The Lactobacillus MRS Broth Test is a microbiological test that is used for the cultivation enrichment and isolation of Lactobacillus species and other lactic acid bacteria. It is based on the use of MRS broth which is a selective liquid culture medium developed by de Man Rogosa and Sharpe in 1960. It is mainly used for organisms that are fastidious in nature and require enriched nutrients for proper growth. This medium is commonly applied in food microbiology dairy microbiology and clinical laboratories.
It is the process where the MRS broth provides a rich nutrient base containing proteose peptone beef extract and yeast extract which support the growth of lactic acid bacteria. Dextrose is present as a fermentable carbohydrate source for energy production. The medium also contains Polysorbate 80 (Tween 80) which supplies fatty acids required for membrane synthesis. Certain salts like magnesium and manganese are included which acts as essential growth factors for Lactobacillus species.
The selectivity of the MRS broth is maintained by the presence of sodium acetate and ammonium citrate. These components inhibit the growth of unwanted microorganisms such as Gram negative bacteria molds and many other competing flora. Due to this selective nature the growth of lactic acid bacteria is favored while other organisms is suppressed. This is referred to as selective enrichment medium.
In laboratory practice the test is performed by inoculating the given sample into the MRS broth followed by incubation generally at 35°C to 37°C for 24 to 48 hours. A positive result is indicated by the development of turbidity in the broth which shows bacterial growth. Clear broth indicates absence of growth. In some cases a Durham tube is placed inverted inside the broth to detect gas production.
If gas bubbles are observed in the Durham tube it indicates gas producing heterofermentative organisms such as Leuconostoc species. Absence of gas formation generally indicates homofermentative organisms such as most Lactobacillus species. Thus the Lactobacillus MRS Broth Test is useful not only for cultivation but also for preliminary differentiation of lactic acid bacteria based on gas production.
Principle of MRS Broth Test
The principle of Lactobacillus MRS Broth Test is based on providing a selective and enriched liquid medium that supports the growth of Lactobacillus species and other lactic acid bacteria while inhibiting the growth of competing microorganisms. It is the process where a nutrient rich environment is created using proteose peptone beef extract and yeast extract which supplies nitrogen vitamins and other essential growth substances. Dextrose (glucose) is present as the main fermentable carbohydrate source and it is utilised by lactic acid bacteria for energy production.
The medium also contains Polysorbate 80 (Tween 80) which acts as a source of fatty acids required for cellular metabolism and membrane synthesis. Certain salts such as magnesium sulphate and manganese sulphate are included which act as essential growth factors for Lactobacillus species. These components together promote luxuriant growth of fastidious lactic acid bacteria.
The selective nature of the MRS broth is due to the presence of ammonium citrate and sodium acetate. These substances suppress the growth of Gram negative bacteria molds and other commensal organisms that may interfere with the growth of lactobacilli. As a result the target organisms grow preferentially in the medium.
A positive test result is indicated by the development of turbidity in the broth due to bacterial multiplication. In some cases a Durham tube is placed in the broth to detect gas formation. The presence or absence of gas helps in differentiating heterofermentative and homofermentative lactic acid bacteria.
Objectives of MRS Broth Test
- To support the luxuriant growth of Lactobacillus species including fastidious strains that do not grow well on simple media.
- To isolate lactobacilli from different sources such as dairy products food oral cavity feces and other clinical or non clinical samples.
- To enrich samples containing lactic acid bacteria by increasing their number prior to further identification or testing.
- To determine gas production from glucose fermentation using a Durham tube which helps in differentiating heterofermentative and homofermentative organisms.
- To aid in the differentiation of certain Gram positive cocci based on their ability to produce gas in the medium.
- To enumerate mesophilic lactic acid bacteria especially in food and animal feeding stuffs using methods such as Most Probable Number.
- To detect contamination by spoilage organisms such as Lactobacillus brevis and Lactobacillus fermentum in beverages like beer cider soft drinks and bottled water.
Requirements for MRS Broth Test
Media components
- Proteose peptone – it acts as the major nitrogen source for growth of Lactobacillus species.
- Beef extract – it supplies vitamins minerals and additional growth factors.
- Yeast extract – it provides B-complex vitamins and amino acids required for fastidious organisms.
- Dextrose (glucose) – it is the fermentable carbohydrate source used for energy production.
- Sodium acetate – it acts as a selective agent inhibiting growth of unwanted microorganisms.
- Ammonium citrate – it suppresses Gram negative bacteria and molds.
- Dipotassium phosphate – it helps in maintaining the buffering capacity of the medium.
- Polysorbate 80 (Tween 80) – it supplies fatty acids essential for metabolism and membrane synthesis.
- Magnesium sulphate – it acts as a growth factor and enzyme activator.
- Manganese sulphate – it provides trace elements required for Lactobacillus growth.
- Distilled water – it is used as the base solvent for preparation of the medium.
Laboratory equipment and materials
- Autoclave – for sterilization of prepared medium at 121°C for 15 minutes.
- Test tubes flasks or bottles – for dispensing and holding the broth medium.
- Durham tubes – placed inverted inside the test tube for detection of gas production when required.
- Sterile inoculating loop needle or pipette – for transferring the test sample into the broth.
- pH meter – to check and adjust the final pH of the medium (around 6.2 to 6.5).
Incubation requirements
- Incubator – maintained between 30°C and 37°C for optimum growth.
- Atmospheric condition – aerobic conditions are generally sufficient though some organisms grow better in reduced oxygen.
- Incubation time – usually 24 to 48 hours and may extend up to 5 days depending on the organism.
MRS Broth Composition
| Dextrose | 20.0gm |
| Peptic Digest of Animal Tissue | 10.0gm |
| Beef Extract | 10.0gm |
| Yeast Extract | 5.0gm |
| Sodium Acetate | 5.0gm |
| Disodium Phosphate | 2.0gm |
| Ammonium Citrate | 2.0gm |
| Polysorbate 80 | 1.0gm |
| Magnesium Sulfate | 0.1gm |
| Manganese Sulfate | 0.05gm |
Final pH 6.5 +/- 0.3 at 25ºC.
Procedure of MRS Broth Test
- Suspend the required amount of dehydrated MRS broth powder (about 51–55 g) in 1000 mL of distilled water.
- Heat the mixture with frequent agitation until the medium is completely dissolved. If Polysorbate 80 (Tween 80) is not included in the powder it is added separately at this stage.
- Dispense the prepared medium into clean test tubes bottles or flasks in required volumes.
- Place a sterile inverted Durham tube into each test tube if gas production is to be detected.
- Sterilize the medium by autoclaving at 121°C for 15 minutes. In some cases a lower temperature may be used for better growth of certain organisms.
- Allow the sterilized medium to cool to room temperature before inoculation.
- Inoculate the broth aseptically with the test sample or with a fresh pure culture (18–24 hours old).
- Incubate the inoculated tubes at 35°C to 37°C under aerobic conditions.
- Maintain incubation for 24 to 48 hours and extend up to 3–5 days for slow growing strains if required.
- Observe the tubes for turbidity which indicates bacterial growth. Presence of gas bubble in Durham tube indicates gas producing organisms.
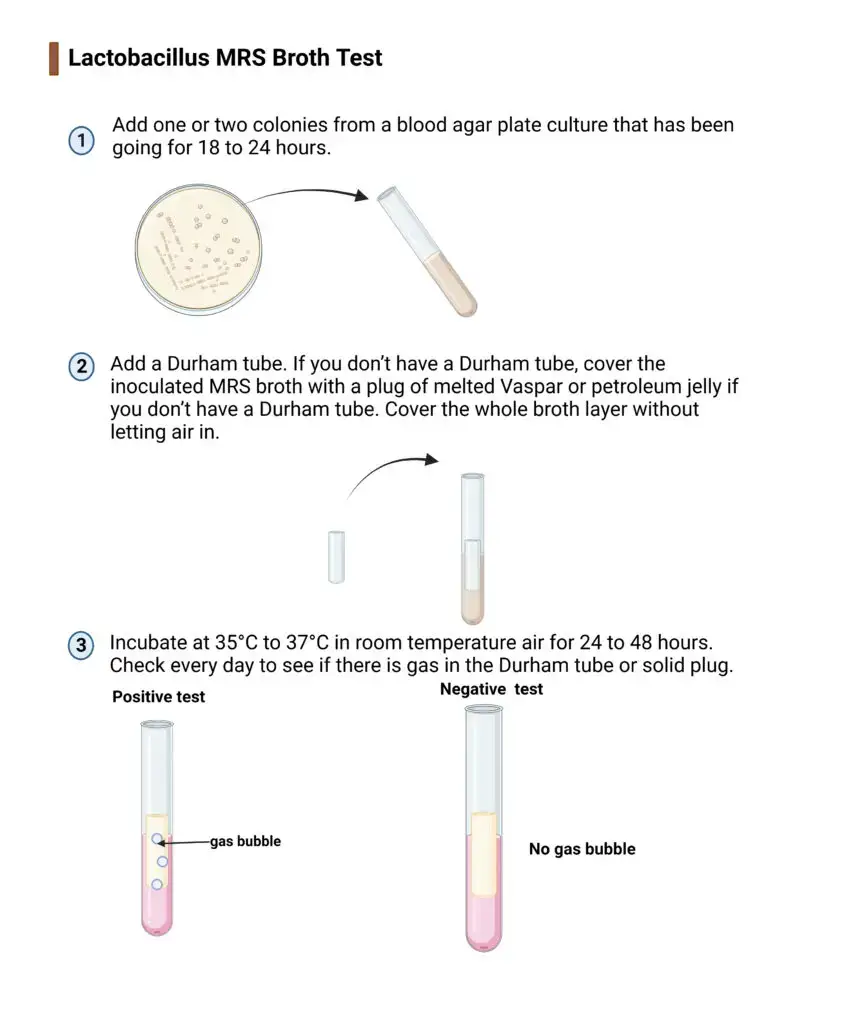
Result of MRS Broth Test
Positive result
The development of turbidity in the MRS broth indicates growth of the organism. This shows that the bacteria is able to utilize the nutrients present in the medium and ferment dextrose in the presence of selective agents. Turbid appearance of the broth is taken as a positive result for lactic acid bacteria growth.
Gas production
If a Durham tube is used and a gas bubble is observed inside it then the organism is gas producing in nature. This indicates heterofermentative organisms such as Leuconostoc species. Gas formation occurs due to fermentation of glucose with release of carbon dioxide.
No gas production
When turbidity is present in the broth but no gas bubble is seen in the Durham tube it indicates growth of homofermentative organisms. Most Lactobacillus species and Pediococcus shows this type of result.
Negative result
If the broth remains clear without any turbidity and without gas formation it indicates that the organism is unable to grow in MRS broth. This is considered as a negative result.
Remarks
Although MRS broth is selective in nature some non target organisms may also show growth. Therefore further biochemical and confirmatory tests are required for definitive identification.
List of organisms showing result in MRS Broth Test
Organisms showing positive result (growth)
Growth without gas production
- Lactobacillus acidophilus
- Lactobacillus casei
- Lactobacillus plantarum
- Lactobacillus delbrueckii subsp. lactis
- Lactobacillus johnsonii
- Lactobacillus rhamnosus
- Lactobacillus sakei
- Lactococcus lactis
- Pediococcus acidilactici
- Pediococcus pentosaceus
Growth with gas production
- Lactobacillus fermentum
- Lactobacillus brevis
- Leuconostoc mesenteroides
- Weissella confusa
- Weissella paramesenteroides
Growth under special conditions
- Bifidobacterium bifidum (growth occurs under anaerobic conditions)
Organisms showing negative result (no growth or inhibited)
- Pseudomonas aeruginosa
- Staphylococcus aureus
- Bacillus cereus
- Enterococcus faecalis
- Escherichia coli (poor growth or inhibited depending on formulation)
Quality Control organisms of MRS Broth Test
Positive control organisms (growth)
- Lactobacillus acidophilus ATCC 4356 – luxuriant growth no gas
- Lactobacillus fermentum ATCC 9338 – luxuriant growth gas positive
- Lactobacillus plantarum ATCC 8014 – luxuriant growth
- Lactobacillus casei ATCC 393 – good to very good growth
- Lactobacillus casei ATCC 9595 – luxuriant growth
- Lactobacillus delbrueckii subsp. lactis ATCC 7830 – good recovery
- Lactobacillus johnsonii ATCC 11506 / ATCC 33200 – good growth
- Lactobacillus sakei ATCC 15521 – good growth
- Lactobacillus rhamnosus ATCC 9595 – good growth
- Lactococcus lactis subsp. lactis ATCC 19435 – good growth
- Leuconostoc mesenteroides ATCC 10830 – growth with gas production
- Pediococcus pentosaceus ATCC 33316 / ATCC 29358 – good growth
- Pediococcus acidilactici ATCC 33314 – good growth no gas
- Weissella paramesenteroides ATCC 33313 – good growth gas positive
- Bifidobacterium bifidum ATCC 11863 – good growth under anaerobic condition
Negative or selectivity control organisms (inhibited or no growth)
- Pseudomonas aeruginosa ATCC 27853 – no growth
- Staphylococcus aureus ATCC 25923 – inhibited
- Bacillus cereus ATCC 11778 – total inhibition
- Enterococcus faecalis ATCC 29212 – no growth
- Escherichia coli ATCC 25922 / ATCC 8739 – inhibited or poor growth depending on formulation
Limitations of MRS Broth Test
- The medium shows low selectivity and may allow growth of organisms other than Lactobacillus such as Pediococcus Leuconostoc and Streptococcus species.
- Some Gram negative bacteria like Escherichia coli may also show fair growth which interferes with interpretation.
- Confirmatory tests are required as growth in MRS broth alone cannot confirm the organism as Lactobacillus species.
- Certain Lactobacillus strains have variable nutritional requirements and may show poor growth or no growth in this medium.
- Bifidobacterium species may require modified sterilization temperature for optimal growth which limits routine use.
- Rapid accumulation of lactic acid and depletion of nutrients may cause self inhibition during heavy growth.
- The medium is relatively costly due to the presence of meat extract and yeast extract.
- Standard sterilization at high temperature may affect pH and degrade some heat sensitive components.
- Gas detection using Durham tubes may sometimes create technical difficulties in heavy gas producing organisms.
- The medium is not intended for diagnostic use in human disease conditions.
Advantages of MRS Broth Test
- It supports luxuriant growth of Lactobacillus species including fastidious strains that do not grow well on ordinary culture media.
- It provides a standardized and consistent medium composition which gives reproducible and reliable results during cultivation and enumeration.
- It contains selective agents such as sodium acetate and ammonium citrate which inhibit the growth of contaminating microorganisms while allowing lactobacilli to grow.
- It supplies essential growth factors like Polysorbate 80 and metallic ions such as magnesium and manganese which are required for normal metabolism of lactic acid bacteria.
- It allows differentiation between homofermentative and heterofermentative lactic acid bacteria when used with a Durham tube.
- It can be used for isolation and cultivation of lactobacilli from a wide range of sources such as dairy products fermented foods feces and oral samples.
- It is widely accepted as a reference or standard medium for laboratory studies and quality control of lactic acid bacteria.
Uses of MRS Broth Test
- It is used for enrichment and cultivation of Lactobacillus species including fastidious strains that show poor growth on ordinary media.
- It is used for isolation of lactobacilli from clinical samples oral cavity fecal samples dairy products and other food materials.
- It is used to differentiate organisms based on gas production during glucose fermentation with the help of a Durham tube.
- It helps in distinguishing heterofermentative organisms such as Leuconostoc and Weissella from homofermentative Lactobacillus species.
- It is used for differentiation of certain Gram positive cocci based on their growth and gas forming ability.
- It is used for enumeration of mesophilic lactic acid bacteria from food and animal feed using methods like Most Probable Number.
- It is applied in detection of spoilage lactobacilli in beverages such as beer cider soft drinks and bottled water.
- It is used for preparation of inoculum for further microbiological and biochemical tests.
- Acharya, T. (n.d.). MRS broth test: Principle, procedure, and results. Microbe Online.
- AES Chemunex. (2013). MRS agar [Package insert].
- Alpha Biosciences. (n.d.). Lactobacillus MRS broth w/o dextrose [Product information].
- Aryaee, H. (2020, March 9). Cheaper culture media instead of MRS for probiotic bacteria [Online forum post]. ResearchGate.
- Aryal, S. (2022, August 10). MRS broth test – principle, procedure, uses and interpretation. Microbiology Info.com.
- Brownlie, E. J. E., Chaharlangi, D., Wong, E. O., Kim, D., & Navarre, W. W. (2022). Acids produced by lactobacilli inhibit the growth of commensal Lachnospiraceae and S24-7 bacteria. Gut Microbes, 14(1), 2046452. https://doi.org/10.1080/19490976.2022.2046452
- Chlebicz-Wójcik, A., & Śliżewska, K. (2020). Growth kinetics of probiotic Lactobacillus strains in the alternative, cost-efficient semi-solid fermentation medium. Biology, 9(12), 423. https://doi.org/10.3390/biology9120423
- Comparative analysis of de Man, Rogosa, and Sharpe (MRS) media: Biochemical foundations, industrial standards, and analytical applications in lactic acid bacteria microbiology. (n.d.).
- Cornell CALS Milk Quality Improvement Program. (n.d.). Lactic acid bacteria – Homofermentative and heterofermentative.
- Fitzpatrick, J., Ahrens, M., & Smith, S. (2001). Effect of manganese on Lactobacillus casei fermentation to produce lactic acid from whey permeate. Process Biochemistry, 36(7), 671–675. https://doi.org/10.1016/S0032-9592(00)00265-X
- Formedium. (n.d.). MRS broth [Product information].
- Garg, A. P., Bamal, A., & Goley, R. (2025). A better and cheaper culture medium for isolation of lactic acid bacteria from milk and its products. Biosciences Biotechnology Research Asia, 22(1). http://dx.doi.org/10.13005/bbra/3348
- Grosseron. (n.d.). Lactobacilli MRS broth (Difco & BBL Manual, 2nd ed.) [Product information].
- Hayek, S. A., Gyawali, R., Aljaloud, S. O., Krastanov, A., & Ibrahim, S. A. (2019). Cultivation media for lactic acid bacteria used in dairy products. Journal of Dairy Research, 86(4), 490–502. https://doi.org/10.1017/S002202991900075X
- HiMedia Laboratories. (2015). Lactobacillus MRS broth (MRS broth) M369 [Technical data].
- Huynh, U., Qiao, M., King, J., Trinh, B., Valdez, J., Haq, M., & Zastrow, M. L. (2022). Differential effects of transition metals on growth and metal uptake for two distinct Lactobacillus species. Microbiology Spectrum, 10(1), e01006-21. https://doi.org/10.1128/spectrum.01006-21
- International Organization for Standardization. (1998). Microbiology of food and animal feeding stuffs — Horizontal method for the enumeration of mesophilic lactic acid bacteria — Colony-count technique at 30 °C (ISO Standard No. 15214:1998).
- Lactobacillus. (n.d.). In Wikipedia.
- Leroy, F., & De Vuyst, L. (2001). Growth of the bacteriocin-producing Lactobacillus sakei strain CTC 494 in MRS broth is strongly reduced due to nutrient exhaustion: A nutrient depletion model for the growth of lactic acid bacteria. Applied and Environmental Microbiology, 67(10), 4407–4413. https://doi.org/10.1128/AEM.67.10.4407-4413.2001
Liofilchem. (2022). MRS broth ISO 15214 [Technical sheet]. - McDonald, L. C., McFeeters, R. F., Daeschel, M. A., & Fleming, H. P. (1987). A differential medium for the enumeration of homofermentative and heterofermentative lactic acid bacteria. Applied and Environmental Microbiology, 53(6), 1382–1384.
- Merck KGaA. (2019). GranuCult™ MRS agar (de MAN, ROGOSA and SHARPE) acc. ISO 15214 [Technical data sheet].
- Merck Millipore. (n.d.). MRS broth (Lactobacillus broth acc. to DE MAN, ROGOSA and SHARPE) [Microbiology manual].
- Micromaster Laboratories. (2018). Lactic bacteria differential agar (DM404) [Product specification sheet].
- Micromaster Laboratories. (n.d.). Lactobacillus MRS broth (MRS broth) (DM141) [Product specification sheet].
- Mohammad, G. A., & Taha Daod, S. (2021). Impact of Tween 80 on fatty acid composition in two bacterial species. Archives of Razi Institute, 76(6), 1617–1627. https://doi.org/10.22092/ari.2021.356359.1826
- Nduna, B. (2015, January 27). Which is the best culture medium for growing lactic acid bacteria producing bacteriocin? [Online forum post]. ResearchGate.
- Neogen Corporation. (2025). Neogen® Lactobacilli MRS broth [Product information].
- Remel. (2010). MRS broth [Instructions for use].
- Remel. (2010). MRS broth w/ and w/o Durham tube [Instructions for use].
- Reshape. (n.d.). Method validation report: Enumeration of lactic acid bacteria following ISO 15214; Comparing manual and automated counts [White paper].
- Sigma-Aldrich. (2013). 69966 MRS broth (Lactobacillus broth acc. to De Man, Rogosa and Sharpe) [Product information].
- Sigma-Aldrich. (n.d.). De Man, Rogosa and Sharpe (MRS) broth [Product information].
- Sigma-Aldrich. (n.d.). Microbiology Lactobacillus species medium for Lactobacilli [Product guide].
- Sigma-Aldrich. (n.d.). MRS broth modified, Vegitone, suitable for microbiology, NutriSelect® Plus [Product information].
- Tientcheu, M. L. T., Kaktcham, P. M., Kouam, E. M. F., Piame, L. T., Kamega, L. C. D., Aarzoo, Shekhar, A., Singh, B. P., & Ngoufack, F. Z. (2025). Development of a low-cost culture medium from industrial and environmental by-products for sustainable cultivation of lactic acid bacteria. PLoS ONE, 20(12), e0337684. https://doi.org/10.1371/journal.pone.0337684
- Tong, Y., Zhai, Q., Wang, G., Zhang, Q., Liu, X., Tian, F., Zhao, J., Zhang, H., & Chen, W. (2017). System-wide analysis of manganese starvation-induced metabolism in key elements of Lactobacillus plantarum. RSC Advances, 7, 12959–12968. https://doi.org/10.1039/C7RA00072C