Tollens’ test is a chemical test used to distinguish aldehydes from ketones. It is the process where aldehydes reduce silver nitrate to metallic silver forming a mirror on the test tube wall. The reagent contain ammoniacal silver nitrate solution. Ketones do not give positive result except alpha-hydroxy ketones. The test is also known as silver mirror test.
Tollens’ test include preparation of Tollens’ reagent by mixing silver nitrate with sodium hydroxide and then ammonia. The reagent form diamminesilver(I) complex [Ag(NH3)2]+. When aldehyde is added, it is oxidized to carboxylic acid. The silver ion is reduced to silver metal. This deposit as shiny mirror on clean glass surface. The reaction occur in alkaline medium. Positive result show silver mirror formation within few minutes. Negative result show no mirror or black precipitate.
History of Tollens’ test dates back to 1880s. It is developed by German chemist Bernhard Tollens. The test is first described for detection of aldehydes in organic compounds. Tollens work on carbohydrate chemistry lead to this test. It become standard method in qualitative organic analysis. The test is widely used in textbooks and laboratories since then.
Importance of Tollens’ test lie in its specificity for aldehydes. It help differentiate aldehydes from ketones in unknown samples. The test is simple and require small amount of sample. It is part of systematic organic compound identification. The silver mirror is visually striking and confirm result clearly. This test aid in study of reducing sugars which contain aldehyde group. It is useful in pharmaceutical and food industry for quality control.
Tollens’ Reagent
Tollens’ reagent is the alkaline solution that contain the diamminesilver complex [Ag(NH3)2]+ and it is used for detecting aldehyde group in organic compounds. It is prepared when silver nitrate is treated with sodium hydroxide to form silver oxide and this oxide is then dissolved in ammonia solution. It is a colourless reagent and it is the process where aldehydes is oxidised into carboxylate ions while the silver ions is reduced into metallic silver. This is referred to as the silver-mirror test because a bright silver coating is formed on the inner wall of the test tube when the reagent is warmed with an aldehyde. Ketones usually do not react in this test except some α-hydroxy ketones which can form aldehydic structure in alkaline medium. It is always prepared fresh because old solution may decompose and form explosive residues.
Preparation of Tollens’ Reagent
- Take an aqueous solution of Silver nitrate (AgNO₃).
- Add a few drops of dilute Sodium hydroxide (NaOH) solution dropwise. A light brown precipitate of Silver(I) oxide (Ag₂O) will form.
- Then add dilute (or concentrated) Ammonia solution (NH₃ / NH₄OH) drop-by-drop with gentle shaking until the brown precipitate dissolves completely. The solution becomes clear and colourless.
- The resulting solution contains the diamminesilver(I) complex ion [Ag(NH₃)₂]⁺ — this is the active species of Tollens’ reagent.
- Use the reagent immediately after preparation. It should not be stored because it decomposes on standing and may form unstable compounds.
Principle of Tollens’ Test
The principle of the Tollens’ test is based on redox reaction between the reagent Tollens’ reagent and an aldehyde (or other suitably oxidisable group). The active species in Tollens’ reagent is the diamminesilver(I) complex [Ag(NH₃)₂]⁺ in alkaline medium.
When an aldehyde (R–CHO) is present and warmed with Tollens’ reagent, the aldehyde is oxidised to corresponding carboxylate ion (which on acidification yields carboxylic acid). At same time the silver(I) ions from the complex are reduced to metallic silver (Ag⁰).
The metallic silver so formed deposits on the inner surface of the test-tube (or reaction vessel) producing a shiny “silver mirror”. This visible deposition of silver indicates a positive Tollens test.
Most ketones do not react under these conditions because ketonic carbonyl does not have hydrogen attached to carbonyl carbon and is not easily oxidised to carboxylate under mild conditions. Thus ketones give negative test.
However some special compounds (for example α-hydroxy ketones, or sugars capable of forming aldehyde under alkaline conditions) may also give positive result because under test conditions they undergo structural transformations that permit oxidation.
Thus the test rests on difference in oxidizability: aldehyde being readily oxidizable while ketone generally not. The change in oxidation state of silver (from +1 to 0) accompanied by oxidation of carbon in aldehyde is the chemical basis of this reagent’s ability to distinguish them.
Reactions of Tollens’ Test
The reactions of the Tollens’ test is based on the oxidation of an aldehyde group while the silver ions of the reagent is reduced to metallic silver. It is the process where the aldehyde (R–CHO) is converted into the corresponding carboxylate ion in alkaline medium and at the same time the diamminesilver complex [Ag(NH3)2]+ is reduced.
The reaction is as follows– R–CHO + 2[Ag(NH3)2]+ + 3OH– → R–COO– + 2Ag(s) + 4NH3 + 2H2O.
In this reaction the silver atoms is deposited on the inner wall of the test tube forming the silver-mirror which indicates a positive result. Ketones usually do not undergo this reaction because these molecules is not easily oxidised under mild alkaline condition. Some α-hydroxy ketones and reducing sugars can also give this reaction because these compound is able to form aldehydic structure in the medium. Thus the Tollens’ reaction occurs when an oxidisable aldehyde group is present and the reduction of silver ion into metallic silver gives the visible confirmation.
Requirements for Tollens’ Test
- A freshly prepared Tollens’ Reagent — that is an alkaline ammoniacal solution containing the diamminesilver(I) complex [Ag(NH₃)₂]⁺.
- A solution of the test substance (possibly in a neutral solvent such as alcohol if necessary) to avoid interfering acidity or strong reactivity.
- Clean, inert glassware (test tube or flask) free from metal contaminants or previous residues — because the glass surface must allow silver deposition for visible “mirror” formation.
- Warm water bath (gentle heating) to accelerate the redox reaction between aldehyde and Tollens reagent.
- Alkaline medium: presence of hydroxide ions (OH⁻) — typically ensured by the reagent preparation (using NaOH) — to maintain basic pH required for the diamminesilver complex stability.
- Immediate disposal or acidification after test (if required) because reagent is unstable — unused reagent should not be stored.
Procedure of Tollens’ Test
- A clean dry test tube is taken because the silver deposit can be disturbed by impurities.
- In this tube the solution of silver nitrate is placed and dilute NaOH is added dropwise. A brown precipitate of silver oxide is formed in this step.
- Now aqueous ammonia is added slowly until the brown precipitate is dissolved completely. It is the process where the diamminesilver complex [Ag(NH3)2]+ is formed.
- The reagent must be prepared fresh because old solution is not stable.
- A small amount of the test sample is taken in another clean test tube.
- Tollens’ reagent is added to the sample and the tube is warmed in a water bath.
- In this step the aldehyde present in the sample is oxidised and silver ions is reduced into metallic silver.
- A shining silver mirror is formed on the inner wall of the test tube which shows a positive Tollens’ test.
- If no silver deposit is formed then the test is negative under these conditions.
- After the test the remaining reagent is destroyed immediately because the residue can become harmful if kept for long time.
Result of Tollens’ Test
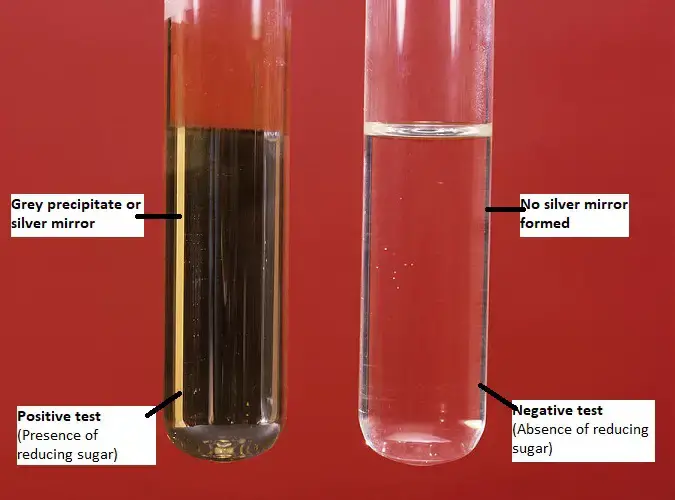
When a compound containing an aldehyde group (or certain special reducible groups) is treated with freshly prepared Tollens’ Reagent and gently warmed, the silver ions from the reagent is reduced to metallic silver. The metallic silver deposits on the inner surface of the glass container. This deposition appears as a shining “silver mirror” or as a dark grey/black precipitate of silver. That observation indicates a positive Tollens’ test.
If no silver deposit or mirror is formed — that is, no visible change — then the test is negative, which implies that the compound does not contain an easily oxidisable aldehyde (or other reducible group under these conditions).
In special cases, some α-hydroxy ketones or reducing sugars may also give positive result (silver mirror or precipitate) because under basic medium they is capable to behave as aldehyde. But general ketones remain unreactive, yielding negative result.
- Positive result: If a dark grey precipitate or silver mirror forms on the bottom and sides of the test tube. It indicates the given sample contains reducing sugars/ aldoses.
- Negative result: If no precipitate is formed. It indicates the given sample doesn’t contain any reducing sugars/ aldoses/ α-hydroxy ketoses.
Uses of Tollens’ test
- It is used for detecting the presence of aldehyde group in organic compounds because aldehydes is easily oxidised by the diamminesilver complex.
- It is used to distinguish aldehydes from ketones since most ketones do not give the silver-mirror reaction under these conditions.
- It is used for identifying reducing sugars because these sugars can form aldehydic structure in alkaline medium and give the silver deposit.
- It is used in demonstrating oxidation–reduction reactions in laboratory studies where silver ion is reduced into metallic silver.
- It is used in preparing silver-mirror coatings on glassware for demonstration purpose because the metallic silver forms a uniform layer on the inner wall.
Limitations of Tollens’ test
- The test cannot clearly distinguish aldehydes from some α-hydroxy ketones because these compounds can form aldehydic structure in alkaline medium and give a silver deposit.
- The reagent is not stable and it must be prepared fresh, so it cannot be stored for later use.
- Old Tollens’ reagent may form explosive silver compounds which makes handling and disposal difficult.
- Ketones generally do not react, but certain sugars and reducing compounds can still give positive results which may cause confusion in interpretation.
- The test requires a clean glass surface because any impurity or oily layer prevents the formation of a proper silver-mirror.
- Strongly coloured or impure samples can mask the appearance of silver deposition and make observation inaccurate.
Advantages of Tollens’ test
- It is a simple qualitative test because the formation of the silver-mirror can be observed directly without any instrument.
- The reagent is a mild oxidising agent, so aldehydes is oxidised easily without causing further unwanted oxidation of other groups.
- It helps to distinguish aldehydes from most ketones since ketones generally do not react under these conditions.
- It is useful in detecting reducing sugars, as these sugars can form aldehydic form in alkaline medium and give positive reaction.
- The visible silver coating provides a clear confirmation, making the test suitable for quick laboratory identification.
- It can also be used for demonstrating redox principles because silver ion is reduced into metallic silver during the process.
- Text Highlighting: Select any text in the post content to highlight it
- Text Annotation: Select text and add comments with annotations
- Comment Management: Edit or delete your own comments
- Highlight Management: Remove your own highlights
How to use: Simply select any text in the post content above, and you'll see annotation options. Login here or create an account to get started.