What is Lysine decarboxylase test?
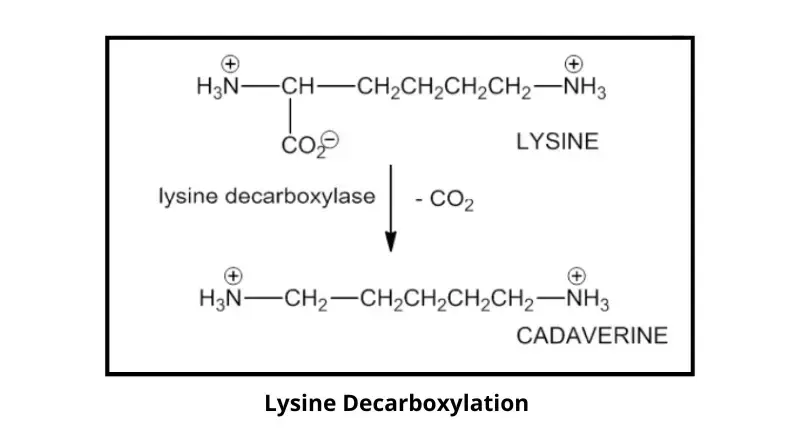
The Lysine decarboxylase test is a biochemical test that is used to identify and differentiate bacteria based on their ability to produce the enzyme lysine decarboxylase. It is mainly applied for the differentiation of members of Enterobacteriaceae family in microbiology laboratory. It is the process in which the amino acid lysine is decarboxylated by removal of carboxyl group (–COOH) resulting in the formation of an alkaline amine known as cadaverine along with carbon dioxide. This change in reaction leads to alteration in the pH of the medium which is used for interpretation of the test.
It is performed by inoculating the test organism into a suitable decarboxylase medium containing glucose lysine peptones and a pH indicator such as bromocresol purple. The medium is overlaid with sterile mineral oil to create anaerobic condition since decarboxylation occurs in absence of oxygen. Initially glucose present in the medium is fermented by bacteria producing acid which lowers the pH and the medium turns yellow. This acidic condition is necessary for induction of lysine decarboxylase enzyme.
If the organism possess lysine decarboxylase enzyme the lysine present in the medium is broken down into cadaverine which is alkaline in nature. Due to this the acidity is neutralized and the medium again turns purple after incubation. This indicates a positive lysine decarboxylase test. If the organism does not produce the enzyme the medium remains acidic and yellow in colour which indicates negative result. A control tube without lysine is usually kept to confirm that the colour change in the test medium is due to lysine metabolism and not because of alkaline end products of glucose fermentation.
This test is important in routine identification of pathogenic bacteria since many organisms shows characteristic reactions in lysine decarboxylase test which helps in their differentiation and confirmation.
Principle of Lysine decarboxylase test
The principle of Lysine decarboxylase test is based on the ability of certain bacteria to produce the enzyme lysine decarboxylase under acidic and anaerobic conditions. It is the process in which lysine present in the medium is decarboxylated by removal of carboxyl group (–COOH) to form an alkaline amine called cadaverine along with carbon dioxide. The test medium contains glucose lysine peptones and a pH indicator such as bromocresol purple which helps in detecting the change in pH during the reaction.
In this test initially the organism ferments glucose present in the medium producing acid which lowers the pH and changes the colour of the indicator from purple to yellow. This acidic condition is essential as it induces the synthesis of lysine decarboxylase enzyme in organisms possessing the specific gene. Once the enzyme is produced lysine is broken down into cadaverine which is alkaline in nature. Due to accumulation of cadaverine the pH of the medium increases and the indicator again turns purple indicating a positive lysine decarboxylase test.
If the organism lacks lysine decarboxylase enzyme the lysine is not degraded and no alkaline amine is formed. As a result the medium remains acidic due to glucose fermentation and retains the yellow colour. Thus the principle relies on initial acid production followed by enzyme mediated alkaline reaction which is detected by change in colour of the pH indicator.
Objectives of Lysine decarboxylase test
- To determine the ability of bacteria to produce the enzyme lysine decarboxylase.
- To study the capacity of organism to decarboxylate the amino acid lysine into alkaline amine cadaverine.
- To differentiate members of Enterobacteriaceae family based on lysine decarboxylation reaction.
- To help in distinguishing Salmonella species from Shigella species during identification.
- To assist in identification of gram negative enteric bacteria when used with other biochemical tests.
- To confirm utilization of lysine by bacteria under anaerobic and acidic condition.
Requirements for Lysine decarboxylase test
- Decarboxylase broth base (Moeller) containing peptones and beef extract as nutrient source.
- L-lysine added to the medium which acts as the specific substrate for lysine decarboxylase enzyme.
- Glucose in small quantity to produce acid initially and to induce enzyme production.
- pH indicator such as bromocresol purple (or cresol red) to detect acidic and alkaline reaction.
- Pyridoxal phosphate which acts as coenzyme for decarboxylation reaction.
- Sterile mineral oil or liquid paraffin to provide anaerobic condition in the medium.
- Control medium without lysine to compare glucose fermentation reaction.
- Fresh pure culture of the test organism for inoculation.
- Sterile inoculating loop or needle for transferring culture.
- Test tubes with caps for holding the medium.
- Incubator maintained at 35–37°C for incubation of inoculated tubes.
Composition of decarboxylase broth medium
| Ingredients | Gms / Litre |
| Peptic digest of animal tissue | 5.000 |
| Yeast extract | 3.000 |
| Dextrose | 1.000 |
| L-Lysine hydrochloride | 5.000 |
| Bromocresol purple | 0.020 |
| Final pH ( at 25°C) | 6.8±0.2 |
Preparation of decarboxylase broth medium
- Weigh 14.02 g of decarboxylase broth medium powder.
- Dissolve it in 1000 ml of distilled water.
- Heat the medium gently if required to dissolve completely.
- Dispense about 5 ml of the prepared medium into clean screw capped test tubes.
- Sterilize the medium by autoclaving at 121°C (15 lbs pressure) for 15 minutes.
Procedure of Lysine decarboxylase test
- Take a fresh pure culture of the test organism (18–24 hours old).
- Inoculate the organism into a tube containing Moeller’s decarboxylase broth with lysine using sterile inoculating loop.
- Inoculate a control tube containing decarboxylase broth base without lysine with the same organism.
- Add a layer of sterile mineral oil over the surface of medium in both test and control tubes to create anaerobic condition.
- Tighten the caps and incubate the tubes at 35–37°C.
- Observe the tubes after 24 48 72 and up to 96 hours for colour change in the medium.
- Record the result based on colour of the test and control tubes.
Lysine decarboxylase test results
Positive result – The medium turns purple or violet in colour after incubation. It indicates that the organism produces lysine decarboxylase enzyme and lysine is converted into alkaline cadaverine.
Negative result – The medium turns yellow and remains yellow. It indicates that glucose is fermented but lysine decarboxylase enzyme is absent.
Non fermenter reaction – The medium shows no colour change or remains light straw colour. It indicates that the organism does not ferment glucose present in the medium.
Invalid test – The control tube turns purple in colour. This indicates improper reaction and the test result is not valid.

| negative reaction | yellow colored media |
| positive reaction | purple colored |
Organisms showing Lysine decarboxylase test result
Lysine decarboxylase positive organisms
- Salmonella species (except Salmonella Paratyphi A).
- Escherichia coli.
- Klebsiella pneumoniae.
- Klebsiella oxytoca.
- Enterobacter aerogenes (Klebsiella aerogenes).
- Serratia marcescens.
- Vibrio cholerae.
- Vibrio parahaemolyticus.
- Edwardsiella tarda.
- Plesiomonas shigelloides.
- Morganella morganii.
Lysine decarboxylase negative organisms
- Shigella species.
- Salmonella Paratyphi A.
- Enterobacter cloacae.
- Proteus species.
- Citrobacter freundii.
- Pseudomonas aeruginosa.
- Providencia species.
Uses of Lysine decarboxylase test
- It is used to differentiate members of Enterobacteriaceae family based on lysine decarboxylation reaction.
- It helps in distinguishing Salmonella species from Shigella species during routine identification.
- It is useful in differentiation of Klebsiella Enterobacter and Serratia group of organisms.
- It is used in identification of Vibrio species and other related gram negative rods.
- It helps in differentiation of Citrobacter species from Salmonella species when used with lysine iron agar.
- It is used to determine the ability of organism to utilize lysine as a substrate for metabolic activity.
Advantages of Lysine decarboxylase test
- It helps in differentiation of enteric bacteria especially members of Enterobacteriaceae family.
- It is useful in distinguishing Salmonella species from Shigella species during routine identification.
- It assists in separation of Klebsiella Enterobacter and Serratia group of organisms.
- It helps in identification of gram negative rods such as Vibrio Aeromonas and Plesiomonas.
- It detects the ability of organism to survive acidic condition by producing lysine decarboxylase enzyme.
- It gives clear and easy interpretation due to distinct colour change in the medium.
- When used with lysine iron agar it allows multiple reactions to be studied in a single test.
Limitations of Lysine decarboxylase test
- The test should not be read before 18–24 hours as early reading may give false negative result.
- Anaerobic condition is essential and improper mineral oil layer may lead to false positive reaction.
- It is not reliable for glucose non fermenting organisms as acid production is required to induce enzyme.
- Some organisms may reduce or destroy the pH indicator causing unclear colour reaction.
- Certain strains require prolonged incubation due to slow permeability of lysine into the cell.
- The test only gives qualitative result and does not measure the exact enzyme activity.
- Weak glucose fermenters may fail to produce sufficient acid resulting in false negative result.
- Sometimes two colour layers appear in the medium making interpretation difficult.
- If the control tube turns purple the test result becomes invalid.
- Alfa Chemistry. (n.d.). Accurate determination of pH transition range of acid-base indicators: A practical protocol. https://www.alfa-chemistry.com/resources/accurate-determination-of-ph-transition-range-of-acid-base-indicators-a-practical-protocol.html
- Aryal, S. (2022, August 10). Amino acid decarboxylase test – Procedure, uses and interpretation. Microbiology Info. https://microbiologyinfo.com/amino-acid-decarboxylase-test/
- Aryal, S. (2022, January 4). Lysine iron agar (LIA)- Composition, principle, preparation, results, uses. Microbe Notes. https://microbenotes.com/lysine-iron-agar-lia/,
- Biolife Italiana. (2025). Decarboxylase Moeller base broth [Instructions for use]. https://gest.joyadv.it/public/cartellina-allegati-schede-certificazioni/schede-tecniche-inglese/ts-4013662.pdf
- BioVendor. (n.d.). Lysine decarboxylase test [Instructions for use]. https://www.biovendor.cz/d-doc/2/14058/638519847350000000/N%C3%A1vod_88014_EN.pdf
- Central Drug House (P) Ltd. (n.d.). Technical information: Moeller decarboxylase broth base. https://www.cdhfinechemical.com/images/product/specs/DM%201393.pdf
- Comprehensive analysis of the lysine decarboxylase test: Biochemical mechanisms, diagnostic standardization, and industrial biotechnology. (n.d.). [Provided text].
- Cooper, C. R., Jr. (2018). BIOL 3702 lab exercise – Decarboxylation test. Youngstown State University. http://crcooper01.people.ysu.edu/microlab/decarboxylation-test.pdf
- Elston, H. R. (1971). Lysine decarboxylase activity in broth and agar media. Applied Microbiology, 22(6), 1091–1095. https://pmc.ncbi.nlm.nih.gov/articles/PMC376490/
- Funnekotter, B., Mancera, R. L., & Bunn, E. (2023). A simple but effective combination of pH indicators for plant tissue culture. Plants, 12(4), 740. https://doi.org/10.3390/plants12040740
- Han, L., Yuan, J., Ao, X., Lin, S., Han, X., & Ye, H. (2018). Biochemical characterization and phylogenetic analysis of the virulence factor lysine decarboxylase from Vibrio vulnificus. Frontiers in Microbiology, 9, 3082. https://doi.org/10.3389/fmicb.2018.03082
- HiMedia Laboratories. (2015). Decarboxylase test medium base (Falkow) [Technical Data]. https://exodocientifica.com.br/_technical-data/M912.pdf
- HiMedia Laboratories. (2015). Moeller decarboxylase broth base [Technical Data]. http://exodocientifica.com.br/_technical-data/M393.pdf
- HiMedia Laboratories. (2024). Decarboxylase broth base, Moeller (Moeller decarboxylase broth base) [Technical Data]. https://www.himedialabs.com/us/m393-decarboxylase-broth-base-moeller-moeller-decarboxylase-broth-base.html
- HiMedia Laboratories. (2024). Technical data: Moeller decarboxylase broth base. https://www.himedialabs.com/media/TD/M393.pdf
- Kroemer, T. (2021). Ultimate guide to cresol red: What it is, what it’s for, how to use it. Gold Biotechnology. https://www.goldbio.com/blogs/articles/ultimate-guide-to-cresol-red
- Lal, A., & Cheeptham, N. (2015). Decarboxylase broth protocol. American Society for Microbiology. https://asm.org/asm/media/protocol-images/decarboxylase-broth-protocol.pdf?ext=.pdf
- Liofilchem. (2023). Lysine decarboxylase test [Instructions for use]. https://www.liofilchem.net/login/pd/ifu/88014_IFU.pdf
- Lv, X., Ma, Z., Li, X., & Zhang, Y. (2021). Highly efficient decarboxylation of L-lysine to cadaverine catalyzed by supported ruthenium oxide. Catalysis Communications, 158, 106339. https://doi.org/10.1016/j.catcom.2021.106339
- National Center for Biotechnology Information. (n.d.). Cadaverine (CID 273). PubChem Compound Summary. https://pubchem.ncbi.nlm.nih.gov/compound/Cadaverine
- Rao, S. P. N. (n.d.). Amino acid metabolism tests. Microrao. https://www.microrao.com/micronotes/pg/amino_acids.pdf
- Sagong, H.-Y., & Kim, K.-J. (2017). Lysine decarboxylase with an enhanced affinity for pyridoxal 5-phosphate by disulfide bond-mediated spatial reconstitution. PLOS ONE, 12(1), e0170163. https://doi.org/10.1371/journal.pone.0170163
- Sapkota, A. (2022, January 20). Decarboxylase test: Principle, procedure, results, uses. Microbe Notes. https://microbenotes.com/decarboxylase-test-principle-procedure-and-result-interpretation/
- Sigma-Aldrich. (2018). D2935 decarboxylase broth base, Moeller [Data sheet]. https://www.sigmaaldrich.com/deepweb/assets/sigmaaldrich/product/documents/299/486/d2935dat.pdf
- Tankeshwar, A. (n.d.). Decarboxylation test: Types, principles, uses. Microbe Online. https://microbeonline.com/decarboxylation-test-types-uses-principles-procedure-results/
- Uyirgene International. (n.d.). Decarboxylases test. https://uyirgene.com/nexus/f/decarboxylases-test?blogcategory=Microbiology+
- VUMIE. (2022, November 1). Lysine decarboxylase test. https://vumicro.com/docs/lysine-decarboxylase-test/
- Wikipedia contributors. (n.d.). Cadaverine. In Wikipedia, The Free Encyclopedia. https://en.wikipedia.org/wiki/Cadaverine