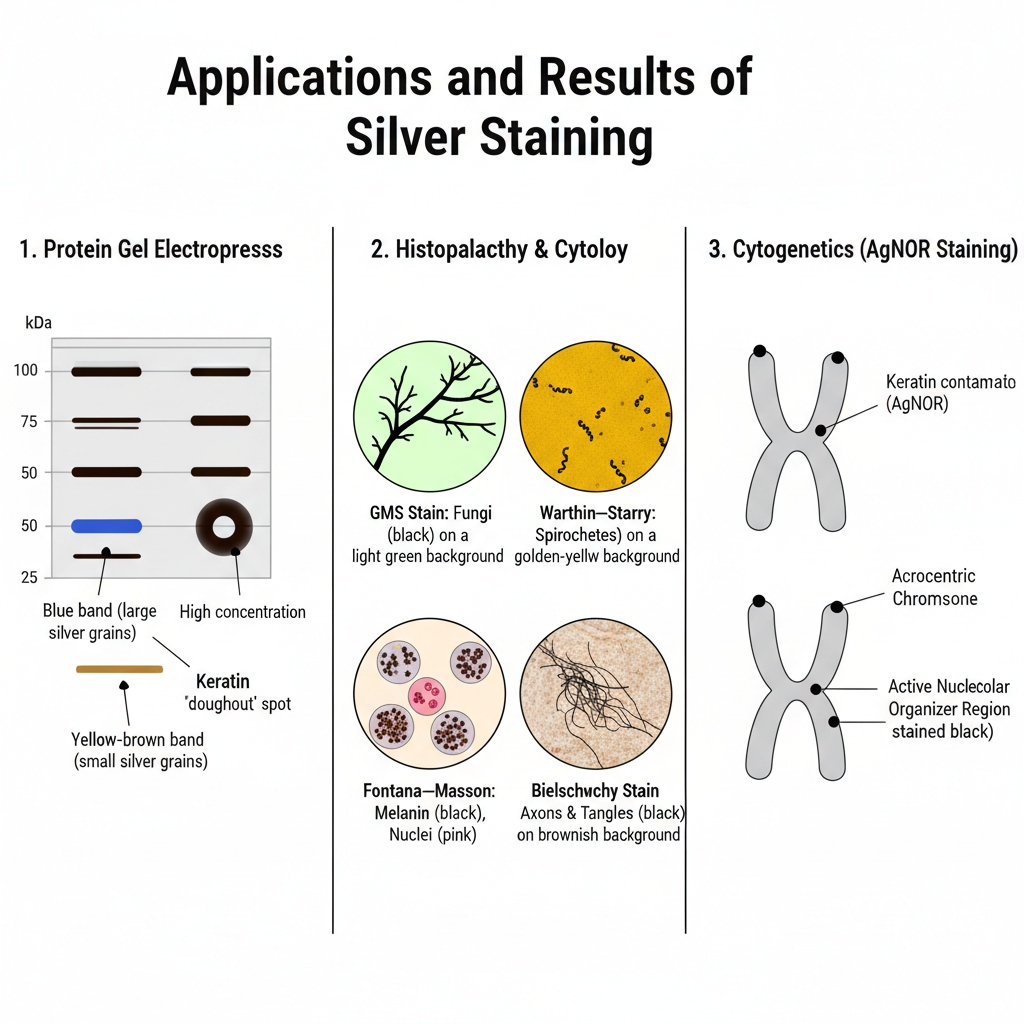
1. Results in Protein Gel Electrophoresis
- Protein bands appear as dark brown to black deposits formed by metallic silver on the gel surface.
- The colour may vary from yellow, red, brown to blue depending on the size of the silver grains formed during development.
- Larger grains can produce blue coloured bands, while smaller grains usually appear yellow or brown.
- High concentration protein spots sometimes show hollow or doughnut-shaped centres due to uneven silver deposition.
- Contamination can produce unwanted bands, especially keratin bands which appear around the 50–68 kDa region.
2. Results in Histopathology and Cytology
- In GMS staining, fungal elements and Pneumocystis cysts appear sharply black against a light green background.
- Reticulin fibres are stained black, and after toning the background appears purplish-grey.
- In Warthin–Starry method, spirochetes and Helicobacter pylori appear black on a golden-yellow background.
- Melanin granules and argentaffin cells stain brown to black in Fontana–Masson staining, while nuclei appear pink or red.
- Neurofibrillary tangles, plaques and neuropil threads appear black in Gallyas staining, and Bielschowsky stain shows axons and tangles in black against a brownish background.
- In Grimelius staining, argyrophil granules appear black while the background appears yellow to gold.
3. Results in Cytogenetics
- In AgNOR staining, the active nucleolar organizer regions appear as distinct black or dark brown dots on the acrocentric chromosomes.
- These dots help in assessing the nucleolar activity of the cell.
Uses of Silver staining
- It is used for detecting very low amount of proteins in gels, and it helps in identifying low-abundance proteins in complex samples.
- It is applied for visualizing DNA and RNA fragments in gels, especially in studies involving different nucleic acid patterns.
- It is used before mass spectrometry to observe protein bands, and aldehyde-free methods are sometimes used when MS compatibility is required.
- It is used in histopathology for detecting fungal organisms like Candida, Aspergillus, Histoplasma and Pneumocystis, mainly by GMS staining.
- It is used for identifying bacteria such as Helicobacter pylori, spirochetes and Bartonella in tissue sections.
- It is used in neuropathology for detecting senile plaques and neurofibrillary tangles in Alzheimer’s disease.
- It is used for studying tauopathies because Gallyas method clearly shows neurofibrillary structures and glial inclusions.
- It is applied to assess connective tissue arrangement by staining reticulin fibres in liver and bone marrow sections.
- It is used to observe basement membranes in renal biopsies, especially in conditions like membranous glomerulonephritis.
- It is used for tumour differentiation by detecting argentaffin substances like melanin or argyrophil cells in neuroendocrine tumours.
- It is used in cytogenetics to stain nucleolar organizer regions (AgNORs) which helps in identifying active chromosomes and studying cellular activity.
Advantages of silver staining
- It is highly sensitive and can detect proteins in very low nanogram levels, making it more sensitive than general protein stains.
- It produces a clear high-contrast image which helps in visualizing faint and low-abundance proteins in gels.
- It is useful in early exploratory studies where rare proteins need to be detected before further analysis.
- It is superior in detecting fungal organisms in tissue sections because it gives stronger contrast and can show even degenerated fungal cells.
- It helps in identifying bacteria like Helicobacter pylori and different spirochetes which are not easily seen in routine staining.
- It is helpful in differentiating argentaffin and argyrophil reactions which assist in diagnosing some tumour types.
- It is important in neuropathology for detecting neurofibrillary tangles, plaques and other structures related to degenerative diseases.
- It gives high resolution in visualizing pathological features which may not be detected by other staining methods.
- It can provide permanent records of samples when toning steps like gold chloride are used, which stabilizes the stained structures.
- It is chemically versatile since it can stain proteins, nucleic acids and some polysaccharide structures in different tissues and gels.
- It has aldehyde-free variants which maintain compatibility with mass spectrometry while still providing clear visualization of protein bands.
Limitations of Silver staining
- It is not suitable for mass spectrometry analysis because formaldehyde or glutaraldehyde used in the procedure causes protein cross-linking which prevents proper digestion of proteins.
- It shows a sensitivity and compatibility trade-off since aldehyde-free methods reduce cross-linking but the sensitivity becomes lower.
- It has narrow linear range, so the stain intensity does not always represent the actual protein concentration, making it less useful for quantitative studies.
- It shows low reproducibility because the staining process depends strongly on timing and temperature conditions.
- It is technically demanding and needs careful handling because the reduction reaction is self-catalytic and can cause background staining if not stopped correctly.
- It requires very clean glassware and pure water, since small contaminants can produce non-specific silver deposition and artifacts.
- It may produce hollow or doughnut-shaped bands, especially in high concentration protein samples, and some protein types like glycoproteins do not stain well.
- In histopathology some silver methods like Bielschowsky stain are costly and difficult to perform, and they show high background staining which affects identification of lesions.
- GMS staining can show background artifacts and is sensitive to time and temperature, so experienced technicians are needed.
- Warthin–Starry method is difficult to standardize and requires proper pH and clean surfaces for correct staining.
- It involves hazardous chemicals like silver nitrate and formaldehyde, and the reagents are more expensive compared to common staining methods.
Precautions
- It is important to use high-purity water because any impurity can start non-specific silver deposition and form background staining.
- It is necessary to clean all glassware properly, and acid washing is often required so that no residue is left which may act as nucleation sites.
- It is important to avoid keratin contamination from fingerprints, so clean gloves must be worn and the gel surface should not be touched directly.
- It is preferred to use plastic trays and tools because metal objects can interfere with the silver reaction.
- It involves hazardous chemicals like silver nitrate which can irritate skin and eyes, so proper protective measures are needed.
- Ammoniacal silver solutions must be handled carefully because they can form explosive salts when dried, and unused solution should be inactivated immediately.
- Formaldehyde is toxic and must be handled in a fume hood, and contact with skin or inhalation should be avoided.
- The developing and silver solutions should be prepared fresh because old solutions can cause background staining or complete staining failure.
- It is necessary to monitor the development step carefully because the reaction proceeds fast, and yellow coloration of the solution means it must be discarded.
- For mass spectrometry-related work, aldehydes should not be used in fixation because they cause cross-linking of proteins, and aldehyde-free protocols should be followed instead.
FAQ
Q1. What is silver staining?
Silver staining is the process used for detecting proteins and nucleic acids in very small quantity, and it forms dark metallic silver deposits on the gel which makes the bands visible.
Q2. How does silver staining work?
It works by binding silver ions to different chemical groups of the proteins, and then these ions is reduced into metallic silver during development which produces a visible image.
Q3. What does silver stain stain?
It stains proteins, DNA, RNA, reticulin fibres, fungi, bacteria and some argentaffin or argyrophil tissue structures.
Q4. What are the advantages of silver staining?
It is highly sensitive, gives clear contrast, detects very low-abundance proteins, helps in identifying microorganisms in tissues and provides permanent stained records when toned.
Q5. What are the key stages of silver staining?
The main stages are fixation, sensitization, silver impregnation, development and stopping of the reaction.
Q6. What is the sensitivity range of silver staining?
It detects proteins in the low nanogram range, usually between 0.1 ng to 1 ng.
Q7. What color do protein bands appear in silver staining?
The protein bands usually appear dark brown to black after development.
Q8. Which functional groups in proteins does silver bind to?
It binds to sulfhydryl groups, carboxyl groups, imidazole groups and amine groups present in amino acids.
Q9. What is the role of formaldehyde in silver staining?
Formaldehyde acts as the reducing agent which converts silver ions into metallic silver forming the visible bands.
Q10. Can silver-stained gels be used for mass spectrometry?
It is difficult with aldehyde-based methods because proteins become cross-linked, but aldehyde-free silver staining can be used for mass spectrometry.
Q11. What are the disadvantages of silver staining?
It shows low reproducibility, narrow linear range, formation of artifacts, contamination issues, and aldehydes interfere with mass spectrometry.
Q12. How long can a silver-stained gel remain stable for observation?
The stained gel can remain stable for several weeks before losing its clarity.
Q13. What type of water should be used in silver staining?
High-purity water like MilliQ water is required to avoid background staining.
Q14. How is background staining minimized in silver staining?
It is minimized by using clean glassware, pure water, proper washing steps and careful monitoring of the development reaction.
Q15. What is the stop solution in silver staining?
The stop solution is usually acetic acid or EDTA which stops further reduction of silver and prevents background formation.
- Bloom, S. E., & Goodpasture, C. (1976). An improved technique for selective silver staining of nucleolar organizer regions in human chromosomes. Human Genetics, 34(2), 199–206. https://doi.org/10.1007/BF00278889
- Cancer Diagnostics Inc. (n.d.). GMS Stain Kit (Modified Gomori Methenamine Silver Nitrate Stain). https://www.cancerdiagnostics.com/SS1046-VO-GMS-Stain-Kit-Modified-Gomoris-Volume-Optimized
- Cell Marque. (n.d.). Fontana-Masson Stain Kit. https://www.cellmarque.com/cms/fontana_masson.php
- Chevallet, M., Luche, S., Diemer, H., Strub, J.-M., Van Dorsselaer, A., & Rabilloud, T. (2008). Sweet silver: A formaldehyde-free silver staining using aldoses as developing agents, with enhanced compatibility with mass spectrometry. Proteomics, 8(23-24), 4853–4861. https://doi.org/10.1002/pmic.200800321
- Children’s Hospital of Philadelphia Research Institute. (n.d.). Special Stain Protocol: Gordon and Sweet’s Reticulin Method. Pathology Core. https://www.research.chop.edu/sites/default/files/2024-03/PathCore_Special_Stain_Protocol_Gordon_Sweets_Reticulin_Method.pdf
- CliniSciences. (n.d.). Warthin-Starry stain. https://www.clinisciences.com/en/buy/cat-warthin-starry-stain-3969.html
- Creative Proteomics. (n.d.). Protein Gel Staining Methods: Coomassie, Silver, Fluorescent, Zinc, and Functional Group-Specific. https://www.creative-proteomics.com/resource/protein-gel-staining-methods.htm
- D’Hue, Z., Perkins, S. M., & Billings, S. D. (2008). GMS is superior to PAS for diagnosis of onychomycosis. Journal of Cutaneous Pathology, 35(8), 745–747. https://doi.org/10.1111/j.1600-0560.2007.00890.x
- Doan, C. (n.d.). Special Stains – Which One, How and Why? Part II: Connective Tissue. Leica Biosystems. https://www.leicabiosystems.com/knowledge-pathway/special-stains-which-one-how-and-why-part-ii-connective-tissue/
- Doan, C. (n.d.). Special Stains – Which One, Why and How? Part III: Microorganisms – Bacteria and Fungi. Leica Biosystems. https://www.leicabiosystems.com/us/knowledge-pathway/special-stains-which-one-why-and-how-part-iii-microorganisms-bacteria-and-fungi/
- Expert Report on Silver Staining: Principles, Procedures, and Specialized Applications. (n.d.). [Provided source document].
- Hempelmann, E. (2017). The mechanism of silver staining of proteins separated by SDS polyacrylamide gel electrophoresis. Biotechnic & Histochemistry, 92(2), 79-85. https://doi.org/10.1080/10520295.2016.1265149
- Kermanshahi, T. R., & Rhatigan, R. (2010). Comparison between PAS and GMS stains for the diagnosis of onychomycosis. Journal of Cutaneous Pathology, 37(10), 1041–1044. https://doi.org/10.1111/j.1600-0560.2009.01468.x
- Kumar, G. (2018). Principle and Method of Silver Staining of Proteins Separated by Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis. Methods in Molecular Biology, 1853, 231–236. https://doi.org/10.1007/978-1-4939-8745-0_26
- Kumar G., P. (2016, March 16). Good protocol for silver staining? ResearchGate. https://www.researchgate.net/post/good_protocol_for_silver_staining
- Lamy, C., Duyckaerts, C., Delaere, P., Payan, C., Fermanian, J., Poulain, V., & Hauw, J. J. (1989). Comparison of seven staining methods for senile plaques and neurofibrillary tangles in a prospective series of 15 elderly patients. Neuropathology and Applied Neurobiology, 15(6), 563–578. https://doi.org/10.1111/j.1365-2990.1989.tb01255.x
- Mavrogiorgou, P., Gertz, H.-J., Ferszt, R., Wolf, R., Bär, K.-J., & Juckel, G. (2011). Are routine methods good enough to stain senile plaques and neurofibrillary tangles in different brain regions of demented patients? Psychiatria Danubina, 23(4), 334–339.
- Merril, C. R., Bisher, M. E., Harrington, M., & Steven, A. C. (1988). Coloration of silver-stained protein bands in polyacrylamide gels is caused by light scattering from silver grains of characteristic sizes. Proceedings of the National Academy of Sciences, 85(2), 453–457.
- Parry, N. (2025, March 19). How To Find Fungi In Your Histology Samples- Go For GMS! Bitesize Bio. https://bitesizebio.com/13430/why-go-for-gms/
- Protocols Online. (2012, July 15). Gallyas Silver Stain. https://www.protocolsonline.com/histology/dyes-and-stains/neurohistology/gallyas-silver-stain/
- Sasse, J., & Gallagher, S. R. (2009). Staining proteins in gels. Current Protocols in Molecular Biology, 85(1), 10.6.1-10.6.26. https://doi.org/10.1002/0471142727.mb1006s85
- Schwarzacher, T. (n.d.). Silver Staining for Nucleolar Organizer Regions. University of Leicester. https://www.le.ac.uk/bl/phh4/agnor.htm
- Sigma-Aldrich. (2022). Fontana-Masson Stain Kit: Procedure No. HT200 [Instructions for Use]. https://www.sigmaaldrich.com/deepweb/assets/sigmaaldrich/product/documents/222/695/procedure-ht200-fontana-masson-stain-kit-ms.pdf
- Silver Stain for Polyacrylamide Gels [Protocol]. (n.d.). (Based upon Shevchenko et al., 1996).
- Silver Staining of Protein Gels [Protocol]. (n.d.). (Based upon Morrissey, 1981).
- StainsFile. (2025). Silver Impregnation. https://www.stainsfile.com/theory/methods/metal-impregnation/silver-impregnation/
- The Protein Man. (2015, March 3). Which Stain is Most Compatible with Mass Spectrometry? G-Biosciences. https://info.gbiosciences.com/blog/which-stain-is-most-compatible-with-mass-spectrometry
- The Rockefeller University. (n.d.). Protocol for Silver Staining. Proteomics Resource Center. https://www.rockefeller.edu/proteomics/protocol-silver-staining/
- Turku Bioscience Centre. (2009, April 14). Mass Spectrometry Compatible Silver Staining (Version w.1.0). https://bioscience.fi/wp-content/uploads/2018/11/MS_Compatible_Silver_Staining_version_w.1.0.pdf
- UT Health San Antonio Department of Pathology and Laboratory Medicine. (n.d.). Special Stains. https://lsom.uthscsa.edu/pathology/reference-labs/histology-immunohistochemistry-laboratory/laboratory-services-2/special-stains/
- Warwick, T. (2022, March 4). Protein Staining Methods: An Overview of 3 of the Best. Bitesize Bio. https://bitesizebio.com/9386/protein-staining-methods/
- WebPath. (n.d.). Grimelius – Pascual’s Modified – Argyrophil Cells [Procedure Card]. https://webpath.med.utah.edu/HISTHTML/MANUALS/GRIMEL.PDF
- Wikipedia. (n.d.). Silver staining. Retrieved from https://en.wikipedia.org/wiki/Silver_staining
- Text Highlighting: Select any text in the post content to highlight it
- Text Annotation: Select text and add comments with annotations
- Comment Management: Edit or delete your own comments
- Highlight Management: Remove your own highlights
How to use: Simply select any text in the post content above, and you'll see annotation options. Login here or create an account to get started.