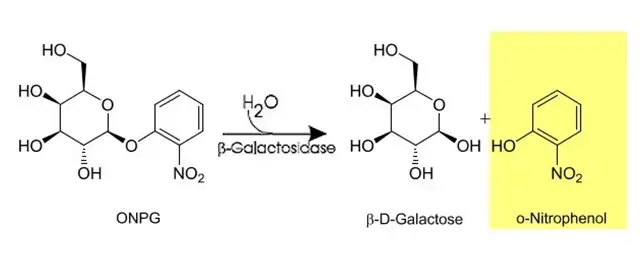
The o-Nitrophenyl-β-D-Galactopyranoside (ONPG) test is a biochemical test used for the detection of the enzyme β-galactosidase in bacteria. It is mainly applied for differentiating late lactose fermenting organisms from non-lactose fermenters. It is based on the ability of the organism to hydrolyse a synthetic lactose analogue when the required enzyme is present. The test is especially useful when organisms fail to ferment lactose rapidly on routine culture media.
Lactose fermentation normally requires two enzymes. One enzyme is β-galactoside permease which helps in the transport of lactose into the bacterial cell and the other enzyme is β-galactosidase which splits lactose into glucose and galactose. In some bacteria β-galactosidase is present but permease is absent. ONPG is a colourless compound that can enter the bacterial cell without the help of permease. Once inside the cell it is acted upon by β-galactosidase and is hydrolysed into galactose and o-nitrophenol. The o-nitrophenol produced gives a yellow colour which indicates a positive test result.
The test is performed by inoculating a heavy bacterial suspension into a medium containing ONPG and incubating it at suitable temperature. If β-galactosidase is present the colourless medium turns yellow within one to twenty-four hours. A yellow colour indicates a positive ONPG test whereas absence of colour change is taken as a negative result. Thus the ONPG test helps in distinguishing late lactose fermenters from true non-lactose fermenting organisms based on enzyme activity rather than sugar fermentation.
Objectives of ONPG Test
- To detect the β-galactosidase activity in organisms by using ONPG as the substrate.
- To identify the late lactose fermenters that lack the permease enzyme but have β-galactosidase.
- To differentiate members of Enterobacteriaceae on the basis of ONPG positivity.
- To identify Neisseria lactamica from other Neisseria species which are ONPG negative.
- To classify some non-fermenting Gram-negative bacilli that show ONPG activity.
- To differentiate species of Vibrionaceae where Vibrio cholerae is ONPG positive and Vibrio parahaemolyticus is negative.
Principle of ONPG Test
It is the principle of the ONPG test that the presence of β-galactosidase enzyme is detected by using a synthetic substrate which is o-Nitrophenyl-β-D-Galactopyranoside (ONPG). It is the process where ONPG acts as a structural analogue of lactose and it can enter inside the bacterial cell without the requirement of permease. In this step the organism that has β-galactosidase will hydrolyse the colorless ONPG molecule into galactose and o-nitrophenol. The reaction is as follows– ONPG → Galactose + o-Nitrophenol. It is the o-nitrophenol that is released and gives a yellow colour in the solution. These are the bacteria referred to as late lactose fermenters because permease is absent or very less active though β-galactosidase is present. This process occurs when ONPG is cleaved directly by the enzyme, and the yellow colour formation is the indication of a positive result.
Requirements of ONPG Test
- A fresh bacterial culture is required, and it is taken as a heavy suspension for the test.
- The organism is generally grown on lactose-containing medium for inducing the β-galactosidase enzyme.
- Known positive and negative control strains are needed for checking the validity of the test.
- ONPG substrate is required in the form of broth, disks, or tablets.
- Sterile saline or distilled water is used for emulsifying the colonies before adding ONPG.
- A suitable buffer may be needed when the medium is not pre-buffered.
- Toluene can be used in some rapid methods for releasing the intracellular enzyme.
- Sterile test tubes are required for preparing the reaction mixture.
- A sterile inoculating loop is used for transferring the bacterial growth.
- An incubator or water bath is needed to maintain 35°C to 37°C for the reaction.
- A McFarland standard can be used when turbidity adjustment of the inoculum is required.
ONPG broth Composition
| Ingredients | Gms / Litre |
| Casitose (Equivalent to Casein Peptone) | 7.500 |
| O-Nitrophenyl-β-D-galactopyranoside | 1.500 |
| Disodium hydrogen phosphate | 0.350 |
| Sodium chloride | 3.750 |
| Final pH ( at 25°C) | 7.5±0.2 |
ONPG Test Procedure
Preparation of Inoculum
- The test organism is grown for 18–24 hours on lactose containing medium such as Triple Sugar Iron agar, Kligler Iron Agar or MacConkey agar.
- Culture grown on glucose containing medium is avoided as glucose inhibits synthesis of β-galactosidase enzyme.
- A heavy suspension of the organism is prepared in sterile physiological saline (0.85% NaCl) or sterile distilled water. The turbidity should be equivalent to McFarland 2 or 3 standard.
- Heavy inoculum is required for rapid reaction and proper enzyme activity.
Inoculation (Any one method is followed)
Method A – ONPG Disk / Tablet Method
- One ONPG disk or tablet is placed into a sterile test tube.
- About 0.1–0.5 mL of sterile physiological saline or distilled water is added to the tube.
- A large amount of bacterial colony is picked with sterile loop and emulsified properly in the tube containing ONPG disk and liquid.
Method B – ONPG Broth Method
- The ONPG broth is brought to room temperature.
- The broth is heavily inoculated with isolated bacterial colonies.
Method C – Rapid Toluene Method
- A large loopful of culture is emulsified in 0.25 mL of physiological saline.
- One drop of toluene is added and the tube is shaken vigorously to disrupt the cell membrane and release enzyme.
- The tube is allowed to stand for 5 minutes at 37°C and then 0.25 mL of buffered ONPG solution is added.
Incubation and Observation
- The inoculated tubes are incubated aerobically at 35–37°C.
- The tubes are observed after 1 hour for colour development.
- If no colour change is observed, incubation is continued up to 24 hours to detect late lactose fermenters.
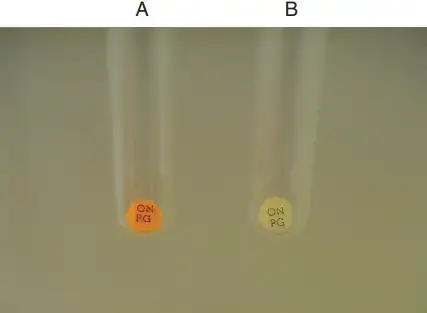
ONPG Test Result
Positive Result
- The solution turns yellow in colour.
- It indicates that the organism produces β-galactosidase enzyme.
- The enzyme hydrolyses the colourless ONPG substrate and releases o-nitrophenol which is yellow in colour.
- It indicates lactose fermenter including late lactose fermenters which possess β-galactosidase but lack permease enzyme.
- Common examples are Escherichia coli, Klebsiella spp., Enterobacter spp., Citrobacter spp., Serratia spp., Vibrio cholerae and Neisseria lactamica.
Negative Result
- The solution remains colourless even after incubation.
- It indicates absence of β-galactosidase enzyme in the organism.
- The organism is considered as true non-lactose fermenter.
- Common examples are Proteus spp., Providencia spp., Salmonella spp. (e.g. S. Typhimurium) and Pseudomonas aeruginosa (except serogroup O:11).
Observation Timing
- Rapid positive reaction shows yellow colour within few minutes to few hours due to strong enzyme activity.
- Late positive reaction is detected after incubation up to 24 hours and indicates late lactose fermenters.
ONPG Test – List of Organisms
ONPG Positive Organisms
- Escherichia coli
- Citrobacter spp.
- Klebsiella spp. (except Klebsiella rhinoscleromatis)
- Enterobacter spp.
- Serratia spp.
- Shigella sonnei
- Shigella dysenteriae serotype 1
- Salmonella subgenus II
- Salmonella subgenus III (Arizonae group)
- Salmonella choleraesuis
- Hafnia spp.
- Yersinia spp.
- Erwinia carotovora
- Levinea malonatica and Levinea amalonatica
- Neisseria lactamica
- Vibrio cholerae
- Aeromonas hydrophila
- Aeromonas sobria
- Plesiomonas shigelloides
- Burkholderia cepacia
- Stenotrophomonas maltophilia
- Pseudomonas aeruginosa serogroup O:11
ONPG Negative Organisms
- Proteus spp.
- Providencia spp.
- Morganella spp.
- Salmonella spp. (most serotypes including S. Typhimurium and S. paratyphi A)
- Shigella spp. (except S. sonnei and S. dysenteriae type 1)
- Edwardsiella spp.
- Alcalescens dispar
- Neisseria gonorrhoeae
- Neisseria meningitidis
- Pseudomonas aeruginosa (other serogroups)
- Vibrio parahaemolyticus
- Vibrio alginolyticus
Quality Control of ONPG Test
Test Frequency and Reagent Inspection
- Quality control is performed with every new batch of ONPG reagent, broth or disk before routine use.
- In laboratories with high workload, quality control is carried out at least once in a week to check substrate stability.
- Before inoculation, ONPG solution or disk is examined visually.
- If the substrate appears yellow in colour before use, it is discarded as it indicates spontaneous hydrolysis and may give false positive result.
Control Organisms (Enterobacteriaceae)
- Positive control (Rapid) – Escherichia coli (ATCC 25922 or NCTC 10418) is used to show rapid yellow colour development.
- Positive control (Late) – Citrobacter freundii (ATCC 8090) is used to confirm detection of late lactose fermenters.
- Negative control – Proteus vulgaris (ATCC 13315) or Proteus mirabilis (ATCC 12453 or NCTC 10975) is used to demonstrate negative reaction.
- Negative control (Food safety) – Salmonella Typhimurium (ATCC 14028) is commonly used in food microbiology laboratories.
Control Organisms (Neisseria)
- Positive control – Neisseria lactamica (ATCC 23971 or NCTC 10617).
- Negative control – Neisseria gonorrhoeae (ATCC 43069 or NCTC 8375).
Expected Results
- Positive control shows yellow colour within incubation period indicating presence of β-galactosidase enzyme.
- Negative control remains colourless or pale yellow indicating absence of β-galactosidase enzyme.
Precautions of ONPG Test
- A heavy inoculum is used for the test as light inoculum may give false negative result.
- The test organism is grown on lactose containing medium to induce β-galactosidase enzyme production.
- Culture grown on glucose containing medium is avoided as glucose suppresses enzyme synthesis.
- Fresh culture of 18–24 hours is used and old culture is avoided due to reduced enzyme activity.
- ONPG reagent or disk is examined before use and discarded if yellow colour is already present.
- The test is not performed on yellow pigmented organisms as colour change may be masked.
- Nichrome or stainless steel inoculating loop is not used as it may give false positive reaction.
- ONPG broth is not autoclaved because the substrate is heat sensitive.
- Proper buffering of ONPG solution is maintained to avoid false positive or false negative result.
- Known positive and negative control organisms are tested with every new batch of reagent.
Uses of ONPG Test
- It is used to detect production of β-galactosidase enzyme in microorganisms.
- It helps in identification of late lactose fermenters which possess β-galactosidase but lack permease enzyme.
- It is used for differentiation of members of Enterobacteriaceae especially Citrobacter spp. from Salmonella spp.
- It is useful in identification of Neisseria lactamica and differentiation from other Neisseria species.
- It aids in characterization of non-fermenting gram negative bacilli such as Stenotrophomonas maltophilia and Burkholderia cepacia.
- It is used to identify Pseudomonas aeruginosa serogroup O:11 which shows ONPG positive reaction.
- It helps in differentiation of Vibrio cholerae from Vibrio parahaemolyticus.
- It is also used in clinical chemistry for estimation of serum sodium using sodium dependent β-galactosidase system.
Advantages of ONPG Test
- It is useful for detection of late lactose fermenters which appear negative on routine lactose fermentation media.
- The test does not require permease enzyme as ONPG enters the bacterial cell directly.
- It gives rapid results within few minutes to few hours as compared to conventional lactose fermentation tests.
- It helps in differentiation of Enterobacteriaceae especially Citrobacter spp. from Salmonella spp.
- It is useful in identification of Neisseria lactamica and differentiation from other Neisseria species.
- It aids in identification of certain non-fermenting gram negative bacilli such as Stenotrophomonas maltophilia and Burkholderia cepacia.
- It is simple to perform and available in disk, tablet and broth form which makes the test convenient.
Limitations of ONPG Test
- The test cannot be performed on yellow pigmented organisms as the colour change may be masked.
- Organisms grown on glucose containing medium may give false negative result due to repression of β-galactosidase enzyme.
- A light inoculum may result in false negative reaction and hence heavy inoculum is required.
- Use of nichrome or stainless steel inoculating loop may lead to false positive reaction.
- ONPG reagent is unstable and may undergo spontaneous hydrolysis if exposed to heat light or moisture.
- The test only detects β-galactosidase activity and is not sufficient for complete identification of organism.
- Rarely ONPG may be hydrolysed by enzymes other than β-galactosidase which may affect interpretation.
- Alfa Chemistry. (n.d.). β-Galactosidase in in vitro diagnostics: Precision enzymology for clinical sodium detection.
- Aryal, S. (2022, August 10). ONPG test – Principle, procedure, uses and interpretation. Microbiology Info. https://microbiologyinfo.com/onpg-test/
- Bio-Rad. (2003). O.N.P.G. 53822: Disks for testing for β-galactosidase [Product insert].
- Comprehensive analysis of the o-Nitrophenyl-β-D-Galactopyranoside (ONPG) test: Principles, methodological frameworks, and clinical diagnostic significance. [Source text].
- Dahal, P. (2023, March 31). ONPG test- Principle, procedure, results, uses. Microbe Notes. https://microbenotes.com/o-nitrophenyl-%CE%B2-d-galactopyranoside-onpg-test/
- Gohara, D. W., & Di Cera, E. (2016). Molecular mechanisms of enzyme activation by monovalent cations. Journal of Biological Chemistry, 291(40), 20840–20848. https://doi.org/10.1074/jbc.R116.737833
- HiMedia Laboratories. (2014). ONPG broth (M1930) [Technical Data].
- HiMedia Laboratories. (2022). ONPG discs (DD008) [Technical Data].
- Juers, D. H., Matthews, B. W., & Huber, R. E. (2012). LacZ β-galactosidase: Structure and function of an enzyme of historical and molecular biological importance. Protein Science, 21(12), 1792–1807. https://doi.org/10.1002/pro.2165
- Juers, D. H., Rob, B., Dugdale, M. L., Rahimzadeh, N., Giang, C., Lee, M., Matthews, B. W., & Huber, R. E. (2009). Direct and indirect roles of His-418 in metal binding and in the activity of β-galactosidase (E. coli). Protein Science, 18(6), 1281–1292. https://doi.org/10.1002/pro.140
- Key Scientific Products. (n.d.). K490/K1490 ONPG test [Product insert].
- Oliveira, J., & Reygaert, W. C. (2023). Gram-negative bacteria. In StatPearls. StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK538213/
- Public Health England. (2014). ONPG (β-galactosidase) test (UK Standards for Microbiology Investigations TP 24 Issue 3).
- Sigma-Aldrich. (n.d.). 49940 ONPG disks (2-Nitrophenyl β-D-galactopyranoside disks, β-galactosidase test disks) [Product information].
- Tankeshwar, A. (n.d.). ONPG (β-galactosidase) test: Principle, procedure and results. Microbe Online. https://microbeonline.com/onpg-test-galactosidase-principle-procedure-results/
- Thermo Fisher Scientific. (2010). ONPG broth [Instructions for Use]. Remel.
- TM Media. (n.d.). TM 1918 – ONPG broth [Product Data Sheet].
- UK Health Security Agency. (2025). ONPG (β-galactosidase) test (UK Standards for Microbiology Investigations TP 24 Issue 4.1).
- U.S. Food and Drug Administration. (2001). BAM R53: ONPG test. Bacteriological Analytical Manual.
- Vinothkumar, K. R., McMullan, G., & Henderson, R. (2014). Molecular mechanism of antibody-mediated activation of β-galactosidase. Structure, 22(4), 621–627. https://doi.org/10.1016/j.str.2014.01.011