What are intermediate filaments?
- Intermediate filaments (IFs) are robust and long-lasting protein fibres found in the cytoplasm of the majority of higher eukaryotic cells.
- 8 nm to 10 nm in diameter, which is “intermediate” between thin and thick filaments in muscle cells, where they were first described; their diameter is also between microfilaments (actin filaments) and microtubules.
- The IFs are resistant to colchicine and cytochalasin B but susceptible to proteolysis. In the majority of animal cells, IFs form a “basket” around the nucleus and stretch outward in gently curved arrays.
- IFs are especially abundant in areas where cells are subjected to mechanical stress, such as in epithelia, where they are interconnected at desmosomal junctions, along the length of axons, and throughout the cytoplasm of smooth muscle cells.
- Different names have been given to intermediate filaments based on the type of cell in which they are seen.
- Therefore, IFs in epidermal cells are known as tonofilaments, IFs in nerve cells are known as neurofilaments, and IFs in neuroglial cells are known as glial filaments.
- Intermediate filaments have a tubular look in cross-section. Each tubule appears to be composed of four or five protofilaments aligned parallel to one another.
- IFs are made of polypeptides whose sizes vary surprisingly widely (from about 40,000 to 130,000 daltons).
Types of intermediate filaments
The intermediate filaments are extremely varied in terms of their biochemical properties, but can be classified into four major kinds based on their morphology and localization:
1. Type I IF proteins
- Two subfamilies of keratin (also called tono, perakeratin, or cytokeratin) are typically present in epithelial cells: acidic keratin and neutral or basic keratin.
- Keratin filaments are always heteropolymers composed of an equal number of subunits from each subfamily of keratin.
- The keratins are the most complex class of intermediate filament (IF) proteins, having at least 19 different forms in human epithelia and 8 more in hair and nail keratins.
- Mammalian cytokeratins are -fibrous proteins that are generated in cells of live epidermis layers and compose the majority of stratum corneum’s dead layers.
2. Type II IF proteins
- Four types of polypeptides are present: vimentin, desmin, synemin, and glial fibrillary acidic protein (or glial filaments).
- Desmin is found in both striated (skeletal and cardiac) and smooth muscle cells. Vimentin is broadly dispersed in cells of mesenchymal origin, including fibroblasts, blood vessel endothelial cells, and white blood cells.
- In the nervous system, glial cells such as astrocytes and some Schwann cells include glial filaments.
- Synemin is a 230,000-dalton protein that, together with desmin and vimentin, is present in the intermediate filaments of muscle. IFs containing vimentin and synemin can be found in chicken erythrocytes.
- Each of these IF proteins prefers to self-assemble in vitro to create homopolymers and co-assembles with other Types II IF proteins to form co-polymers and heteropolymers.
- Some types of cells have co-polymers of vimentin and desmin, or vimentin and glial fibrillary acidic protein. Desmin, for instance, stays concentrated in the Z-lines and T-tubule system of the striated or skeletal system, alongside vimentin, synemin, and – actemin.
- Since desmin connects actin to plasma memberane, Lazarides and colleagues derived the name desmin from this fact in 1976. (in Greek desmin means link or bond).
3. Type III IF proteins
- Neurofilament proteins are the IF proteins that assemble form neurofilaments, a key cytoskeletal component in nerve axons and dendrites.
- The so-called neurofilament triplet consists of three different polypeptides in vertebrates.
4. Type IVIF proteins
- The nuclear lamins are the filamentous two-dimensional sheets that are so well-organized.
- At a certain point in the mitotic process, these filaments quickly breakdown and reassemble.
Characteristics of four types of intermediate filament proteins
| Types of intermediate filaments | Component polypeptide (mass in daltons) | Cellular location |
| Type I | Acidic keratins (40,000—70,000) Neutral or basic keratins (40,000—70,000) | Epithelial cells and epidermal derivatives such as hair and nail |
| Type II | Vimentin (53,000) Desmin (52,000) Glial fibrillar acidic protein (glial filaments; 45,000) Synemin (230,000) | Many cells of mesenchymal origin Muscle cells Glial cells (astrocytes and some Schwann cells) Muscle cells |
| Type III | Neurofilament proteins (about 130,000, 100,000 and 60,000) | Neurons |
| Type IV | Nuclear lamins A, B and C (65,000—75,000) | Nuclear lamina of all cells |
General Structure of intermediate filaments
- Although cytoplasmic IF proteins range greatly in size, they are all encoded by the same multigene family.
- Based on their respective amino acid sequences, it may be deduced that the middle 310 amino acid residues of each IF polypeptide chain form an extended α -helix with three brief α -helical disruptions.
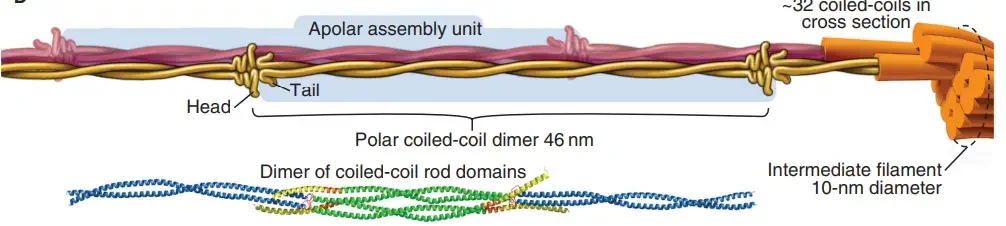
Assembly of intermediate filaments
The following are the processes typically assumed to be taken during construction of an intermediate filament according to current models:
- A dimer is formed when two identical monomers join together to form a coiled coil, with the conserved helical core sections oriented in parallel. The two dimers then align next to one another, forming a protofilament of 48 nm by 3 nm that is comprised of four polypeptide chains.
- The further association of these protofilaments results in progressively more complex structures.
- It is hypothesised that the intermediate filament, which has a diameter of 10 nm, is made up of 8-protofilaments (i.e., 32 polypeptide chains) that are linked end-on-end to neighbours by staggered overlap. If IFs are polar structures (like actin and tubulin) or non-polar structures is still unknown (like the DNA double helix).
Intermediate filaments During Mitosis
- Epithelial cells in culture undergo dramatic alterations in their intermediate filaments of cytokeratin and vimentin during mitosis.
- The 10 nm filaments unravel into 2–4 nm threads and form spheroidal aggregates of both types of proteins during prophase.
- Most vimentin and cytokeratin are found in spheroid bodies during metaphase and anaphase, and the filamentous cytoskeleton is gradually rebuilt during telophase.
- Franke (1982) drew the conclusion from these experiments that living cells include elements that facilitate the reversible disintegration and restoration of intermediate filaments during mitosis.
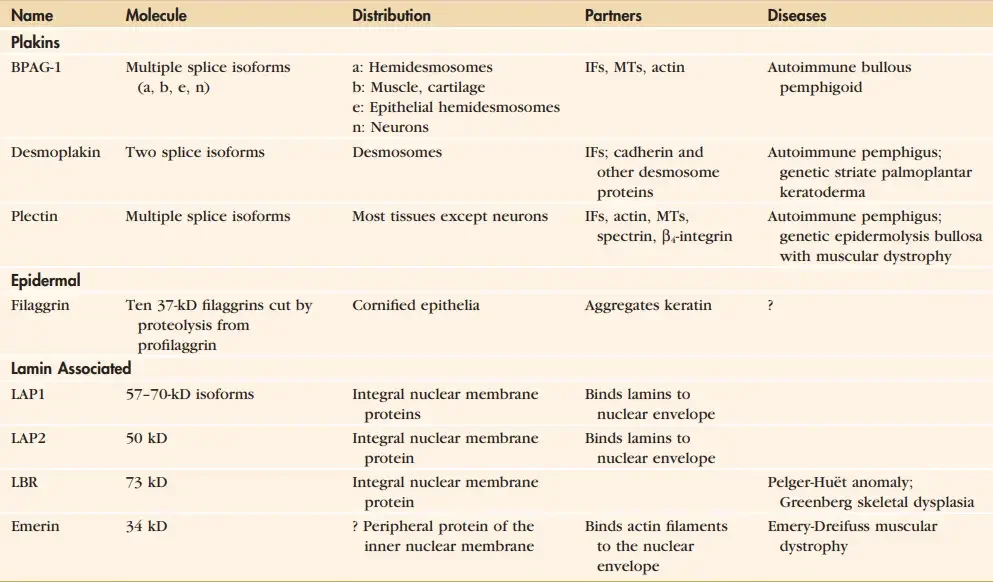
Proteins Associated With Intermediate Filaments
- Several proteins bind intermediate filaments and connect them to membranes and other polymers in the cytoskeleton.
- Nuclear lamins are tethered to the nuclear membrane by integral membrane proteins known as nuclear envelope transmembrane proteins.
- Keratin filaments in the skin’s outer layers are aggregated by filaggrin’s mediation. Plakins are large proteins that serve as binding sites for cytoskeletal polymers and sticky junction proteins, thereby connecting cytoskeletal components and membranes.
- Plectin, like many other plakins, is comprised of a 200-nm coiledcoil with globular domains at either end. Plectin can crosslink intermediate filaments to one another, to actin filaments, and to microtubules via binding sites in both globular domains.
- Rare muscular dystrophy with blistering skin is caused by recessive mutations in human plectin. Having the null mutation in mice is fatal.
- At desmosomes and hemidesmosomes, plectin and two additional plakins connect keratin filaments to three distinct plasma membrane adhesion proteins.
- Desmoplakin also acts like a cadherin to secure keratin filaments to desmosomes. Plakin BPAG1e (bullous pemphigoid antigen 1-e) binds keratin filaments to the transmembrane protein BPAG2, while plectin 1a links keratin to 4-integrins at hemidesmosomes (bullous pemphigoid antigen 2).
- Some people who suffer from neuropathies or a specific type of epidermolysis bullosa have mutations in the dystonin gene, which is expressed in multiple splice variants.
Intermediate filaments function
- Most intermediate filaments’ primary role is to give the cell and its nucleus structural support.
- The transcellular network formed by IFs in epithelia appears to be geared toward withstanding mechanical stress.
- The long, skinny cylinders of cytoplasm in nerve cells (axons) are protected by neurofilaments against stresses brought on by the animal’s movement.
- The sarcomeres of muscle cells are supported mechanically by desmin filaments, while the enormous lipid droplets in adipose tissue are surrounded by vimentin filaments.
References
- Cooper GM. The Cell: A Molecular Approach. 2nd edition. Sunderland (MA): Sinauer Associates; 2000. Intermediate Filaments. Available from: https://www.ncbi.nlm.nih.gov/books/NBK9834/
- Intermediate Filaments. (2017). Cell Biology, 613–622. doi:10.1016/b978-0-323-34126-4.00035-9
- Eng, D. L., & Eng, L. F. (2009). Intermediate Filaments. Encyclopedia of Neuroscience, 173–178. doi:10.1016/b978-008045046-9.01010-x
- Bomont, P. (2016). Degradation of the Intermediate Filament Family by Gigaxonin. Intermediate Filament Associated Proteins, 215–231. doi:10.1016/bs.mie.2015.07.009
- Herrmann, H., Bär, H., Kreplak, L. et al. Intermediate filaments: from cell architecture to nanomechanics. Nat Rev Mol Cell Biol 8, 562–573 (2007). https://doi.org/10.1038/nrm2197
- Zamoner, A., & Pessoa-Pureur, R. (2017). Intermediate Filaments as a Target of Signaling Mechanisms in Neurotoxicity. In (Ed.), Cytoskeleton – Structure, Dynamics, Function and Disease. IntechOpen. https://doi.org/10.5772/66926
- https://study.com/academy/lesson/intermediate-filaments-definition-function-structure.html
- https://micro.magnet.fsu.edu/cells/intermediatefilaments/intermediatefilaments.html
- https://cshperspectives.cshlp.org/content/8/11/a018242
- https://www.nejm.org/doi/full/10.1056/nejmra040319
- https://www.molbiolcell.org/doi/10.1091/mbc.e03-06-0376
- https://www.mechanobio.info/cytoskeleton-dynamics/what-is-the-cytoskeleton/what-are-intermediate-filaments/
- https://www.frontiersin.org/articles/10.3389/fcell.2017.00081/full