What is Immunoelectrophoresis?
Immunoelectrophoresis is defined as the separation of proteins by electrophoresis followed by their identification through immunologic (antigen-antibody) reactions.
- It is a laboratory technique that combines gel electrophoresis and immunoprecipitation to detect and characterise proteins (antigens/ antibodies) in mixtures.
- In the method a sample (for example serum, urine or other body fluid) is first subjected to electrophoresis (in a gel like agarose) so that proteins are separated by charge/size etc.
- After separation a trough or channel containing specific antiserum (antibodies) is introduced adjacent to the separated proteins so that antigen-antibody reaction diffuses and precipitin arcs/lines form where matching antigens meet antibodies.
- The pattern of the precipitin arcs (their position, number, shape) allows identification (and in some variants, semi-quantification) of the proteins present.
- The method is often done under near-native conditions (i.e., proteins not heavily denatured) so that functional/structural features of the proteins may be preserved.
- In clinical labs this technique has been used for analysing immunoglobulins and other serum proteins when abnormalities are suspected.
- It allowed researchers and clinicians to separate and characterise complex mixtures of proteins in biological fluids: for example to show multiple immunoglobulin classes, heterogeneity of proteins in serum.
- The technique gave a way to detect abnormal proteins (for instance monoclonal immunoglobulins) or abnormal patterns in disease states (for example in certain cancers, liver disease, immune disorders) which improved diagnostic capability.
- It remains important in research for checking purity of antigen/antibody preparations and for studying antigen-antibody interactions in a gel setting.
- Although newer, faster methods exist, immunoelectrophoresis still holds value when near-native conditions or specific characterisation (rather than simply amounts) are required.
- The term “immunoelectrophoresis” was coined in the early 1950s.
- The French immunologist Pierre Grabar (along with C.A. Williams) published one of the foundational papers in 1955 describing the method of immuno-electrophoretic analysis of mixtures of antigenic substances.
- Over subsequent decades variants of the method were developed: for example “crossed immunoelectrophoresis”, “rocket immunoelectrophoresis” etc.
- Its usage peaked in mid to late 20th century in clinical and research labs; later replaced in many contexts by higher sensitivity or automated techniques, but the legacy remains.
Immunoelectrophoresis Definition
Immunoelectrophoresis is a biochemical technique that combines electrophoresis and antibody-antigen reactions to separate and identify proteins based on their antigenic properties.
Objectives of Immunoelectrophoresis
- To allow separation of a mixture of antigens (proteins) by gel-electrophoresis and then permit their identification by reaction with antiserum/antibodies.
- To detect presence of specific antigen–antibody interactions (precipitin lines/arcs) so that heterogeneity of proteins can be demonstrated.
- To analyse and characterise different proteins in body fluids (like serum, urine, CSF) for diagnostic purposes (for example identify abnormal immunoglobulins) .
- To check the purity and identity of antigen/antibody preparations in research or quality-control settings.
- To provide a method that works under near-native conditions (so that the structure/charge of proteins is preserved) and so relationships of proteins/antibodies can be studied.
Principle of Immunoelectrophoresis
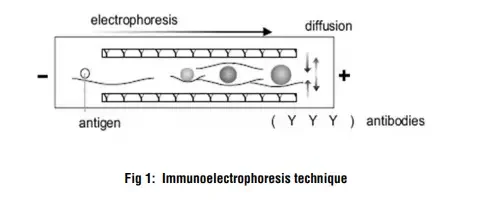
The method is based on combining two processes, electrophoresis and immunodiffusion / antibody-antigen reaction, so that proteins in a mixture are first separated then identified.
Initially a gel (often agarose) is used and an electric current is applied so that the antigen mixture is separated into individual antigen-components according to their charge and to some extent size / shape.
After that separation a trough or channel containing specific antiserum (antibodies) is introduced roughly parallel to the path of separated antigens and the antibodies diffuse (or are driven) to meet the antigen components.
Where the antigen and its matching antibody meet in correct proportions (equivalence zone) a precipitin-line / arc is formed in the gel, because the antigen-antibody complexes aggregate and precipitate.
The precipitin lines’ positions (distance from origin), shapes, number allow inference about which antigen is present, whether multiple antigens exist, heterogeneity etc.
The procedure thus exploits the fact that antigens migrate under electric field in gel (electrophoresis) and that antibodies can diffuse and react, forming visible precipitates under near-native conditions (i.e., proteins not heavily denatured) so structural/functional features are preserved.
In some variants the process is modified (for example counter- or crossed immunoelectrophoresis) where antigen and antibody migration are both facilitated by electric field or second dimension electrophoresis is done.
The time for diffusion and reaction is often 18-24 hrs after electrophoresis for the arcs to appear.
Material Required
- A suitable specimen is required (for example serum, or sometimes urine) drawn by venipuncture or collection of urine.
- The specimen must be separated (for serum, cells removed) and transferred to clean labelled tube within acceptable time after collection.
- A minimum volume of specimen is needed (for example 2 mL of serum in some labs) so that analysis and possible repeats are possible.
- Appropriate container / tube type must be used (for serum: red-top plain tube or gel‐barrier tube) and correct handling of sample (eg centrifugation, refrigeration) must be done.
- The specimen must be stored and transported under proper temperature / stability conditions (eg refrigerated, frozen within certain time, avoid repeated freeze/thaw cycles).
- The sample must be free of gross hemolysis / lipemia / improper sample type (eg plasma when serum required) because these conditions could interfere with the test and cause rejection.
- Laboratory must have the required gel medium (for example agarose), electrophoresis setup, antibody/antiserum reagents, detection stains etc. (implicitly required from principle)
- Proper documentation: Patient’s age, clinical diagnosis may need to be provided on test request form so that interpretation is accurate.
- If urine specimen is used then correct collection method (eg 24-hour urine or random urine, sterile container) must be followed.
Procedure of Immunoelectrophoresis
- The agarose (or another suitable gel) is prepared on a glass slide (horizontal) and allowed to solidify.
- Wells are cut in the gel at the application zone using a sample template and careful handling.
- The sample (antigen mixture) is diluted if required (for example 20 µl antigen +10 µl diluent) and then loaded into the wells (for example 5 µl of sample & control into each slit).
- The gel slide is placed into the electrophoresis chamber with correct orientation (sample on cathodic side) and buffer is added, then electrophoresis is run (for example ~20 minutes at ~100 volts) to separate the proteins by charge/size.
- After electrophoresis, a trough (channel) is cut parallel to the migration path, and corresponding antiserum (antibodies) is added into the trough(s).
- Incubation is done in a moist chamber at room temperature (for example ~18-20 hours) with gel laid horizontally so that antigen and antibody diffuse and interact.
- After incubation, the gel is washed (for example soak in saline for ~10 minutes) and dried (for example at <70°C), then stained (for example with a protein stain, ~3 minutes) and destained (~5 minutes) to visualise precipitin arcs.
- The final gel is evaluated: the presence of elliptical precipitin arcs indicates antigen-antibody reaction, their position/shape/number are interpreted to identify antigens.
Results of Immunoelectrophoresis

- Normal result is given when immunoglobulins (Ig-classes like IgG, IgM, IgA) are present in expected proportions and no unusual resolved band/arc is seen.
- A clear precipitin arc is formed when antigen-antibody reaction takes place, and the presence of elliptical arcs at correct positions indicates identity of an antigen/protein.
- When absence of precipitate or very faint arcs are seen then that can mean no reaction (antigen may be missing or antibody mismatch) and thus a negative result.
- If a monoclonal band (also called M-protein or paraprotein) is seen then a clone of plasma cells is probably producing a single immunoglobulin type, this result is associated with conditions like Multiple myeloma or Waldenström macroglobulinemia.
- A “polyclonal increase” pattern (many arcs or diffuse arc broadening) is seen when many immunoglobulins are increased (eg in chronic infection or liver disease) instead of single clone.
- The position, shape and intensity of arcs allow semi-quantitative interpretation: heavier or sharper arc suggests higher concentration, shifted position suggests variant mobility, multiple arcs suggest heterogeneity.
- The results must be interpreted in context of patient’s history, medications, vaccinations etc, because increases in immunoglobulins may also follow non-malignant causes like recent immunization.
- If abnormal result is found then further more sensitive tests (for example Immunofixation electrophoresis) may be ordered to quantify or identify light chains (kappa/lambda) etc.
Note : For better precipitin lines incubate for longer period at 37oC
Advantages of Immunoelectrophoresis
- The test is highly resolving because the mixture of antigens is first separated by electrophoresis then reacted with antibodies, so many components can be distinguished.
- A good number of antigens can be identified in a single serum / sample, which gives it breadth of application.
- Relatively less sample is required compared to some other methods, so it’s useful when sample volume is limited.
- Near-native conditions are maintained (proteins not heavily denatured) so structural/ functional features of antigen/antibody complexes are preserved.
- The method allows both separation and antibody reaction steps combined, so cost/ complexity is less than doing many separate assays.
- The technique is versatile for qualitative and semi-quantitative detection of abnormal proteins (eg monoclonal immunoglobulins) in clinical labs.
Limitations of Immunoelectrophoresis
- It is slower method when compared with newer techniques, many labs find that IEP takes long time to perform and results are delayed.
- The sensitivity is lower, so small amounts of abnormal proteins (for example small monoclonal M-proteins) may not be detected, they might be masked by major proteins migrating faster.
- The interpretation of results is more difficult and subjective, because the precipitin arcs may be faint or ambiguous and the mobility shifts subtle.
- The technique requires availability of specific antisera / antibodies, and if those are not available then certain antigens cannot be studied; its use in fields like food analysis is limited for that reason.
- Quantification is poor or semi-quantitative at best, accurate measurement of concentration is not easily achieved with classical IEP, so it’s less suited for detailed quantitation.
- It is less efficient for complex mixtures of proteins when many similar antigens exist, resolution may suffer and overlapping arcs may complicate the outcome.
- From operational viewpoint the technique may demand good technical skill, careful control of gel, electrophoresis conditions, diffusion etc., so reproducibility may vary among labs.
Applications of Immunoelectrophoresis
- It is used in clinical diagnostics when abnormal immunoglobulins (Ig’s) are suspected (for example in diseases like Multiple myeloma) and then IEP helps identifying which Ig class / type is present.
- The test is employed for detection of both normal and abnormal proteins in body fluids (serum, urine) like paraproteins, when over-production or deficiency is suspected.
- It is applied in research / quality-control for checking the purity of antigen/antibody preparations, and to identify a single antigen among mixture of antigens, which gives value in biochemical product monitoring.
- For analysis of complex protein mixtures containing many antigens the method is useful because the separation + antibody reaction gives resolving power.
- It is used to monitor the therapeutic response in immune-related disorders (eg gammopathies) because changes in protein / immunoglobulin patterns are shown by IEP.
Precautions of Immunoelectrophoresis
- Specimen handling must be done carefully, the serum or urine sample should be free of hemolysis / lipemia and they must be promptly processed because sample deterioration will affect results.
- The gel (eg agarose) must be poured and solidified on a level surface and the wells/troughs must be made neat and correct spacing must be maintained to avoid diffusion-errors.
- Electrical equipment (power supply, electrodes) must be checked, wet surfaces avoided and gel chamber must be dry before running, because current leakage / short circuit may cause artefacts.
- The antiserum must be carefully applied to troughs after electrophoresis finishes and over-filling of troughs must be avoided because cross contamination between troughs will distort precipitation arcs.
- Incubation must be done in a humid chamber at appropriate temperature (eg ~15-30 °C) and duration (18-24 h) so that antigens and antibodies diffuse properly and incomplete incubation will lead to weak or missing arcs.
- Washing and drying steps must be done gently and thoroughly (eg 0.85% saline wash, drying at ~60-70°C) so unbound proteins are removed and background is cleared, because poor washing will cause fuzzy or non-interpretable arcs.
- All reagents (agarose, buffer, antiserum) must be stored at correct temperatures (eg 2-8 °C for plates/antisera) and inspected for signs of deterioration (cloudy plates, contaminated antiserum) because bad reagents will invalidate the test.
- Safety precautions must be observed (gloves, goggles, no mouth-pipetting, proper handling of heated agarose, correct disposal of azide-containing waste) because chemical/ biological hazards exist.
FAQ
What is immunoelectrophoresis?
Immunoelectrophoresis is a biochemical method that combines electrophoresis and immunodiffusion to separate and characterize proteins based on their electrophoretic mobility and reaction with antibodies.
What is the principle of immunoelectrophoresis?
Immunoelectrophoresis involves the separation of antigens by electrophoresis, followed by reaction with specific antibodies. The formation of precipitin lines indicates the presence of antigen-antibody interactions.
What are the advantages of immunoelectrophoresis?
Immunoelectrophoresis has high resolving power and can identify multiple antigens in a sample. It is useful for protein identification, immunodominant epitope determination, and monitoring antigen-antibody purity.
How does immunoelectrophoresis compare to immunofixation electrophoresis?
Immunoelectrophoresis is slower, less sensitive, and more difficult to interpret compared to immunofixation electrophoresis. Immunofixation electrophoresis is more suitable for detecting small monoclonal M-proteins.
What are the applications of immunoelectrophoresis?
Immunoelectrophoresis is used for protein identification and quantization, detecting gammopathies, analyzing complex protein mixtures, diagnosing immune-related diseases, and evaluating therapeutic responses.
What are the materials required for immunoelectrophoresis?
The materials include glassware (conical flask, measuring cylinder, beaker), reagents (distilled water, alcohol), and equipment (incubator, electrophoresis unit, vortex mixer, micropipettes, gel cutter).
What is the procedure for immunoelectrophoresis?
The procedure involves preparing an agarose gel, loading samples and controls, performing electrophoresis, adding specific antiserum, incubating, drying the gel, staining, and evaluating the results.
How do you interpret immunoelectrophoresis results?
The presence of precipitin lines indicates antigen-antibody interactions. A single continuous line suggests homogeneity of the antiserum, while multiple lines indicate heterogeneity and the presence of different immunodominant epitopes.
What precautions should be taken during immunoelectrophoresis?
Precautions include wearing gloves, following the procedure carefully, properly preparing reagents, maintaining temperature control, cutting wells and troughs neatly, and ensuring a humid atmosphere in the moist chamber.
Are there limitations to immunoelectrophoresis?
Immunoelectrophoresis may fail to detect small M-proteins and has limited use in food analysis due to the availability of specific antibodies. Other advanced techniques like immunofixation electrophoresis or more sensitive immunoassays may be preferred in certain cases.
- Csako, G. (2018). Immunoelectrophoresis: A Method with Many Faces. Electrophoretic Separation of Proteins, 249–268. doi:10.1007/978-1-4939-8793-1_21
- https://en.wikipedia.org/wiki/Immunoelectrophoresis
- https://himedialabs.com/TD/HTI005.pdf
- http://lnmuacin.in/studentnotice/2020/Immunoelectrophoresis.pdf
- http://www.ndvsu.org/images/StudyMaterials/Micro/Immunoelectrophoresis-test.pdf