The Oxidation–Fermentation (OF) test is a biochemical test that is used to differentiate bacteria on the basis of their ability to metabolize carbohydrates by oxidative or fermentative pathways. It is the process that helps in identifying whether the organism utilizes carbohydrate in the presence of oxygen or in the absence of oxygen. This test was developed by Hugh and Leifson and is mainly applied for differentiation of Gram-negative bacilli.
It is carried out using a semisolid OF medium which contains a high concentration of carbohydrate (generally glucose) and a low concentration of peptone. The reduced amount of peptone is used to prevent alkaline reaction due to protein metabolism. A pH indicator bromothymol blue is present in the medium which helps in detecting acid production even in small amount. Initially the medium is green in colour.
In this test two tubes of OF medium are inoculated with the test organism. One tube is kept open to allow oxygen supply while the other tube is covered with sterile mineral oil or paraffin to create anaerobic condition. If the organism is fermentative, acid is produced in both tubes and the colour of medium changes from green to yellow. If the organism is oxidative, the colour change to yellow is observed only in the open tube while the closed tube remains green. If no colour change is observed in both tubes then the organism is considered as non-saccharolytic.
The OF test is important in the identification of non-fermenting bacteria and is widely used in microbiology laboratories. It is especially useful for differentiating organisms such as Pseudomonas, Acinetobacter and Burkholderia species.
Objectives of OF Test
- To determine whether bacteria utilize carbohydrate by oxidative or fermentative pathway.
- To differentiate organisms based on glucose metabolism in presence or absence of oxygen.
- To detect non-saccharolytic organisms which are unable to utilize carbohydrate.
- To classify bacteria into oxidative group and fermentative group on the basis of acid production.
- To help in identification of Gram-negative bacilli especially non-fermenters.
- To study weak acid production by oxidative organisms which cannot be detected by routine fermentation tests.
- To support laboratory identification of bacteria using the principle developed by Hugh and Leifson.
Principle of OF (Oxidation–Fermentation) Test
The principle of Oxidation–Fermentation (OF) test is based on the ability of bacteria to metabolize carbohydrate by oxidative or fermentative mechanism. It is the process used to differentiate organisms depending on whether the carbohydrate is utilized in presence of oxygen or in absence of oxygen. This test was developed by Hugh and Leifson and mainly uses glucose as the carbohydrate source. The medium contains high concentration of carbohydrate and very low concentration of peptone which helps in preventing alkaline reaction due to protein utilization.
In this test two tubes of OF medium are inoculated with test organism. One tube is kept open to allow aerobic condition while the other tube is covered with sterile mineral oil or paraffin to create anaerobic condition. A pH indicator bromothymol blue is present in the medium which indicates acid production by change in colour from green to yellow. Oxidative organisms produce weak acids only in aerobic condition therefore only the open tube becomes yellow. Fermentative organisms produce acid in both aerobic and anaerobic condition and both tubes turn yellow. Non-saccharolytic organisms do not utilize carbohydrate and no acid is produced, so the medium remains green or may turn blue in open tube due to alkaline reaction.
Requirements for OF (Oxidation–Fermentation) Test
- OF basal medium (Hugh and Leifson’s medium) which is a semisolid medium containing low concentration of peptone and agar.
- Carbohydrate source, usually glucose (dextrose) but other sugars may also be used.
- pH indicator bromothymol blue present in the medium to detect acid production.
- Sterile mineral oil or liquid paraffin for creating anaerobic condition in one tube.
- Sterile test tubes taken in duplicate for aerobic and anaerobic setup.
- Inoculating needle or straight wire for stabbing the medium.
- Fresh pure culture of the test organism for inoculation.
- Incubator maintained at suitable temperature for incubation of inoculated tubes.
- Sterilization equipment for preparing and maintaining sterile conditions during the test.
Hugh and Leifson’s OF basal medium
Composition
- Peptone (tryptone) – 2.0 g
- Sodium chloride – 5.0 g
- Dipotassium phosphate – 0.30 g
- Bromothymol blue – 0.03 g
- Agar – 3.0 g
- Glucose or other carbohydrate – 10.0 g
- Distilled water – up to 1000 ml
Preparation
- All the ingredients except carbohydrate are dissolved in distilled water and the volume is made up to 1 litre.
- The pH of the medium is adjusted to 7.1 before sterilization.
- The medium is heated by boiling or steaming to dissolve agar completely.
- The medium is then sterilized by autoclaving at 121°C for 15 minutes.
- After sterilization, a sterile 10% carbohydrate solution is added aseptically to obtain a final concentration of 1%.
- The medium is mixed properly and dispensed into sterile test tubes aseptically.
- The tubes are allowed to cool in upright position so that semisolid consistency is maintained.
- In some methods carbohydrate is added before sterilization and the tubed medium is steamed instead of autoclaving to prevent breakdown of sugar.
- Commercially prepared OF basal medium is also available and carbohydrate is added separately as required.
This medium was formulated by Hugh and Leifson for differentiation of oxidative and fermentative bacteria.
Procedure of OF (Oxidation–Fermentation) Test
- A fresh pure culture of the test organism (18–24 hours old) is taken for inoculation.
- Two tubes of OF basal medium containing glucose are selected for each organism.
- Both tubes are inoculated by stabbing the medium vertically with a sterile straight inoculating needle.
- The stab is made in the centre of the medium and should reach near the bottom of the tube.
- One of the inoculated tubes is overlaid with sterile mineral oil or liquid paraffin to create anaerobic condition.
- The second tube is left without oil overlay to maintain aerobic condition.
- The tubes are incubated at 35–37°C for 24–48 hours.
- The tubes are observed daily for colour change from green to yellow indicating acid production.
- In slow growing organisms incubation may be continued for several days before final result is recorded.
This procedure is followed according to the method described by Hugh and Leifson.
Interpretation and results of OF (Oxidation-Fermentation) Test

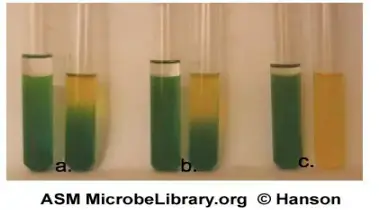

Oxidative reaction
- The open tube shows colour change from green to yellow while the oil-covered tube remains green.
- This indicates acid production only in presence of oxygen.
- The organism utilizes carbohydrate by oxidative pathway.
- Example – Pseudomonas aeruginosa.
Fermentative reaction
- Both open tube and oil-covered tube show colour change from green to yellow.
- This indicates acid production in both aerobic and anaerobic conditions.
- The organism utilizes carbohydrate by fermentative pathway.
- Example – Escherichia coli.
Non-saccharolytic reaction
- No yellow colour is produced in both tubes.
- The oil-covered tube remains green and the open tube may remain green or turn blue due to alkaline reaction.
- This indicates the organism does not utilize carbohydrate and metabolizes peptone instead.
- Example – Alcaligenes faecalis.
Quality Control of OF (Oxidation–Fermentation) Test
- Fermentative control organism is inoculated into two OF medium tubes.
Expected result is colour change from green to yellow in both open and oil-covered tubes indicating acid production. - Oxidative control organism is inoculated into two OF medium tubes.
Expected result is yellow colour in open tube only while oil-covered tube remains green. - Non-saccharolytic control organism is inoculated into two OF medium tubes.
Expected result is no colour change in oil-covered tube and open tube remains green or turns blue due to alkaline reaction. - Uninoculated control tubes with and without oil overlay are incubated along with test tubes.
No colour change should be observed after incubation. - The prepared medium should appear greenish blue and free from turbidity before inoculation.
- The pH of the medium should be maintained around neutral range before use for accurate result.
Precautions of OF (Oxidation–Fermentation) Test
- The inoculating needle should be cooled properly after flaming before touching the culture to avoid killing the organism.
- A fresh and pure culture of the test organism should be used for inoculation.
- Sterile mineral oil or liquid paraffin used should be non-acidic to prevent false positive reaction.
- The oil layer in anaerobic tube should be sufficient to completely cover the surface of the medium.
- The cap of the open tube should be kept loose to allow oxygen entry during incubation.
- The carbohydrate solution should be sterilized properly and added aseptically to the medium.
- The inoculum should be light and a straight stab should be made in the centre of the medium.
- The tubes should not be interpreted before adequate incubation period.
- The surface of the open tube should be observed carefully as oxidative reaction begins from top.
- Proper incubation temperature should be maintained throughout the test.
Uses of OF (Oxidation–Fermentation) Test
- It is used to determine whether bacteria utilize carbohydrate by oxidative or fermentative pathway.
- It helps in identification and classification of Gram-negative bacteria based on glucose metabolism.
- It is used to differentiate fermentative Enterobacteriaceae from oxidative non-fermenters.
- It helps in identification of non-fermenting Gram-negative bacilli such as Pseudomonas aeruginosa, Acinetobacter and Burkholderia cepacia.
- It is useful for detecting non-saccharolytic organisms like Alcaligenes faecalis which do not utilize carbohydrate.
- It can be used to test utilization of other carbohydrates apart from glucose.
- It helps in differentiating Gram-positive cocci such as Staphylococcus and Micrococcus in modified OF media.
- It may also indicate motility due to semisolid nature of the medium.
- It is widely used in routine diagnostic microbiology laboratories for bacterial identification.
Advantages of OF (Oxidation–Fermentation) Test
- It helps in detection of weak acid production by oxidative organisms due to low peptone and high carbohydrate concentration.
- It allows clear differentiation between fermentative and oxidative bacteria.
- It is useful in identification of non-fermenting Gram-negative bacilli such as Pseudomonas aeruginosa, Acinetobacter and Burkholderia cepacia.
- The semisolid nature of the medium helps in concentration of acid at the site of reaction making colour change easily visible.
- Motility of organism can also be observed in the same medium due to semisolid agar.
- It can be used with different carbohydrates other than glucose for metabolic study.
- It is a simple and reliable test for routine laboratory identification of bacteria.
Limitations of OF (Oxidation–Fermentation) Test
- It indicates only the metabolic pathway of carbohydrate utilization and does not identify the organism completely.
- Slow growing organisms may require prolonged incubation before giving visible reaction.
- The medium may not support growth of fastidious organisms leading to false negative result.
- Acidic mineral oil can produce false positive reaction by lowering the pH of the medium.
- Improper inoculum size may affect the result and give misleading interpretation.
- Excess acid production may inhibit motility and lead to wrong motility observation.
- Alkaline products formed due to peptone utilization may mask weak acid production.
- Further biochemical tests are required for confirmation of bacterial identity.
- Bugno, A., & Pinto, T. J. A. (2003). The Influence of Incubation Conditions in Sterility Tests. PDA Journal of Pharmaceutical Science and Technology, 57(6), 399–403.
- Bouvet, P. J., & Bouvet, O. M. (1989). Glucose dehydrogenase activity in Acinetobacter species. Research in Microbiology, 140(8), 531–540. https://doi.org/10.1016/0923-2508(89)90085-5
- Conway, T. (1992). The Entner-Doudoroff pathway: history, physiology and molecular biology. FEMS Microbiology Reviews, 103(1), 1–28.
- Dahal, P. (2023, March 6). OF Test- Oxidation/Oxidative-Fermentation/Fermentative Test. Microbe Notes.
- [deleted]. (2015). Inoculation Failure [Online forum post]. Reddit. https://www.reddit.com/r/microbiology/comments/6mp7bh/inoculation_failure/
- DrChika. (2023, January 2). BAIRD–PARKER AGAR. Microbiology Class. https://microbiologyclass.net/baird-parker-agar/
- El Husseini, N., Mekonnen, S. A., Hall, C. L., Cole, S. J., Carter, J. A., Belew, A. T., El-Sayed, N. M., & Lee, V. T. (2024). Characterization of the Entner-Doudoroff pathway in Pseudomonas aeruginosa catheter-associated urinary tract infections. Journal of Bacteriology, 206(1), e00361-23. https://doi.org/10.1128/jb.00361-23
- Gautam, V., Singhal, L., & Ray, P. (2011). Burkholderia cepacia complex: Beyond pseudomonas and acinetobacter. Indian Journal of Medical Microbiology, 29(1), 4–12. https://doi.org/10.4103/0255-0857.76516
- Gupta, N., Gandham, N., Jadhav, S., & Mishra, R. N. (2015). Isolation and identification of Acinetobacter species with special reference to antibiotic resistance. Journal of Natural Science, Biology and Medicine, 6(1), 159–162. https://doi.org/10.4103/0976-9668.149116
- Häfliger, E., Atkinson, A., & Marschall, J. (2020). Systematic review of healthcare-associated Burkholderia cepacia complex outbreaks: presentation, causes and outbreak control. Infection Prevention in Practice, 2(3), 100082. https://doi.org/10.1016/j.infpip.2020.100082
- Hanson, A. (2008, September 8). Oxidative-Fermentative Test Protocol. American Society for Microbiology.
- He, L., Groom, J. D., & Lidstrom, M. E. (2021). The Entner-Doudoroff Pathway Is an Essential Metabolic Route for Methylotuvimicrobium buryatense 5GB1C. Applied and Environmental Microbiology, 87(3), e02481-20. https://doi.org/10.1128/AEM.02481-20
- Henderson, T. (2025, November 7). Sterility Testing Guide: Direct Inoculation vs. Membrane Filtration. Contract Laboratory.
- HiMedia Laboratories. (2017). Hugh Leifson Medium [Technical Data, M826].
- HiMedia Laboratories. (2018). Baird Parker Agar Base [Technical Data].
- HiMedia Laboratories. (2024). Hugh Leifson Medium [Technical Data, M826S].
- Huang, C. (2020). Extensively drug-resistant Alcaligenes faecalis infection. BMC Infectious Diseases, 20, 833. https://doi.org/10.1186/s12879-020-05557-8
- Kim, S.-H., & Mun, S. J. (2025). Comparative Analysis of Clinical Characteristics and Antimicrobial Resistance Between Acinetobacter baumannii and Other Acinetobacter Species. Pathogens, 14(1), 46. https://doi.org/10.3390/pathogens14010046
- Merck KGaA. (n.d.). 11705 Baird Parker Agar [Data sheet]. Sigma-Aldrich.
- Peekhaus, N., & Conway, T. (1998). What’s for Dinner?: Entner-Doudoroff Metabolism in Escherichia coli. Journal of Bacteriology, 180(14), 3495–3502. https://doi.org/10.1128/jb.180.14.3495-3502.1998
- Periaiah, P., Antony, T., & Samuel, S. (2024). Identification of Burkholderia cepacia Complex: Comparing Conventional, Automated, and Molecular Methods in a Tertiary Care Center. Cureus, 16(10), e70847. https://doi.org/10.7759/cureus.70847
- PharmaState Academy. (2017, November 7). Why Sterility Test Require 14 Days of Long Incubation Time.
- Plongla, R., Panagea, T., Pincus, D. H., Jones, M. C., & Gilligan, P. H. (2016). Identification of Burkholderia and Uncommon Glucose-Nonfermenting Gram-Negative Bacilli Isolated from Patients with Cystic Fibrosis by Use of Matrix-Assisted Laser Desorption Ionization–Time of Flight Mass Spectrometry (MALDI-TOF MS). Journal of Clinical Microbiology, 54(12), 3071–3072. https://doi.org/10.1128/JCM.01623-16
- Sandle, T. (2015, March 2). Oxidation-fermentation test explained. Pharmaceutical Microbiology Resources.
Sandle, T. (2018, October 29). Burkholderia cepacia complex: review of origins, risks and test methodologies. European Pharmaceutical Review. - Shamsi, S. S., Elzen, A. A., & Ahmad, K. M. (2021). Burkholderia cepacia Complex, an Emerging Nosocomial Pathogen at Health Care Facilities in Sebha, Libya. Journal of Medical Microbiology and Infectious Diseases, 9(4), 178–184. https://doi.org/10.52547/JoMMID.9.4.178
- Siegrist, J. (2007). Pseudomonas Media and Tests. Sigma-Aldrich.
- Tankeshwar, A. (2022, August 10). Oxidative fermentative (OF) test: Principle, Procedure, Results. Microbe Online.
- Thermo Fisher Scientific. (2018). OF King Medium w/ and w/o Carbohydrates [Instructions for Use].
- TM Media. (2019). TM 125 – Hugh Leifson Medium [Product Data Sheet].
- Valency Lab Equipments. (2024, October 5). Top 5 Common Mistakes When Using Petri Dishes And How To Avoid Them.
- Watson, R. (n.d.). Tests used to identify Gram Negative Bacteria. University of Wyoming. https://www.uwyo.edu/molb2210_lab/info/biochemical_tests.htm
- Wikipedia contributors. (2022, November 8). Oxidative/fermentation glucose test. Wikipedia. https://en.wikipedia.org/wiki/Oxidative/fermentation_glucose_test
- Wikipedia contributors. (2025, January 10). Baird-Parker agar. Wikipedia. https://en.wikipedia.org/w/index.php?title=Baird-Parker_agar
- Xu, N., Zuo, J., Li, C., Gao, C., & Guo, M. (2024). Reconstruction and Analysis of a Genome-Scale Metabolic Model of Acinetobacter lwoffii. International Journal of Molecular Sciences, 25(17), 9321. https://doi.org/10.3390/ijms25179321
- Yelimeshyn, S. (2025, December 24). Common Mistakes When Working with Petri Dishes and How to Avoid Them. BostonMed Supply.
- Your Article Library. (n.d.). Oxidation-Fermentation Test on Bacteria to find-out their Ability to Utilize Glucose.
- Text Highlighting: Select any text in the post content to highlight it
- Text Annotation: Select text and add comments with annotations
- Comment Management: Edit or delete your own comments
- Highlight Management: Remove your own highlights
How to use: Simply select any text in the post content above, and you'll see annotation options. Login here or create an account to get started.