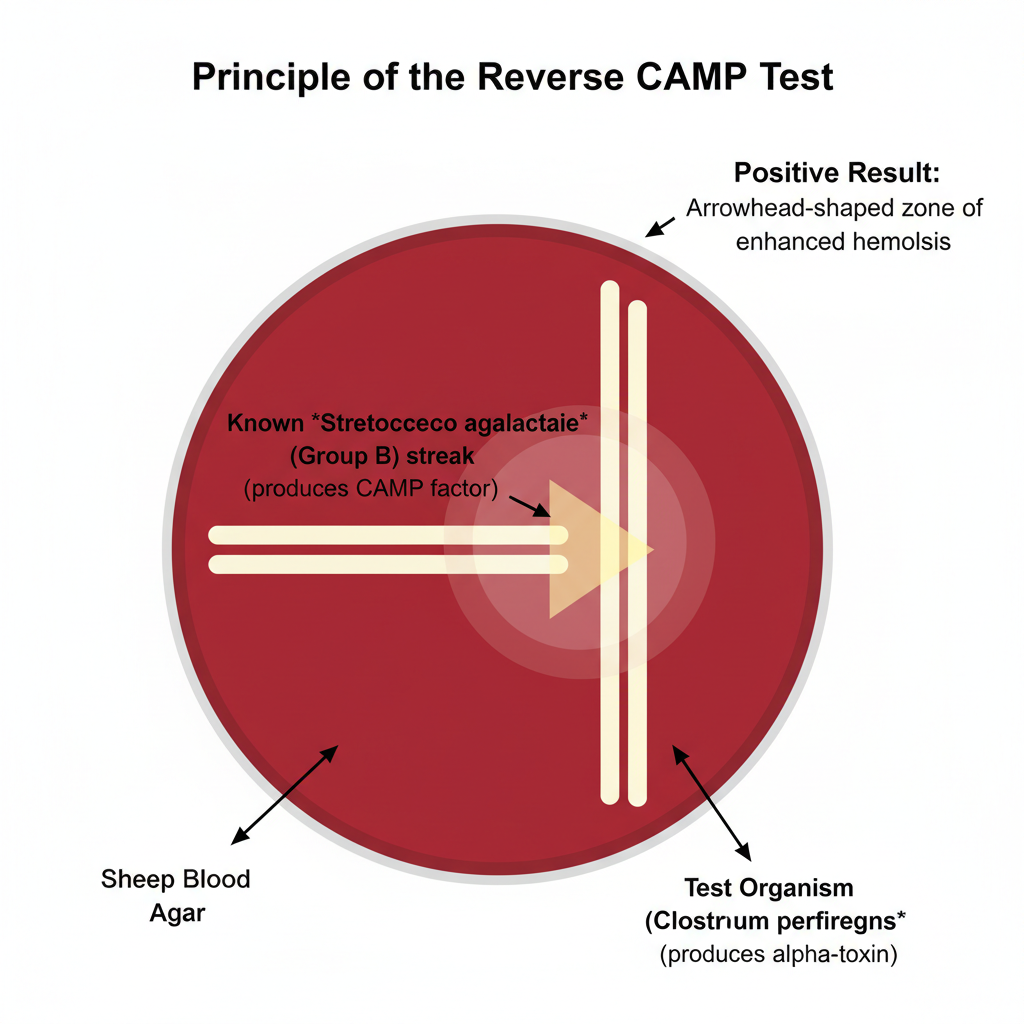
The Reverse CAMP test is a microbiological test which is used for the presumptive identification of Clostridium perfringens. It is the process where the reaction is based on the interaction between the alpha-toxin of C. perfringens and the CAMP factor of Group B Streptococcus (Streptococcus agalactiae). In this test a known strain of Group B Streptococcus is streaked in the centre of a sheep blood agar plate and the test organism is streaked at a right angle to it leaving a small gap. After incubation at 37°C under anaerobic condition for about 24–48 hours an enhanced hemolysis is produced at the junction of the two streaks. It is referred to as the “arrowhead’’ or sometimes a bow-tie shaped zone. This is referred to as the positive Reverse CAMP reaction. It is termed reverse because here the known Streptococcus agalactiae is used to identify the unknown Clostridium perfringens whereas in the normal CAMP test Staphylococcus aureus is used for identifying the Group B Streptococcus.
Objective of Reverse CAMP test
- To differentiate the Clostridium perfringens from other Clostridium species.
Principle of Reverse CAMP test
The principle of the Reverse CAMP test is based on the synergistic hemolytic reaction that occurs when the alpha-toxin of Clostridium perfringens diffuses toward the CAMP factor produced by Streptococcus agalactiae. It is the process where a known strain of Group B Streptococcus is streaked on sheep blood agar and the test organism is placed perpendicularly at a short distance so that both organisms release their extracellular products into the medium. As these diffusible factors move through the agar they meet at the junction site, and the alpha-toxin (phospholipase C) interacts with the CAMP factor producing a marked enhancement of red blood cell lysis. After incubation in anaerobic condition at 37°C the area where the two factors overlap shows a characteristic arrowhead-shaped zone of hemolysis. This reaction is referred to as the Reverse CAMP effect and it is used as the principle to identify C. perfringens from other Clostridium species.
Material Required for Reverse CAMP test
- Sheep blood agar plate (Trypticase soy agar with 5% sheep blood) is required for observing the synergistic hemolysis.
- A known CAMP-positive strain of Streptococcus agalactiae is needed as the reference strain supplying the CAMP factor.
- The suspected Clostridium perfringens isolate is used as the test organism for detecting the alpha-toxin activity.
- A positive control strain of C. perfringens is required to check the proper performance of the test system.
- A negative control strain such as Clostridium septicum or Clostridium sporogenes is also used to compare the absence of the Reverse CAMP effect.
- Sterile loops or inoculating needles are used to make straight streaks on the agar surface.
- An anaerobic system (anaerobic jar or gas-pak system) is required because the test organism needs strict anaerobic condition for toxin production.
- An incubator adjusted around 35°C to 37°C is required for growth and expression of hemolytic activity.
- Strict anaerobic conditions must be maintained during incubation for 24–48 hours.
- In the inoculation pattern the Group B Streptococcus is streaked along the centre and the test organism is streaked perpendicularly at a small gap of about 1–2 mm without touching the reference streak.
Procedure of Reverse CAMP Test
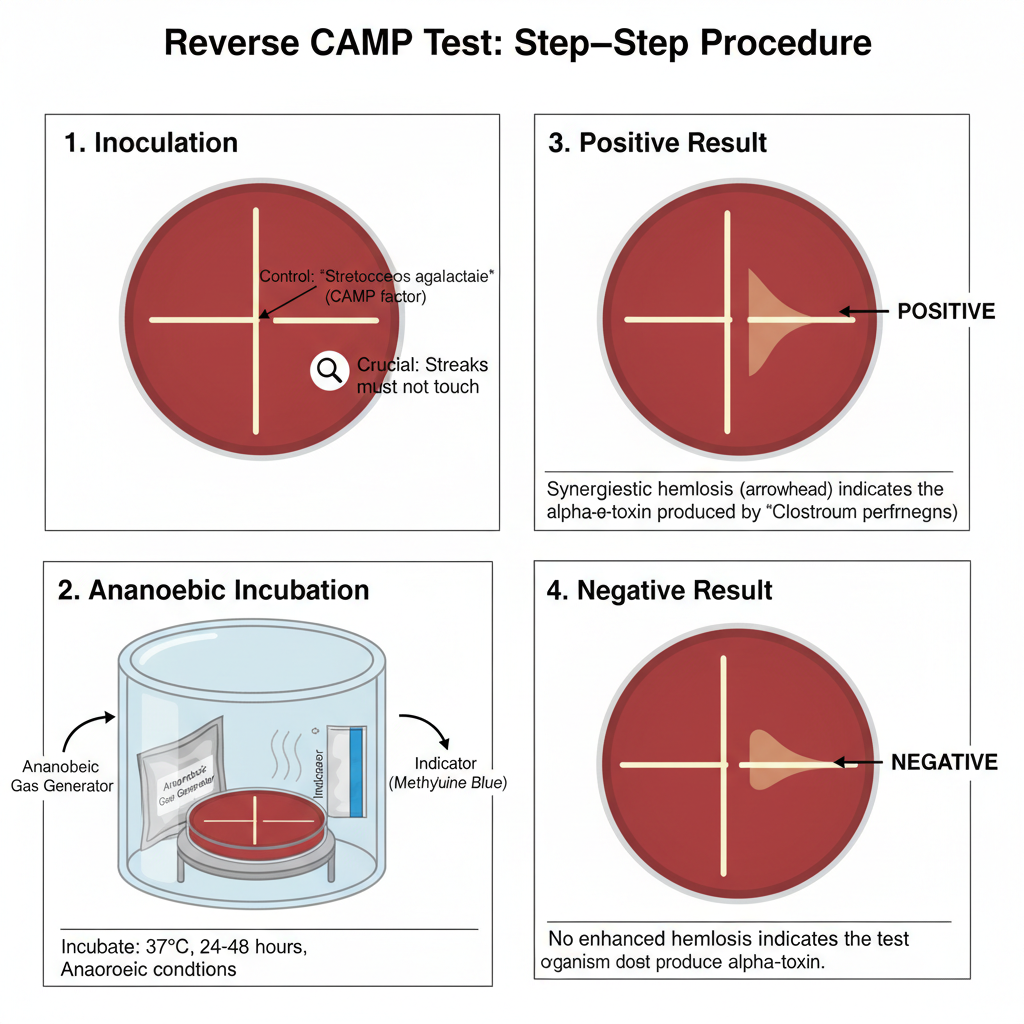
- Preparation of the medium
- It is done by selecting Sheep Blood Agar (5% sheep blood) because the reaction is dependent on the specific lipid present in sheep erythrocytes.
- Other blood sources is avoided since the synergistic hemolysis is not formed properly.
- A known CAMP-positive Streptococcus agalactiae is used as the control organism and sometimes a positive C. perfringens culture is also kept for reference.
- Inoculation of the reference strain
- The known CAMP-positive Streptococcus agalactiae is streaked as a single straight line on the center of the plate.
- It is the central line that helps in observing the reaction when different isolates is tested.
- Inoculation of the test organism
- The suspected Clostridium perfringens isolate is streaked at a right angle to the central line.
- The streak is kept 1–2 mm away from the S. agalactiae streak.
- These two lines must not touch because the synergistic zone is formed only when diffusion of enzymes occur through the agar.
- Incubation
- The plate is incubated in strict anaerobic conditions because the organism is an obligate anaerobe and the alpha-toxin expression is maximum in anaerobic environment.
- The temperature is maintained at 37°C.
- The incubation time is usually 24–48 hours (the reaction sometimes appears late in weakly toxigenic strains).
- Observation and interpretation
- The plate is examined for an enhanced zone of β-hemolysis at the region where the two streaks approach each other.
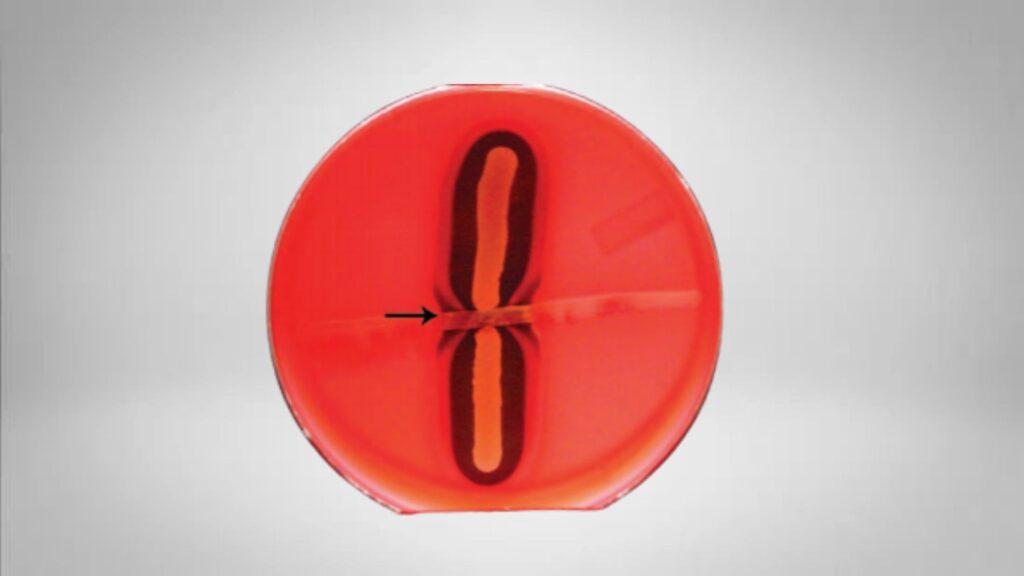
- A positive test shows an arrowhead-like or bow-tie shaped hemolysis due to the interaction of CAMP factor with alpha-toxin.
- A negative test shows no increased hemolysis at the junction, although individual hemolysis around colonies may be present.
Results of Reverse CAMP Test
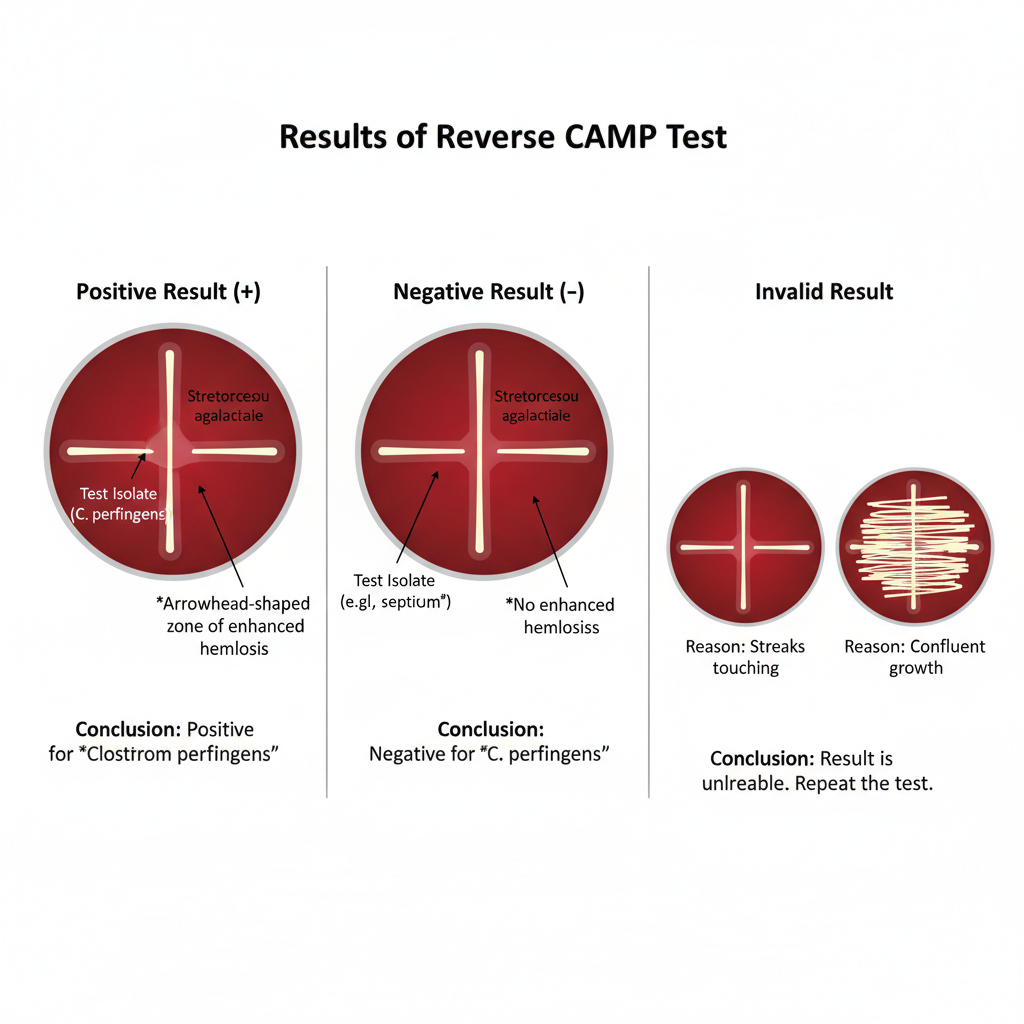
Positive Result (+)
- It is the indication that the organism is Clostridium perfringens because the synergistic hemolysis is clearly formed.
- A sharp arrowhead-shaped or bow-tie zone of enhanced β-hemolysis is seen at the region where the test isolate come close to the Streptococcus agalactiae streak.
- This enhanced zone is formed when the alpha-toxin of C. perfringens diffuse and interact with the CAMP factor, and it is the characteristic reaction.
Negative Result (–)
- It is observed when no enhanced hemolysis is present at the intersection region.
- The arrowhead pattern is absent although some hemolysis around individual colonies may still appear.
- It is the sign that the isolate may be some other Clostridium species (like C. septicum), where the synergistic reaction is not produced.
Invalid Result
- It is obtained when the streaks touch each other or when unsuitable blood agar is used since the proper diffusion pattern is not formed.
- Lack of anaerobic condition or confluent growth also disturb the reaction and the hemolytic zone cannot be interpreted.
- In this case the test is repeated because the result is not reliable.
Uses of Reverse CAMP test
- It is used for the presumptive identification of Clostridium perfringens from clinical samples because most isolates show a clear positive Reverse CAMP reaction.
- It is used to differentiate C. perfringens from other Clostridium species like C. septicum and C. bifermentans which generally does not produce the synergistic hemolysis.
- It is used as a functional test to detect the biologically active alpha-toxin (phospholipase C) secreted by C. perfringens which is an important virulence factor in gas gangrene.
- It can also help in identifying some other Gram-positive rods that show similar synergistic reactions such as Corynebacterium pseudotuberculosis, Corynebacterium ulcerans, Arcanobacterium haemolyticum, and Mycoplasma hyorhinis.
- A variation of this synergistic hemolysis test can differentiate Listeria ivanovii from other Listeria species when used with Rhodococcus equi.
- It is used in rapid diagnostic screening of suspected clostridial myonecrosis cases because early identification of C. perfringens helps in quick medical and surgical management.
Advantages of Reverse CAMP test
- It is highly sensitive for detecting Clostridium perfringens because most isolates produce a clear positive Reverse CAMP reaction which makes the test reliable for presumptive identification.
- It helps in specific differentiation of C. perfringens from other Clostridium species that do not show the synergistic hemolysis with Group B Streptococcus.
- It offers a functional confirmation that the organism is actively producing the alpha-toxin since the synergistic hemolysis only occurs when the toxin is secreted in a biologically active form.
- It produces a very distinct arrowhead-shaped zone of hemolysis which makes interpretation easy because the enhanced hemolysis amplifies the visible signal.
- It can be applied directly to clinical specimens in some cases which helps in quick presumptive identification without waiting for a full subculture.
- It is a cost-effective and simple method that reduces the need for complex biochemical identification in the early stage of laboratory diagnosis.
Limitations of Reverse CAMP test
- It is only a presumptive test and cannot be used alone for confirming Clostridium perfringens because further biochemical tests and staining characteristics are required for the final identification.
- It must be performed strictly on sheep blood agar since other blood sources do not support the proper synergistic hemolysis needed for the Reverse CAMP reaction.
- There can be confusion in interpretation because some descriptions refer to a different type of “reverse” reaction, so the expected arrowhead-shaped hemolysis must be clearly defined in the laboratory.
- Strict anaerobic condition is required since exposure to oxygen reduces the growth of C. perfringens and also inhibits alpha-toxin production.
- Reading the results too early may give false-negative reactions because adequate toxin diffusion requires at least 24–48 hours of incubation.
- The streaks must be placed carefully because touching the two streak lines destroys the proper pattern of toxin diffusion and produces invalid results.
- Very thick agar plates may interfere with the diffusion of hemolytic factors which can reduce the clarity of the arrowhead zone and affect the interpretation.
- Aryal, S. (2022, August 10). CAMP Test- Principle, Uses, Procedure and Result Interpretation. Microbiology Info.com. https://microbiologyinfo.com/camp-test-principle-uses-procedure-result-interpretation/
- Buchanan, A. G. (1982). Clinical laboratory evaluation of a reverse CAMP test for presumptive identification of Clostridium perfringens. Journal of Clinical Microbiology, 16(4), 761–762. https://doi.org/10.1128/jcm.16.4.761-762.1982
- Flores-Díaz, M., & Alape-Girón, A. (2003). Role of Clostridium perfringens phospholipase C in the pathogenesis of gas gangrene. Toxicon, 42(8), 979–986. https://doi.org/10.1016/j.toxicon.2003.11.013
- Fontana, C., Jezzi, T., Testore, G. P., & Dainelli, B. (1995). Differentiation of Clostridium difficile, Clostridium bifermentans, Clostridium sordellii, and Clostridium perfringens from diarrheal stool by API ZYM and API LRA oxidase test. Microbiology and Immunology, 39(4), 231–235. https://doi.org/10.1111/j.1348-0421.1995.tb02194.x
- Hansen, M. V., & Elliott, L. P. (1980). New presumptive identification test for Clostridium perfringens: reverse CAMP test. Journal of Clinical Microbiology, 12(4), 617–619. https://doi.org/10.1128/jcm.12.4.617-619.1980
- Hanson, A. (2006, October 9). CAMP Test Protocols. American Society for Microbiology. https://asm.org/asm/media/protocol-images/camp-test-protocols.pdf
- Kumari, S. (2020, December 31). CAMP Tests (Standard and Rapid) and Reverse CAMP test [Lecture notes]. Department of Veterinary Microbiology, Bihar Veterinary College.
Microbe Canvas. (n.d.). CAMP-test revers_Clostridium perfringens. Retrieved from https://microbe-canvas.com/tests/tests/camp-test-revers_clostridium-perfringens.html - Rao, S. P. N. (n.d.). CAMP TEST. Microrao. Retrieved from https://www.microrao.com/micronotes/camp_test.pdf
- Sapkota, A. (2022, January 27). CAMP Test- Principle, Procedure, Types, Results, Uses, Limitations. Microbe Notes. https://microbenotes.com/camp-test-principle-procedure-and-result-interpretation/
- Sigma-Aldrich. (n.d.). Detection, Identification and Differentiation of Clostridium perfringens. Retrieved from https://www.sigmaaldrich.com/US/en/technical-documents/technical-article/microbiological-testing/pathogen-and-spoilage-testing/clostridium-perfringens
- The Reverse CAMP Test: Principle, Standardization, and Clinical Utility for the Presumptive Identification of Clostridium perfringens. (n.d.). [Provided Source Document].
- Urbina, P., Collado, M. I., Alonso, A., Goñi, F. M., Flores-Díaz, M., Alape-Girón, A., Ruysschaert, J. M., & Lensink, M. F. (2011). Unexpected wide substrate specificity of C. perfringens α-toxin phospholipase C. Biochimica et Biophysica Acta (BBA) – Biomembranes, 1808(10), 2618–2627. https://doi.org/10.1016/j.bbamem.2011.06.008
- Wikipedia. (n.d.). CAMP test. Retrieved from https://en.wikipedia.org/wiki/CAMP_test
- Yao, P. Y., & Annamaraju, P. (2023, August 8). Clostridium perfringens Infection. In StatPearls. StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK559049/