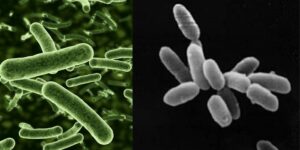
What is Clostridium perfringens?
- Clostridium perfringens, previously called C. welchii or Bacillus welchii, is a Gram-positive, rod-shaped bacterium that thrives in anaerobic conditions and forms spores. Belonging to the genus Clostridium, it is found abundantly in nature, existing in environments like decaying vegetation, marine sediments, soil, and within the intestines of humans and animals. Remarkably, it exhibits one of the fastest generation times of any organism, replicating in just 6.3 minutes under optimal conditions in a thioglycolate medium.
- This bacterium is a significant cause of foodborne illness in the United States, ranking alongside pathogens such as norovirus, Salmonella, Campylobacter, and Staphylococcus aureus. While ingestion of C. perfringens often leads to food poisoning, it can sometimes pass through the digestive system without causing harm. However, in more severe cases, infections can result in tissue necrosis, gas gangrene, and bacteremia. Gas gangrene, also termed clostridial myonecrosis, involves extensive tissue destruction caused by the alpha toxin produced by the bacterium. This toxin alters the structure of cell membranes, disrupting their normal functions and leading to cell death.
- The Latin origin of its name reflects its role in tissue damage; perfringens is derived from “per” (through) and “frango” (to burst), highlighting the destructive nature of its infections. Aside from causing infections, C. perfringens can exist harmlessly as part of the normal microbial flora, particularly in polymicrobial anaerobic infections where its contribution to disease is minimal.
- Genetically, C. perfringens demonstrates significant variability due to horizontal gene transfer, particularly through plasmids from neighboring cells. This genetic flexibility allows it to adapt and develop novel pathogenic traits. Based on the toxins they produce, populations of C. perfringens are classified into different strains, a feature critical for understanding its role in foodborne illnesses and guiding prevention efforts in the food industry.
- Historically considered non-motile, recent studies suggest C. perfringens may exhibit hyper-motility, challenging prior assumptions about its behavior. Ongoing research into its metabolic processes and genetic mechanisms continues to provide deeper insights into the bacterium’s pathogenicity and its potential impact on public health.
Scientific classification of Clostridium perfringens
| Domain: | Bacteria |
| Phylum: | Bacillota |
| Class: | Clostridia |
| Order: | Eubacteriales |
| Family: | Clostridiaceae |
| Genus: | Clostridium |
| Species: | C. perfringens |
Geographical Distribution and Habitat of Clostridium perfringens
Clostridium perfringens is found globally, with varying types responsible for different diseases in humans and animals.
- Geographical Distribution
- C. perfringens is widely distributed across the world.
- Type A is the most common strain, causing a majority of human diseases, including food poisoning, soft tissue infections, gas gangrene, and primary septicemia.
- Type C is associated with necrotizing enteritis (also known as enteritis necroticans), a severe and often fatal condition.
- Habitat
- C. perfringens type A is a part of the normal intestinal flora in humans and animals.
- These bacteria are excreted through feces, which can lead to contamination of the skin around the perianal region, buttocks, and thighs.
- The spores of C. perfringens type A are widespread and can be found in soil, dust, and air.
- These spores are remarkably resilient, capable of surviving for extended periods even in harsh environmental conditions.
- Other types of C. perfringens—namely B, C, D, and F—are typically found in the intestines of animals and occasionally humans.
- Unlike type A, the spores of these other types do not usually persist in soil.
Morphology of Clostridium perfringens
Clostridium perfringens is a Gram-positive bacterium with distinct structural and morphological characteristics that make it recognizable under microscopic and cultural examination. Here’s a detailed breakdown of its features:
- Shape and Size
- It is a large, rectangular bacillus (rod-shaped) with parallel sides and rounded or truncated ends.
- The size ranges from 3–8 µm in length and 0.4–1.2 µm in diameter, showing variability depending on the environment.
- While typically straight, it can occasionally appear curved, displaying pleomorphic tendencies.
- Arrangement
- The bacilli can be found as single cells, in chains, or grouped in bundles, depending on the sample and growth conditions.
- Capsule
- These bacteria are capsulated, providing them with additional protection and contributing to their pathogenicity.
- Motility
- Despite being classified as non-motile and lacking flagella, they display a characteristic “spreading colony” growth pattern on media, mimicking motile clostridia.
- This phenomenon may be linked to filamentous structures lining their body, which allow them to glide across surfaces.
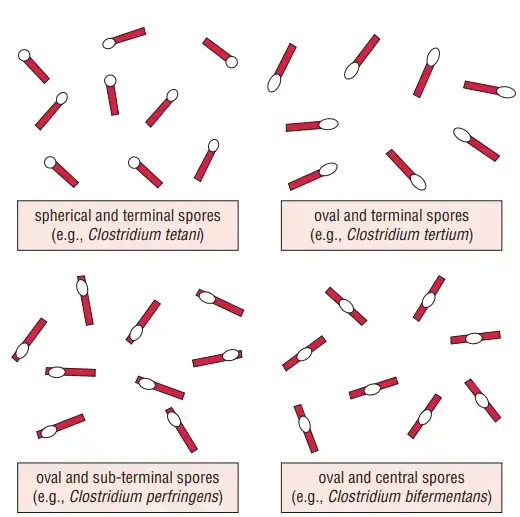
- Spore Formation
- C. perfringens produces central or subterminal spores, although spores are rarely observed in clinical specimens or culture media.
- The spores are wider than the bacterial body, causing the bacillus to appear swollen, resembling a spindle.
- These endospores are highly resistant, capable of surviving prolonged exposure to heat, air, and adverse environmental conditions.
- Cell Wall
- It has a thick cell wall composed of peptidoglycan, which provides structural integrity and resistance against environmental stressors.
Genome Structure of Clostridium perfringens
The genome of Clostridium perfringens is well-studied and exhibits features that contribute to its adaptability, pathogenicity, and resistance. Its genomic composition reflects high diversity and unique structural elements. Below are the key aspects:
- Chromosomal Organization
- The bacterium has a single circular chromosome with an average size of 3.5 million base pairs (Mb).
- The genome contains about 6 million base pairs in some strains, highlighting size variability.
- Its guanine-cytosine (G+C) content is relatively low, ranging from 27–28%, though some reports extend this range to 24–55%.
- Core and Accessory Genes
- Only 12.6% of the genes are classified as core genes, making it one of the most genetically diverse Gram-positive bacteria.
- The remaining genes are part of the accessory genome, which varies between strains and contributes to its adaptability.
- Conserved and Variable Regions
- Despite its diversity, the 16S rRNA regions among C. perfringens strains show over 99.1% sequence identity, indicating strong conservation in this area.
- This balance between conserved and variable regions underpins its ability to survive in diverse environments and adapt to changing conditions.
- Plasmid DNA
- C. perfringens harbors plasmids that encode vital traits, including toxin production and antibiotic resistance.
- The pCW3 plasmid is central to antibiotic resistance, carrying genes for tetracycline resistance, aminoglycoside resistance, and efflux proteins.
- Plasmids also encode major toxins, such as the Clostridium perfringens enterotoxin (CPE), essential for food poisoning. The CPE gene can exist on both plasmids and chromosomal DNA.
- Ribosomal and Transfer RNA Genes
- The genome contains 10 rRNA genes and 96 tRNA genes, enabling efficient protein synthesis and adaptation to various environments.
- Role in Food Production and Public Health
- Genomic sequencing has revealed antibiotic-resistant strains impacting industries like poultry production.
- Meta-genomic analysis is used to identify emerging pathogenic strains, such as C. perfringens B20, which can affect food safety.
Culture of Clostridium perfringens
Clostridium perfringens is a facultative anaerobe capable of surviving exposure to oxygen, growing efficiently under microaerophilic conditions. It thrives within a temperature range of 20–44°C, with an optimal temperature of 37°C, and can tolerate a pH range of 5.5–8.0. Below are the culture techniques and conditions used to grow this bacterium:
- Growth in Robertson’s Cooked Meat (RCM) Broth
- This medium supports rapid bacterial growth and is particularly useful in tissue and culture studies.
- C. perfringens produces a distinctive sour odor and an acidic reaction in RCM broth, turning the meat pink without digesting it.
- Certain strains can grow at 45°C, reducing their generation time to about 10 minutes. This feature is exploited for isolating the bacterium in specimens contaminated with other clostridial species.
- The process involves inoculating the specimen in RCM broth, incubating it at 45°C for 4–6 minutes, and then culturing on blood agar to produce pure colonies.
- Culturing on Blood Agar
- Blood agar supplemented with human, sheep, or rabbit blood is another reliable medium for C. perfringens.
- Colonies exhibit a dual zone of hemolysis upon prolonged incubation:
- A narrow zone of complete hemolysis caused by theta-toxin.
- A wider zone of incomplete hemolysis resulting from alpha-toxin activity.

Biochemical Reactions of Clostridium perfringens
Clostridium perfringens exhibits a variety of distinct biochemical activities, many of which are used for its identification in laboratory settings. These reactions reflect its metabolic capabilities and unique enzymatic properties.
- Carbohydrate Fermentation
- Actively ferments glucose, lactose, sucrose, and maltose.
- Acid and gas are the primary end products during this process.
- Hydrogen Sulfide and Nitrate Reduction
- Produces H₂S as a metabolic byproduct.
- Reduces nitrate to nitrite, which is a standard reaction for this bacterium.
- Methyl Red and Voges-Proskauer Tests
- Positive for the Methyl Red (MR) test, indicating stable acid production.
- Negative for the Voges-Proskauer (VP) test, showing no acetoin production.
- Indole Production
- Indole test results are negative, meaning it does not produce indole from tryptophan degradation.
- Litmus Milk Fermentation
- Ferments lactose in litmus milk, leading to acid production.
- The medium’s color shifts from blue to red due to the acidic environment.
- Stormy Fermentation in Litmus Milk
- Acid production coagulates casein in the milk, creating a solidified texture.
- Large volumes of gas disrupt the coagulated milk, breaking it apart violently.
- This activity pushes the paraffin plug upward, leaving fragments of the clot adhered to the sides of the glass tube.
- Known as stormy fermentation, this is a hallmark reaction of C. perfringens.

Virulence Factors of Clostridium perfringens
Clostridium perfringens is a highly pathogenic bacterium due to its production of over 12 toxins and enzymes. These virulence factors play a crucial role in disease development, tissue damage, and bacterial survival.
- Major Toxins
- Alpha-Toxin
- Produced by all strains, particularly in large amounts by type A strains.
- Functions as a lecithinase (phospholipase C), breaking down lecithin into phosphoryl choline and diglyceride in the presence of calcium and magnesium ions.
- Causes toxemia in gas gangrene, increasing vascular permeability and triggering hemolysis, tissue destruction, and myocardial dysfunction.
- Lyses erythrocytes, leukocytes, platelets, and endothelial cells.
- Heat-stable; partially inactivated by boiling for five minutes.
- Red cell lysis is most evident when incubated at 37°C and then at 4°C (hot-cold lysis).
- Beta-Toxin
- Lethal toxin responsible for necrotic lesions in necrotizing enteritis.
- Epsilon-Toxin
- Produced as a protoxin and activated by trypsin.
- Increases vascular permeability in the gastrointestinal wall.
- Iota-Toxin
- Causes necrotic lesions and enhances vascular permeability.
- Alpha-Toxin
- Minor Toxins
- Delta-Toxin: Hemolytic, particularly against red cells from sheep, goats, and cattle.
- Theta-Toxin: An oxygen-sensitive hemolysin that also functions as a cytolytic toxin.
- Kappa-Toxin: Acts as a collagenase, aiding tissue destruction.
- Lambda-Toxin: Exhibits proteinase and gelatinase activity.
- Mu-Toxin: Functions as a hyaluronidase, breaking down hyaluronic acid to spread infection.
- Nu-Toxin: Deoxyribonuclease that degrades DNA.
- Enterotoxin
- Produced mainly by type A strains during sporulation in an alkaline environment, such as the small intestine.
- Binds to epithelial receptors in the small intestine, disrupting ion transport and altering membrane permeability.
- Antigenic, though antibodies are not protective.
- Enzymes and Soluble Substances
- Neuraminidase
- Alters cell surface ganglioside receptors, promoting capillary permeability.
- Bursting Factor
- Acts on muscle tissue, potentially causing muscle lesions characteristic of gas gangrene.
- Circulating Factor
- Increases adrenaline sensitivity in capillary membranes and inhibits phagocytosis.
- Hyaluronidase
- Degrades intercellular cement substances, allowing infection to spread along tissue planes.
- Neuraminidase

Pathogenesis of Clostridium perfringens
Clostridium perfringens is capable of causing a range of infections, from gas gangrene to food poisoning, through its ability to produce a variety of potent toxins. The bacteria’s pathogenesis involves multiple stages, including spore germination, tissue invasion, and the release of toxins that lead to severe tissue damage.
- Invasive Infection and Gas Gangrene
- Clostridium perfringens spores typically enter tissues through contaminated traumatic injuries, either from soil, feces, or the intestinal tract.
- Once inside a low-oxygen environment, spores germinate into vegetative cells.
- These cells begin multiplying and ferment the carbohydrates present in the affected tissue.
- The fermentation process produces gas, which leads to distention of tissues and interferes with blood supply.
- The secretion of necrotizing toxins and hyaluronidase promotes the spread of infection by breaking down tissue barriers.
- This results in further tissue necrosis, which provides an environment for increased bacterial growth.
- As the infection progresses, Clostridium perfringens releases α-toxin, which disrupts the plasma membrane of host cells, leading to cell death and dysfunction.
- The consequences include hemolytic anemia, severe toxemia, and, without intervention, death.
- Diarrheal Disease (Type A Food Poisoning)
- The illness is triggered by the ingestion of food contaminated with high levels of Clostridium perfringens bacteria.
- These bacteria can survive high cooking temperatures and, when food is improperly cooled or held at unsafe temperatures (54°F–140°F), they can proliferate.
- The bacteria grow most rapidly at 109°F–117°F (43°C–47°C), and if the food isn’t properly reheated, live bacteria may be consumed.
- Upon reaching the intestines, the vegetative cells of the bacteria multiply and then begin sporulating.
- The process of sporulation and the production of the CPE toxin (Clostridium perfringens enterotoxin) is tightly controlled by Spo0A and alternate sigma factors.
- The toxin accumulates within the mother cell and is released upon cell lysis at the end of sporulation.
- Once released, the toxin damages the intestinal lining, leading to symptoms such as diarrhea and abdominal cramping.
Clinical Syndromes of Clostridium perfringens
Clostridium perfringens is a pathogen capable of causing various clinical syndromes, each with distinct manifestations and levels of severity. These include soft tissue infections, food poisoning, necrotizing enteritis, and septicemia.
- Soft Tissue Infections
- Cellulitis:
- C. perfringens can invade the fascial planes of the skin, leading to anaerobic cellulitis.
- This infection involves gas formation in the soft tissues but does not invade the muscle tissue, and the bacteria produce minimal toxin.
- Fasciitis and Suppurative Myositis:
- As cellulitis progresses, it can develop into suppurative myositis, characterized by pus accumulation in muscle planes.
- This condition does not involve muscle necrosis or systemic symptoms.
- Gas Gangrene (Clostridial Myonecrosis):
- This severe, life-threatening infection can develop rapidly, sometimes within as little as 7 days after trauma or surgery.
- The early signs include increasing pain, tenderness, and edema in the affected area, along with systemic toxemia.
- The bacteria’s metabolic activity produces gas, which accumulates in tissues, causing crepitus (a crackling sound).
- The discharge from the wound starts as watery and becomes serosanguineous over time.
- In untreated cases, the disease progresses quickly, leading to muscle necrosis, shock, renal failure, and death within 48 hours.
- The production of clostridial toxins typically causes extensive hemolysis and bleeding, ultimately resulting in circulatory failure and death.
- Cellulitis:
- Food Poisoning
- Caused by C. perfringens type A strains, food poisoning occurs when individuals ingest large amounts of the bacteria, commonly from contaminated meat dishes.
- These strains produce heat-resistant spores that survive cooking, then germinate and produce enterotoxins in the intestine.
- The incubation period is short—between 8 and 24 hours.
- Symptoms include abdominal cramps and watery diarrhea, without nausea, vomiting, or fever.
- The disease is self-limiting, and symptoms resolve within 24–48 hours.
- Toxin production in the intestines is primarily responsible for the symptoms, with minimal production of alpha and theta toxins in this syndrome.
- Necrotizing Enteritis
- Caused by C. perfringens type C, necrotizing enteritis is an acute and severe infection affecting the jejunum.
- The condition is marked by intense abdominal pain, bloody diarrhea, shock, and peritonitis.
- It is often fatal without prompt intervention.
- Known as “Pigbel” in Papua New Guinea and “Darmbrand” in Germany, this condition is more prevalent in certain regions like East Africa, Thailand, and Nepal.
- Immunization with type C toxoid has been shown to offer protection against this condition.
- Septicemia
- Septicemia caused by C. perfringens is a life-threatening situation where the bacteria are isolated in the bloodstream.
- This syndrome is associated with severe systemic effects and requires immediate medical attention.
Reservoir, Source, and Transmission of Infection of Clostridium perfringens
Clostridium perfringens can cause a range of infections depending on the source and route of transmission. Understanding where these bacteria live and how they spread is key to controlling their effects.
- Reservoirs of Infection
- C. perfringens is naturally found in the intestines of both humans and animals.
- It is also present in the soil, dust, and air, with spores capable of surviving in harsh conditions for extended periods.
- Sources of Infection
- Exogenous Sources (External)
- C. perfringens can contaminate wounds from environmental sources like soil, especially manure soil or cultivated soil.
- Road dust or bits of clothing contaminated with these spores can also introduce the bacteria into a wound.
- This is common in traumatic injuries like road traffic accidents, military injuries, or any incidents involving crushing trauma to large muscle groups.
- In rare cases, surgical operations can lead to contamination and subsequent infection.
- Endogenous Sources (Internal)
- The bacteria are part of the normal flora of human skin, especially in areas like the perineum and thighs.
- C. perfringens can invade through wounds or surgical incisions, causing infections.
- Exogenous Sources (External)
- Transmission of Infection
- Gas Gangrene
- This occurs when C. perfringens spores from contaminated external sources infect traumatic wounds, particularly those involving large muscle areas.
- Contamination can happen through environmental contact, where soil or dust with C. perfringens enters the wound site.
- Clostridial Food Poisoning
- C. perfringens type A causes food poisoning when spores from contaminated food, especially meat dishes, are ingested.
- The spores are heat-resistant, so improperly stored or reheated food can harbor these bacteria.
- Foods exposed to a large number of C. perfringens spores are the primary source of infection.
- Necrotizing Enteritis (Pigbel)
- Large numbers of C. perfringens spores in contaminated food can cause necrotizing enteritis, a severe and often fatal disease.
- Malnutrition and improper food handling practices increase the risk of infection by providing an environment where the bacteria can thrive.
- Gas Gangrene
Diagnosis of Clostridium perfringens
Diagnosing Clostridium perfringens infection involves a mix of clinical signs, laboratory tests, and post-mortem findings. The goal is to identify the specific toxins and bacterial presence in the body to confirm infection.
- Field Diagnosis
- The diagnosis starts with clinical history and clinical signs observed before death.
- Sudden death without warning signs is a key indicator.
- Necropsy reveals acute hemorrhagic enterocolitis, with hemorrhagic intestinal contents, which are highly suggestive of the infection.
- Stress within the 12 to 36 hours before death should be considered as it may contribute to the onset of the disease.
- Post-Mortem Examination
- The ideal sample for bacterial analysis is a ligated intestinal loop from the area where hemorrhagic lesions are found.
- Freshly dead animals provide the best samples for bacteriological culture.
- Quick post-mortem examinations, ideally on-site, help ensure the integrity of samples.
- To stabilize toxins, chloroform may be added to intestinal contents during transport.
- Laboratory Diagnosis
- Isolation of C. perfringens from faeces or gut lumen is essential but tricky, as the bacterium is a normal gut inhabitant in many animals.
- Bacterial counts of 104-107 CFU/g of fecal sample are typically seen in healthy sheep and goats.
- Impression smears from affected tissues reveal short, thick, non-sporulating Gram-positive rods, which can be a clue for further testing.
- Common media for isolating C. perfringens include Robertson’s cooked meat media, thioglycolate, reinforced clostridial media, and TSC-egg yolk agar. Specific patterns like gas production and double zone hemolysis are indicative of C. perfringens.
- Molecular Detection
- Polymerase Chain Reaction (PCR) is used to detect genes responsible for toxin production, especially the epsilon toxin gene (etx), which is crucial for diagnosing enterotoxaemia.
- Multiplex PCR allows for toxinotyping of different C. perfringens strains in samples like faeces and intestinal tissue. It can identify various toxin genes, including beta2, alpha, and epsilon.
- Real-time PCR is another method for detecting toxin genes, used for both diagnosis and understanding prevalence in different animal species.
- Toxin Detection Methods
- Immunohistochemistry can identify clostridial toxins in tissues when PCR or culture are not feasible. It’s particularly useful for detecting C. perfringens enterotoxin (CPE) in various animal tissues.
- Biochemical tests for C. perfringens help identify specific characteristics of the bacterium, such as sugar fermentation and H2S production.
- Serological and Biological Neutralization Tests:
- Mouse Neutralization Test (MNT) is used to detect epsilon toxin in body fluids and intestinal contents. However, its use has declined with the rise of more efficient serological tests.
- ELISA is commonly employed for toxin detection in gut contents, with high sensitivity and specificity. Recent advancements include sandwich immunoassay and immunochromatographic tests, which improve detection limits.
- Latex Agglutination Test (LAT) is a quicker, cheaper alternative for detecting epsilon toxin in field settings, although ELISA is more sensitive.
- Indirect Haemagglutination Tests (IHT) are used for measuring antibody titers against C. perfringens toxins, and Indirect Haemagglutination Inhibition Tests (IHIT) for detecting epsilon toxin.
- Mass Spectrometry (MS)
- Techniques like LC-MS and MALDI-TOF are now used in some specialized labs to detect and quantify epsilon toxin. While extremely sensitive, these methods are expensive and not commonly used due to their high costs and inability to assess toxin activity.
Treatment of Clostridium perfringens
Treatment of Clostridium perfringens infections varies depending on the clinical presentation, but swift action is key in managing the infection effectively.
- Gas Gangrene Treatment
- Surgery is the cornerstone of treatment for gas gangrene. Immediate surgical intervention is necessary to remove necrotic tissue, clear out foreign materials, and eliminate blood clots from the affected area.
- The removal of damaged tissue helps to reduce the bacterial load and prevent further toxin release.
- Hyperbaric oxygen therapy has been suggested to help treat gas gangrene, though it is not a substitute for surgery.
- Antiserum against alpha-toxin is no longer recommended, as it has fallen out of favor in clinical practice.
- Antibiotic Therapy
- The first-line antibiotic for treating C. perfringens infections is metronidazole, given intravenously. The recommended dosage is three times a day with an eight-hour interval between doses.
- Broad-spectrum antibiotics, including gentamicin, amoxicillin, and metronidazole, are often used prophylactically. This combination is effective in cases where mixed infections (aerobic and anaerobic bacteria) are suspected.
- Antibiotic use before surgery is crucial to reduce bacterial growth and spread during surgical treatment.
- Food Poisoning
- Antibiotics are generally not recommended for treating C. perfringens food poisoning. The body typically clears the infection without the need for antibiotics, and management focuses on symptom relief.
Prevention and Control of Clostridium perfringens
Preventing Clostridium perfringens infections comes down to a few critical practices focused on hygiene, wound care, and proper use of antibiotics.
- Wound Care and Hygiene
- Proper wound cleaning and debridement are essential. It’s crucial to remove foreign materials, necrotic tissue, and any other debris from the wound to limit bacterial growth.
- Regular inspection of wounds helps catch infections early, especially in cases of trauma or surgery. Cleaning wounds thoroughly prevents the introduction of C. perfringens and other harmful bacteria.
- Antibiotic Use
- Prophylactic use of penicillin is recommended in certain cases, especially in high-risk situations. This helps to prevent infection before it even starts.
- The early administration of antibiotics can also be critical in treating any established infection and preventing its spread, especially when dealing with contaminated wounds.
- Lack of Vaccine
- Currently, no vaccine exists to prevent C. perfringens infections, so attention to wound care and antibiotic use remains the primary defense.
- Miyamoto, K., & Nagahama, M. (2016). Clostridium: Food Poisoning by Clostridium perfringens. Encyclopedia of Food and Health, 149–154. doi:10.1016/b978-0-12-384947-2.00171-9
- Yao P, Annamaraju P. Clostridium Perfringens. [Updated 2022 Oct 3]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK559049/
- Obana, N., Nakamura, K., & Nomura, N. (2014). A Sporulation Factor Is Involved in the Morphological Change of Clostridium perfringens Biofilms in Response to Temperature. Journal of Bacteriology, 196(8), 1540–1550. doi:10.1128/jb.01444-13
- Labbé, R. G. (2003). CLOSTRIDIUM | Occurrence of Clostridium perfringens. Encyclopedia of Food Sciences and Nutrition, 1398–1401. doi:10.1016/b0-12-227055-x/00252-2
- McClane, B. A. (2014). Clostridium perfringens. Encyclopedia of Toxicology, 987–988. doi:10.1016/b978-0-12-386454-3.00081-6
- Pawaiya, Rajveer & Kumaresan, Gururaj & Gangwar, Neeraj & Singh, D.D. & Kumar, Rahul & Kumar, Ashok Kumar & Gururaj, S & Gangwar, K & Singh, N & Kumar, R & Pawaiya, R. (2020). The Challenges of Diagnosis and Control of Enterotoxaemia Caused by Clostridium perfringens in Small Ruminants Open Access. Advances in Microbiology. 10. 238-273. 10.4236/aim.2020.105019.
- Labbe, R., Juneja, V. K., & Blaschek, H. P. (2014). CLOSTRIDIUM | Clostridium perfringens. Encyclopedia of Food Microbiology, 463–467. doi:10.1016/b978-0-12-384730-0.00068-9
- Nagahama, M., Oda, M., Tsuge, H., & Kobayashi, K. (2015). Enteric Toxins of Clostridium perfringens. Molecular Medical Microbiology, 997–1013. doi:10.1016/b978-0-12-397169-2.00056-1
- Labbé, R., & Juneja, V. (2016). Clostridium: Occurrence and Detection of Clostridium perfringens. Encyclopedia of Food and Health, 146–148. doi:10.1016/b978-0-12-384947-2.00169-0
- Popoff, M. R. (2014). CLOSTRIDIUM | Detection of Enterotoxin of Clostridium perfringens. Encyclopedia of Food Microbiology, 474–480. doi:10.1016/b978-0-12-384730-0.00069-0
- Labbé, R. G. (2003). CLOSTRIDIUM | Food Poisoning by Clostridium perfringens. Encyclopedia of Food Sciences and Nutrition, 1403–1407. doi:10.1016/b0-12-227055-x/00254-6
- https://www.thermofisher.com/blog/food/fact-sheet-on-clostridium-perfringens/#:~:text=C.,for%20long%20periods%20of%20time.
- http://microbesinfo.com/2015/02/clostridium-perfringens-morphology-cultural-characteristics-classification-and-laboratory-diagnosis/
- https://microbeonline.com/clostridium-perfringens-properties-diseases-and-diagnosis/
- https://en.wikipedia.org/wiki/Clostridium_perfringens
- https://www.embopress.org/doi/full/10.15252/embr.202254600
- https://www.canada.ca/en/public-health/services/laboratory-biosafety-biosecurity/pathogen-safetydata-sheets-risk-assessment/clostridium-perfringens.html
- https://www.academia.edu/3690750/Isolation_identification_and_characterization_of_Clostridium_perfringens_from_lamb_dysentery_in_Dinajpur_district_of_Bangladesh
- https://www.muhadharaty.com/lecture/6425/%D8%BA%D9%8A%D8%B1-%D9%85%D8%B9%D8%B1%D9%88%D9%81/Bacteria-pdf
- https://www.cdc.gov/foodsafety/diseases/clostridium-perfringens.html
- https://www.fda.gov/food/laboratory-methods-food/bam-chapter-16-clostridium-perfringens