What is Hypersensitivity?
- hypersensitive, also known as hypersensitive reaction or intolerance, is an abnormal physiological condition in which the body’s immune system reacts negatively to an antigen. It reflects an immune system malfunction that can lead to immunological disorders such as allergies and autoimmunity. Various particles and chemicals from the external environment or within the body can cause this response because they are recognized as antigens by immune cells.
- Hypersensitivity immunological reactions are frequently characterized as an immune system overreaction. They can be harmful and unpleasant for the person experiencing them. Philip George Houthem Gell and Robin Coombs developed a categorization system in 1963 to identify the various types of hypersensitivity based on the antigens and immune responses involved. The Gell and Coombs classification, sometimes known as the Gell-Coombs classification, distinguishes four categories of hypersensitivity reactions.
- Type I hypersensitivity is an acute reaction caused by immunoglobulin E (IgE). It happens quickly, usually within 24 hours of being exposed to the antigen. Allergies, such as hay fever, asthma, and food allergies, are frequently connected with type I hypersensitivity. Symptoms can range from mild discomfort to severe reactions, such as anaphylaxis, a potentially fatal illness.
- Type II hypersensitivity is characterized by an antibody-mediated reaction involving immunoglobulin G (IgG) or immunoglobulin M (IgM). It can result in a variety of disorders, including autoimmune diseases and adverse medication reactions. Type II hypersensitivity occurs when the immune system misidentifies normal cells or tissues as foreign and targets them for destruction.
- Type III hypersensitivity is an immune complex-mediated reaction involving IgG, the complement system, and phagocytes. This type of hypersensitivity arises when immune complexes composed of antigens and antibodies build up in tissues, causing inflammation and damage. Type III hypersensitivity reactions include diseases such as systemic lupus erythematosus and rheumatoid arthritis.
- Type IV hypersensitivity, also known as delayed hypersensitivity or cell-mediated hypersensitivity, is a T-cell-mediated cytotoxic reaction. Unlike the other three types of hypersensitivity, type IV hypersensitivity takes longer to manifest, usually more than 12 hours after contact to the allergen. Typically, the maximum reaction time is between 48 and 72 hours. This sort of hypersensitivity is linked to problems including contact dermatitis and organ transplant rejection.
- Hypersensitivity is a rather common occurrence, with an estimated 15% of people suffering at least one sort of hypersensitivity reaction at some point in their lives. Indeed, hypersensitivity has become more common since the second half of the twentieth century. It is crucial to remember that the severity and impact of hypersensitivity reactions can vary from person to person, and proper diagnosis and care are critical for persons afflicted by hypersensitivity diseases.
Definition of Hypersensitivity
Hypersensitivity is an abnormal and exaggerated response of the immune system to a substance (antigen) that is usually harmless. It can lead to allergic reactions and immune-related diseases.
Causes Of Hypersensitivity Diseases
Hypersensitivity is caused by various immune responses that can be triggered by different types of antigens. The causes of hypersensitivity can be broadly categorized into three main groups: autoimmunity, reactions against microbes, and reactions against non-microbial environmental antigens.
- Autoimmunity: Autoimmunity refers to immune responses that target and react against self-antigens. In autoimmune disorders, the immune system mistakenly identifies normal cells and tissues as foreign and launches an immune attack against them. This can lead to chronic inflammation and tissue damage. Examples of autoimmune diseases include rheumatoid arthritis, lupus, and multiple sclerosis.
- Reactions against microbes: Reactions against microbes occur when the immune system recognizes and responds to antigens derived from microorganisms. These antigens can be proteins or other molecules present on the surface of bacteria, viruses, fungi, or parasites. The immune response aims to eliminate the invading pathogens and protect the body from infection. However, in some cases, the immune response can become exaggerated or misdirected, resulting in hypersensitivity reactions. Examples include allergic reactions to certain bacteria or viruses.
- Reactions against non-microbial environmental antigens: Reactions against non-microbial environmental antigens involve hypersensitivity responses to substances present in the environment that are not derived from microorganisms. These substances, known as environmental antigens or allergens, can include pollen, dust mites, pet dander, certain foods, and medications. When exposed to these allergens, susceptible individuals may develop hypersensitivity reactions, commonly known as allergies. The immune system produces antibodies, particularly immunoglobulin E (IgE), in response to these allergens, leading to the release of inflammatory substances such as histamine. This release of inflammatory mediators causes symptoms such as itching, sneezing, coughing, and skin rashes.
It’s important to note that the specific causes of hypersensitivity can vary greatly depending on the individual and the type of hypersensitivity reaction involved. Genetic factors, environmental factors, and a complex interplay between the immune system and external triggers all contribute to the development of hypersensitivity diseases. Understanding the causes of hypersensitivity is crucial for diagnosis, prevention, and management of these immune-related conditions.
Mechanisms Of Hypersensitivity Reactions
Hypersensitivity reactions are immune responses that occur in response to specific antigens and can result in cell and tissue injury. These reactions are classified into different types based on the immune mechanisms involved. Understanding these mechanisms is crucial for diagnosing and treating hypersensitivity diseases effectively.
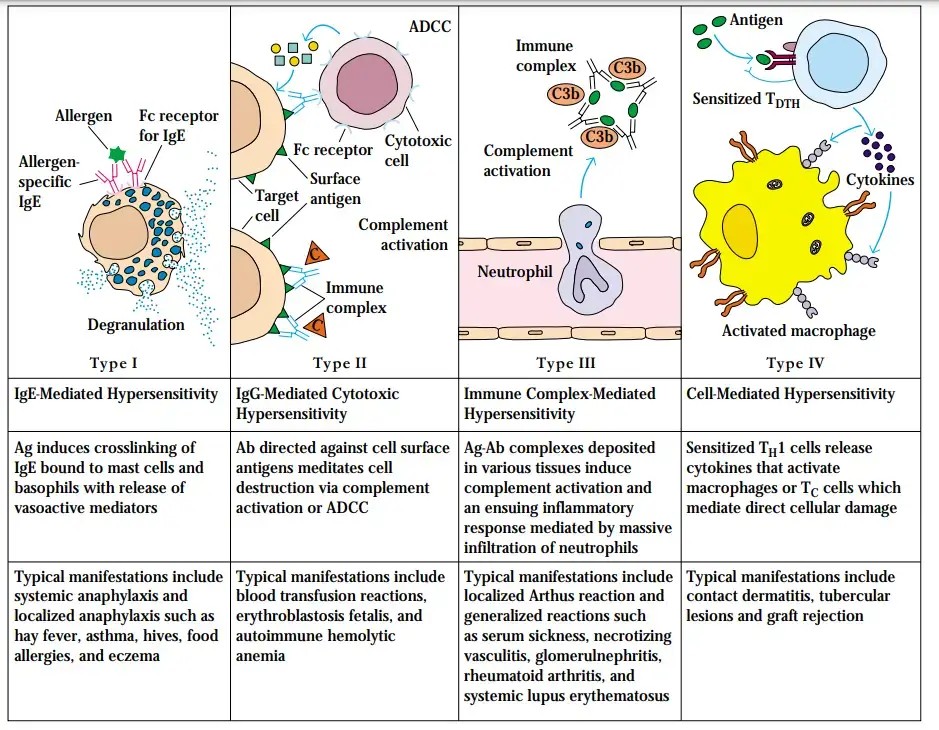
- Immediate or type I hypersensitivity: Type I hypersensitivity, also known as immediate hypersensitivity or allergy, is the most common type of hypersensitivity reaction. It is triggered by the presence of IgE antibodies specific to environmental antigens. When the antigen binds to the IgE antibodies on the surface of mast cells and basophils, it triggers the release of inflammatory mediators such as histamine, leukotrienes, and cytokines. This activation of mast cells and basophils leads to immediate symptoms like itching, sneezing, hives, and in severe cases, anaphylaxis.
- Antibody-mediated or type II hypersensitivity: Type II hypersensitivity reactions are antibody-mediated responses involving IgG and IgM antibodies. These antibodies recognize and bind to antigens present on cell surfaces or in the extracellular matrix. The binding of antibodies to these antigens can activate the complement system, recruit inflammatory cells, and interfere with normal cellular functions. Examples of type II hypersensitivity reactions include autoimmune disorders like autoimmune hemolytic anemia and certain drug-induced immune reactions.
- Immune complex-mediated or type III hypersensitivity: Type III hypersensitivity reactions, known as immune complex-mediated hypersensitivity, occur when IgM and IgG antibodies form immune complexes with soluble antigens in the blood. These immune complexes can then deposit in various tissues, particularly blood vessel walls. The deposition of immune complexes triggers an inflammatory response, leading to tissue damage and injury. Diseases associated with type III hypersensitivity include systemic lupus erythematosus, rheumatoid arthritis, and immune complex glomerulonephritis.
- T cell-mediated or type IV hypersensitivity: Type IV hypersensitivity reactions involve T cell-mediated immune responses. These reactions are primarily driven by CD4+ helper T cells, although cytotoxic CD8+ T cells (CTLs) can also contribute in certain diseases. CD4+ T cells recognize specific antigens presented by antigen-presenting cells and release cytokines that promote inflammation and activate other immune cells, particularly neutrophils and macrophages. Type IV hypersensitivity reactions are characterized by delayed onset, typically occurring within 24 to 48 hours after exposure to the antigen. Examples of type IV hypersensitivity reactions include contact dermatitis, tuberculin skin test reactions, and graft rejection.
Therapeutic Approaches For Immunologic Diseases
The creation of innovative medicines based on an understanding of fundamental science and its application to human disease has been one of the most remarkable achievements of immunology. Therapies can be categorised into numerous main categories.
1. Anti-inflammatory Agents
- Anti-inflammatory medications, namely corticosteroids, have been the cornerstone of hypersensitivity illness treatment for many years.
- These medications aim to reduce tissue damage, specifically the inflammatory component of pathological immune responses.
2. Depletion of Cells and Antibodies
- Antibodies that decrease all lymphoid cells, only B cells, or exclusively T cells are administered.
- Anti-CD20 antibody (rituximab), which depletes exclusively B cells, has been used successfully to treat disorders that were previously believed to be caused primarily by T cell–mediated inflammation.
- Some patients with rheumatoid arthritis and multiple sclerosis have exhibited positive responses to this medication. Plasmapheresis has been utilised to remove autoantibodies and immune complexes from circulation.
3. Anti-Cytokine Therapies
- A significant range of inflammatory cytokines are being addressed by specialised antagonists for the treatment of T cell-mediated chronic inflammatory disorders.
- The first achievement with this class of biologic treatments was a soluble version of the TNF receptor and TNF-neutralizing anti-TNF antibodies.
- Numerous patients with rheumatoid arthritis, Crohn’s disease, and psoriasis benefit greatly from these medications. Antibodies against various proinflammatory cytokines, such as IL-1, the p40 chain found in both IL-12 and IL-23, IL-6, IL-17A, and numerous more, are in use or in clinical development for the treatment of inflammatory illnesses.
4. Agents That Inhibit Cell-Cell Interactions in Immune Responses
- B7 costimulator-blocking agents are licenced for the treatment of rheumatoid arthritis and psoriasis, and are currently being evaluated for SLE and other illnesses.
- Antibodies against CD40 ligand inhibit T cell–mediated activation of B cells and macrophages and have been found to be beneficial in patients with inflammatory bowel disease; however, a small number of treated patients have developed thrombotic episodes, presumably because this molecule is expressed on human platelets (where its function is unknown).
- Multiple sclerosis has been treated using anti-integrin antibodies to prevent leukocyte migration into tissues, particularly the central nervous system (CNS).
5. Intravenous IgG
- In certain hypersensitivity illnesses, large doses of intravenous IgG (IVIG) are useful. It is unclear how this drug inhibits immunological inflammation; nevertheless, it is possible that the IgG binds to the inhibitory Fc receptor (FcRIIB) on macrophages and B cells, thereby dampening inflammatory reactions.
- IVIG may also compete with pathogenic antibodies for binding to the neonatal Fc receptor (FcRn), which in adults serves to protect antibodies from catabolism, hence reducing the half-lives of the pathogenic antibodies.
- Ongoing efforts include developing tolerance in disease-producing T cells and creating regulatory T cells that are selective for self antigens.
- Multiple sclerosis and type 1 diabetes are two immunological illnesses for which the target antigens have been identified; in both cases, antigens to inhibit specific immune responses are being tested in clinical studies.
- Numerous medications that inhibit specific immune system components pose the risk of interfering with the immune system’s natural function in combating germs, hence making persons susceptible to infection.
- Antigen Specific Tolerance circumvents this issue by targeting just the disease-causing lymphocytes. These general ideas are comparable to those upon which transplant rejection management is based.
Types of Hypersensitivity Reactions
Hypersensitivity reactions, as classified by Gell and Coombs, provide a framework for understanding and categorizing immune responses based on their timing and underlying mechanisms. The classification includes four types of hypersensitivity reactions, each characterized by a different latency period and immune response.
- Type 1 hypersensitivity, also known as immediate hypersensitivity, is the most rapid and acute form of hypersensitivity reaction. It occurs within seconds to minutes after exposure to an allergen. This type of reaction involves the activation of IgE antibodies, which are specific to environmental antigens. When the allergen interacts with the IgE antibodies bound to mast cells and basophils, it triggers the release of various inflammatory mediators, such as histamine and leukotrienes. This immediate response can lead to symptoms like itching, sneezing, hives, and in severe cases, anaphylaxis.
- Type 2 hypersensitivity, known as cytotoxic hypersensitivity, occurs within minutes to hours after exposure to an antigen. In this type of reaction, IgG or IgM antibodies bind to specific antigens present on the surface of cells or tissues. The binding of antibodies to these antigens can activate the complement system, leading to cell destruction through mechanisms like antibody-dependent cell-mediated cytotoxicity (ADCC) or complement-mediated lysis. Examples of type 2 hypersensitivity reactions include autoimmune hemolytic anemia and some drug-induced immune reactions.
- Type 3 hypersensitivity is characterized by immune complex-mediated reactions. Symptoms typically appear within several hours after exposure to the antigen. In this type, IgG or IgM antibodies form immune complexes with soluble antigens, and these complexes can deposit in various tissues, particularly in blood vessel walls. The deposition of immune complexes triggers an inflammatory response, causing tissue damage and injury. Type 3 hypersensitivity is associated with diseases like systemic lupus erythematosus, rheumatoid arthritis, and immune complex glomerulonephritis.
- Type 4 hypersensitivity, also known as delayed hypersensitivity or cell-mediated hypersensitivity, has a longer latency period compared to the previous types. It typically takes hours to days for symptoms to appear. Type 4 hypersensitivity involves T cell-mediated immune responses. CD4+ helper T cells recognize specific antigens presented by antigen-presenting cells and release cytokines that promote inflammation and activate other immune cells, particularly neutrophils and macrophages. This delayed response is commonly seen in conditions like contact dermatitis, tuberculin skin test reactions, and graft rejection.
Gell and Coombs’ classification of hypersensitivity reactions provides a useful framework for understanding the different types of immune responses involved in hypersensitivity disorders. These types range from immediate IgE-mediated reactions (type 1) to delayed T cell-mediated reactions (type 4), with distinct mechanisms and latency periods. Understanding these types is essential for diagnosing, treating, and managing hypersensitivity diseases effectively.
Summary of Hypersensitivity Type I, II, III and IV
| Characters | Type I Hypersensitivity (Allergic hypersensitivity) |
|---|---|
| Alternative Name | Allergic hypersensitivity |
| Principle | Antibody-mediated degranulation of granulocytes leads to the destruction of cells. |
| Primary Mediator | IgE |
| Other components as mediators | Mast cells, basophils, histamine, and other pharmacological agents |
| Reaction time | Immediate or within a few hours |
| Antigen | Free in circulation (soluble) |
| Antigen origin | Exogenous |
| Antibody | Fixed on mast cells and basophils |
| Mechanism | Allergen-specific IgE antibodies bind to mast cells via their Fc receptor, leading to mast cell degranulation. |
| Complement activation | No |
| Appearance | Weal and flare |
| Transfer with serum | Passive transfer possible with serum |
| Desensitization | Easy but short-lived |
| Examples | Asthma, rhinitis, atopic eczema, bee sting reaction |
| Characters | Type II Hypersensitivity (Cytotoxic hypersensitivity) |
|---|---|
| Alternative Name | Cytotoxic hypersensitivity |
| Principle | Antibody-mediated destruction of healthy cells |
| Primary Mediator | IgG/IgM |
| Other components as mediators | Complement, neutrophils |
| Reaction time | 5-8 hours |
| Antigen | Fixed on cells |
| Antigen origin | Endogenous or exogenous |
| Antibody | Free in circulation |
| Mechanism | IgG or IgM antibody binds to a cellular antigen, leading to complement activation and cell lysis. IgG can also mediate ADCC with cytotoxic T cells, natural killer cells, macrophages, and neutrophils. |
| Complement activation | Yes |
| Appearance | Lysis and necrosis |
| Transfer with serum | Passive transfer |
| Desensitization | Easy but short-lived |
| Examples | Rhesus incompatibility (Rh hemolytic disease), transfusion reactions, cell destruction due to autoantigens, drug-induced hemolytic anemia |
| Characters | Type III Hypersensitivity (Immune complex hypersensitivity) |
|---|---|
| Alternative Name | Immune complex hypersensitivity |
| Principle | Antigen-antibody complex-mediated destruction of cells |
| Primary Mediator | IgG/IgM |
| Other components as mediators | Complement, phagocytes, and K cells |
| Reaction time | 2-8 hours |
| Antigen | Free in circulation (soluble) |
| Antigen origin | Exogenous or endogenous |
| Antibody | Free in circulation |
| Mechanism | Antigen-antibody complexes are deposited in tissues. Complement activation provides inflammatory mediators and recruits neutrophils. Enzymes released from neutrophils damage tissue. |
| Complement activation | Yes |
| Appearance | Erythema and edema |
| Transfer with serum | Passive transfer |
| Desensitization | Easy but short-lived |
| Examples | Glomerulonephritis, systemic lupus erythematosus, Farmer’s lung arthritis, vasculitis |
| Characters | Type IV Hypersensitivity (Cell-mediated hypersensitivity/Delayed type of hypersensitivity) |
|---|---|
| Alternative Name | Cell-mediated hypersensitivity/Delayed type of hypersensitivity |
| Principle | T lymphocytes mediate the destruction of cells |
| Primary Mediator | Specific subsets of CD4+ helper T cells or CD8+ cytotoxic T cells |
| Other components as mediators | Dendritic cells, macrophages, and cytokines |
| Reaction time | After 24 hours, mostly 48-72 hours after contact |
| Antigen | Soluble or cell-bound |
| Antigen origin | Exogenous or endogenous |
| Antibody | Not applicable |
| Mechanism | Th2 cells secrete cytokines, which activate macrophages and cytotoxic T cells. |
| Complement activation | No |
| Appearance | Erythema and induration |
| Transfer with serum | Cannot be transferred with serum, but possible with T cell transfer |
| Desensitization | Difficult but long-lived |
| Examples | Tuberculin reaction, granuloma formation, allergic contact dermatitis, type 1 diabetes |
Summary Table of Hypersensitivity Type I, II, III and IV
Here’s the information presented in table format:
| Alternative Name | Allergic hypersensitivity | Cytotoxic hypersensitivity | Immune complex hypersensitivity | Cell-mediated hypersensitivity/Delayed type of hypersensitivity |
| Principle | Antibody-mediated degranulation of granulocytes leads to the destruction of cells. | Antibody-mediated destruction of healthy cells | Antigen-antibody complex-mediated destruction of cells | T lymphocytes mediate the destruction of cells |
| Primary Mediator | IgE | IgG/IgM | IgG/IgM | Specific subsets of CD4+ helper T cells or CD8+ cytotoxic T cells |
| Other components as mediators | Mast cells, basophils, histamine, and other pharmacological agents | Complement, neutrophils | Complement, phagocytes, and K cells | Dendritic cells, macrophages, and cytokines |
| Reaction time | Immediate or within a few hours | 5-8 hours | 2-8 hours | After 24 hours, mostly 48-72 hours after contact |
| Antigen | Free in circulation (soluble) | Fixed on cells | Free in circulation (soluble) | Soluble or cell-bound |
| Antigen origin | Exogenous | Endogenous or exogenous | Exogenous or endogenous | Exogenous or endogenous |
| Antibody | Fixed on mast cells and basophils | Free in circulation | Free in circulation | Not applicable |
| Mechanism | Allergen-specific IgE antibodies bind to mast cells via their Fc receptor, leading to mast cell degranulation. | IgG or IgM antibody binds to a cellular antigen, leading to complement activation and cell lysis. IgG can also mediate ADCC with cytotoxic T cells, natural killer cells, macrophages, and neutrophils. | Antigen-antibody complexes are deposited in tissues. Complement activation provides inflammatory mediators and recruits neutrophils. Enzymes released from neutrophils damage tissue. | Th2 cells secrete cytokines, which activate macrophages and cytotoxic T cells. |
| Complement activation | No | Yes | Yes | No |
| Appearance | Weal and flare | Lysis and necrosis | Erythema and edema | Erythema and induration |
| Transfer with serum | Passive transfer possible with serum | Passive transfer | Passive transfer | Cannot be transferred with serum, but possible with T cell transfer |
| Desensitization | Easy but short-lived | Easy but short-lived | Easy but short-lived | Difficult but long-lived |
| Examples | Asthma, rhinitis, atopic eczema, bee sting reaction | Rhesus incompatibility (Rh hemolytic disease), transfusion reactions, cell destruction due to autoantigens, drug-induced hemolytic anemia | Glomerulonephritis, systemic lupus erythematosus, Farmer’s lung arthritis, vasculitis | Tuberculin reaction, granuloma formation, allergic contact dermatitis, type 1 diabetes |
1. Type I hypersensitivity
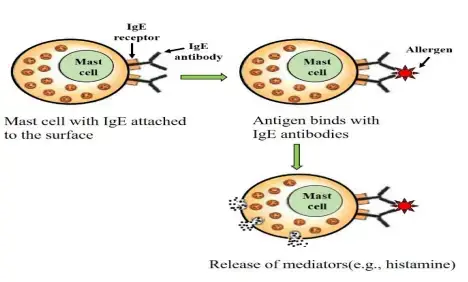
- Type I hypersensitivity, also known as allergic hypersensitivity, is an immediate immune response that occurs within seconds to minutes after exposure to specific antigens, known as allergens. These allergens can include insect venom, foods, pollen, dust mites, and more. Individuals with a predisposition produce IgE antibodies in response to these allergens.
- During sensitization, the IgE antibodies bind to mast cells and basophils via Fc receptors. Upon re-exposure to the allergen, cross-linkage of IgE occurs, leading to the immediate release of mediators such as histamine and kininogen. This release of mediators induces various symptoms, including vasodilation, smooth muscle contraction, mucus secretion, edema, and skin blisters.
- Allergens are often small proteins that can easily penetrate the skin or mucosa and are frequently proteases. The exact mechanism by which B cells produce IgE in response to allergens is not fully understood. However, IL-4 plays a role in promoting the differentiation of TH2 cells, which secrete cytokines that stimulate IgE production by plasma cells.
- Type I hypersensitivity can manifest as systemic or local reactions. Systemic reactions involve skin erythema followed by respiratory difficulties due to bronchial constriction. Local reactions typically occur on the skin or mucosal surfaces at the site of antigen exposure. Examples include allergies to penicillin, Aspergillus spores, or rupture of an Echinococcus cyst.
- Symptoms of type I hypersensitivity can affect different parts of the body, resulting in conditions such as rhinitis (nasal allergy), conjunctivitis (ocular allergy), and dermatological allergies like eczema. The immune response is mediated by immunoglobulin E (IgE), which interacts with the antigen, leading to the release of histamine and other inflammatory mediators.
- The immune response to type I hypersensitivity occurs in two stages. The first stage is the sensitization stage, where the host is initially exposed to the antigen. This contact is asymptomatic as the host recognizes the antigen for the first time. In the second stage, known as the late-phase reaction, the sensitized host is re-exposed to the antigen, triggering the development of a type I hypersensitivity reaction.
- Various types of antigens can induce hypersensitivity reactions, including certain foods (such as nuts, soy, and wheat), animal sources (like bee venom, cat dander, and rat dander), environmental sources (such as dust, pollen, and molds), and drugs (especially antibiotics, but also anesthesia drugs like propofol and isoflurane). These antigens can elicit an exaggerated immune response in susceptible individuals, leading to type I hypersensitivity reactions.
Characteristics
Type I hypersensitivity, also known as allergic hypersensitivity, is characterized by several distinct features:
- Immediate Onset: Type I hypersensitivity reactions occur rapidly, typically within seconds to minutes after exposure to the allergen. This immediate response sets it apart from other types of hypersensitivity reactions.
- IgE-Mediated: Type I hypersensitivity is primarily mediated by immunoglobulin E (IgE) antibodies. Individuals with a predisposition to allergies produce IgE antibodies in response to specific allergens. These IgE antibodies bind to Fc receptors on mast cells and basophils.
- Sensitization: The initial exposure to an allergen triggers a sensitization process. During this stage, the immune system recognizes the allergen as foreign and produces allergen-specific IgE antibodies. These IgE antibodies bind to the surface of mast cells and basophils, sensitizing them for future encounters with the allergen.
- Re-Exposure: Upon re-exposure to the allergen, the allergen binds to the IgE antibodies already attached to mast cells and basophils. This cross-linking of IgE molecules leads to the activation of mast cells and basophils.
- Release of Mediators: Activation of mast cells and basophils triggers the immediate release of various chemical mediators, including histamine, leukotrienes, prostaglandins, and cytokines. Histamine is particularly important and contributes to the characteristic symptoms of type I hypersensitivity, such as vasodilation, increased vascular permeability, smooth muscle contraction, mucus secretion, edema, and pruritus.
- Symptoms and Manifestations: Type I hypersensitivity can manifest in various ways, affecting different organ systems. Common symptoms include skin manifestations like itching, erythema, and hives (urticaria). Respiratory symptoms may include sneezing, rhinorrhea (runny nose), nasal congestion, coughing, and wheezing. Ocular symptoms can include redness, itching, and watering of the eyes. Systemic reactions may lead to anaphylaxis, a severe and potentially life-threatening allergic reaction involving multiple organ systems.
- Allergen Diversity: Type I hypersensitivity can be triggered by a wide range of allergens, including insect venom, pollen, dust mites, certain foods (e.g., nuts, shellfish, dairy), medications (e.g., antibiotics, non-steroidal anti-inflammatory drugs), and environmental factors (e.g., mold spores, pet dander).
Mechanism of Type-I hypersensitivity
The mechanism of Type I hypersensitivity involves a complex cascade of events that occur upon exposure to specific allergens. Here is an overview of the key steps involved:
- IgE Synthesis: Individuals prone to Type I hypersensitivity exhibit a preference for IgE antibody synthesis in response to specific antigens (allergens). This is influenced by the production of interleukin-4 (IL-4) and interleukin-13 (IL-13), which promote the class switch to IgE antibodies.
- Binding of IgE to Mast Cells and Basophils: The Fc component of IgE antibodies binds to FcIII and CD23 receptors located on the surface of mast cells and basophils. This sensitizes these cells for subsequent encounters with the allergen.
- Cross-Linking and Degranulation: Upon re-exposure to the same allergen, the allergen molecules cross-link the cell-bound IgE antibodies on mast cells and basophils. This cross-linking triggers a process called degranulation, where the cells release various pharmacologically active substances stored in their granules. An increase in calcium influx precedes mast cell degranulation.
- Release of Mediators: Degranulation of mast cells and basophils leads to the release of a wide range of inflammatory mediators. These substances include histamine, leukotrienes, bradykinins, prostaglandins, and cytokines. They contribute to the immediate allergic responses observed within minutes after antigen exposure.
- Mast Cell Activation: Cross-linking of the IgE Fc receptors is crucial for mast cell activation. The released inflammatory mediators play a key role in initiating and amplifying the allergic response. For example, platelet-activating factor (PAF) stimulates platelet aggregation and the release of histamine, heparin, and other vasoactive amines, further enhancing the reaction. Eosinophil chemotactic factor of anaphylaxis (ECF-A) and neutrophil chemotactic factors attract eosinophils and neutrophils, respectively, which then release different enzymes contributing to tissue damage.
- Late Phase Reaction: In addition to the immediate phase of Type I hypersensitivity, a late-phase reaction may occur several hours after antigen exposure. During this phase, cell-bound IgE on the surface of basophils binds a chemical called histamine releasing factor, potentially generated by macrophages and B-lymphocytes. This leads to further histamine release and prolonged allergic symptoms.
The intricate interplay between IgE antibodies, mast cells, basophils, and the release of various inflammatory mediators forms the basis of the mechanism of Type I hypersensitivity. Understanding this mechanism is crucial for developing effective treatments and preventive strategies for allergic reactions.
Effects of Type-I Hypersensitivity
Type-I hypersensitivity reactions can have various effects on the body due to the release or production of inflammatory agents. Here are some of the common effects observed:
- Dilation of Blood Vessels: The release of inflammatory mediators, such as histamine, leads to the dilation of blood vessels in the affected area. This results in local redness (erythema) at the site of allergen injection. If the dilation is widespread, it can contribute to lower vascular resistance, a reduction in blood pressure, and potentially lead to shock.
- Increased Capillary Permeability: Inflammatory mediators also increase the permeability of capillaries, leading to leakage of fluid into the surrounding tissues. This causes localized tissue enlargement, known as edema. If the edema is widespread, it can decrease blood volume and potentially result in shock.
- Bronchial Airway Constriction: One of the hallmark effects of Type-I hypersensitivity is the constriction of bronchial airways. The smooth muscles surrounding the airways contract in response to inflammatory mediators, leading to narrowing of the air passages. This can result in wheezing and difficulty breathing, characteristic of conditions like asthma.
- Stimulation of Mucous Secretion: Inflammatory mediators can stimulate the production of excessive mucus in the respiratory tract. This leads to airway congestion and increased production of phlegm, further contributing to breathing difficulties and coughing.
- Stimulation of Nerve Endings: The release of inflammatory mediators can also stimulate nerve endings in the affected area. This stimulation causes skin irritation and discomfort, leading to itching, redness, and localized sensations of heat or pain.
These effects collectively contribute to the symptoms experienced during Type-I hypersensitivity reactions. They include localized and systemic manifestations such as redness, swelling, lowered blood pressure, shock, wheezing, breathing difficulties, mucus production, and skin irritation. Understanding these effects is crucial for managing and treating allergic reactions effectively.
Pathology of Type-I hypersensitivity
- Type I is primarily composed of mast cells and basophils. Platelets, neutrophils, and eosinophils modify and/or amplify the reaction.
- The reaction may affect the skin (urticaria and eczema), the eyes (conjunctivitis), the nasopharynx (rhinorrhea, rhinitis), the bronchopulmonary tissues (asthma), and the gastrointestinal tract (gastroenteritis). The reaction may result in anything from a small annoyance to death.
- In the majority of domesticated species, the lungs are the primary target organs and the portal-mesenteric vasculature is a secondary target; however, in dogs, the roles are inverted.
- The liver is the primary organ affected by anaphylactic shock in dogs, and indications are related with constriction of hepatic veins, which results in portal hypertension and visceral blood pooling. Dogs are more likely to have gastrointestinal than respiratory symptoms.
- Human IgE-mediated illnesses include systemic anaphylactic shock, asthma, allergic rhinitis (hay fever), tropical pulmonary eosionophila, allergic conjunctivitis, skin reactions (urticaria, eczema), and food allergies.
- Systemic anaphylactic shock, urticarial reactions (hives), atopic dermatitis, food allergies, allergic enteritis, atypical interstitial pneumonia in cattle, chronic allergic bronchitis and pulmonary infiltration with eosinophilia in dogs, and allergic bronchiolitis and asthma in cats are IgE-mediated diseases in animals.
Type-I Hypersensitivity Summary Table
| Alternative Name | Allergic hypersensitivity |
| Principle | Antibody-mediated degranulation of granulocytes leads to the destruction of cells. |
| Primary Mediator | IgE |
| Other components as mediators | Mast cells, Basophils, histamine & other pharmacological agents |
| Reaction time | Immediate or within a few hours |
| Antigen | Free in circulation (Soluble) |
| Antigen origin | Exogenous |
| Antibody | Fixed on mast cells and basophils |
| Mechanism | IgE antibodies specific for allergens bind to mast cells via their Fc receptor. When a specific allergen binds to IgE, cross-linking of IgE triggers mast cell degranulation. |
| Complement activation | No |
| Appearance | Weal & flare |
| Transfer with serum | Passive transfer possible with serum |
| Desensitization | Easy but short-lived |
| Examples | Asthma, Rhinitis, Atopic eczema, Bee sting reaction |
2. Type II Hypersensitivity (Antibody-mediated cytotoxic hypersensitivity)
Type II hypersensitivity, also known as antibody-mediated cytotoxic hypersensitivity, involves immune reactions where antibodies (IgG or IgM) target and attack specific antigens on cell surfaces or extracellular matrix. This type of hypersensitivity can lead to cellular destruction, functional loss, or damage to tissues. Here are some key points about Type II hypersensitivity:
- Immunization during pregnancy: A classic example of Type II hypersensitivity is the immunization of individuals to erythrocyte antigens during pregnancy. In cases where the fetus inherits certain erythrocyte antigens from the father, the mother’s immune system can produce antibodies against these antigens, leading to severe hemolysis of fetal erythrocytes.
- Drug-induced reactions: Type II hypersensitivity can occur due to drug-induced immune responses. For example, drugs like penicillin can bind to erythrocytes, and subsequent antibodies against the drug can cause lysis of the erythrocytes. In some cases, antibodies directed against the basement membrane of the glomerulus (a component of the kidney) can cross-react with the basement membrane of the lung, leading to lung damage and glomerulonephritis.
- Mechanisms of damage: Type II hypersensitivity reactions can cause damage through different mechanisms:
- a) Complement-mediated lysis: Antibodies binding to cell surface antigens can activate the complement system, leading to cell lysis. For example, red blood cells can be damaged through this mechanism, resulting in hemolytic anemia.
- b) Antibody-dependent cellular cytotoxicity: Various cell types, such as macrophages, neutrophils, and natural killer cells, can cause lysis of target cells coated with IgG antibodies.
- c) Antibody-mediated cellular dysfunction: In some cases, antibodies can impair the function of cells by binding to cell surface receptors. This can lead to conditions like myasthenia gravis, where antibodies react with acetylcholine receptors on motor endplates, affecting neuromuscular transmission.
- Pathophysiology: The pathophysiology of Type II hypersensitivity reactions can involve different immune-mediated events, which can vary in their manifestations. These events can include cell depletion or destruction without inflammation, inflammation mediated by complement or Fc receptors, and cellular dysfunction caused by antibodies.
Type II hypersensitivity reactions play a role in various conditions, including autoimmune disorders, drug-induced reactions, and immune-mediated tissue damage. Understanding the mechanisms and manifestations of Type II hypersensitivity is crucial for diagnosis, treatment, and management of these immune responses.
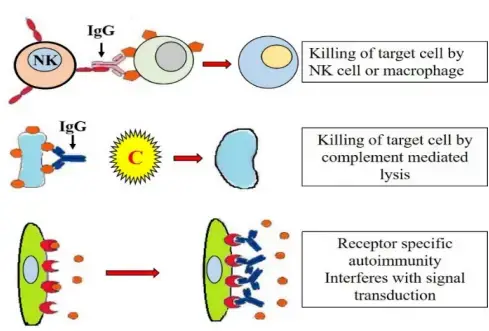
Mechanism of Type II Hypersensitivity
The mechanism of Type II hypersensitivity, also known as antibody-mediated cytotoxic hypersensitivity, involves the production of IgM or IgG antibodies against self-antigens or the development of cross-reactive antibodies during infections. Here is an overview of the mechanisms involved:
- Loss of immunological tolerance or cross-reactivity: In Type II hypersensitivity, the immune system generates IgM or IgG antibodies against self-antigens. This can occur due to a breakdown of immunological tolerance, where the immune system fails to recognize self-antigens as harmless, or through the development of cross-reactive antibodies during infections. Cross-reactive antibodies produced against infectious agents can bind to normal tissue antigens, leading to an immune response against self-tissues.
- Binding of antibodies to host cells: The antibodies produced in Type II hypersensitivity reactions bind to specific antigens present on the surface of host cells. This binding triggers various mechanisms that can result in cell damage or destruction.
- Opsonization: Antibodies, particularly IgG, can act as opsonins, promoting phagocytosis by immune cells such as macrophages and neutrophils. The antibodies coating the host cells act as tags, allowing phagocytes to recognize and adhere to the cells more efficiently. Subsequently, the phagocytes release lysosomal enzymes, leading to the destruction of the opsonized cells.
- Classical complement pathway activation: Antibody-antigen complexes can activate the classical complement pathway. This results in the formation of membrane attack complexes (MACs), which can insert into the cell membranes of target cells, leading to cell lysis.
- Antibody-dependent cellular cytotoxicity (ADCC): ADCC involves the attachment of natural killer (NK) cells to the Fc region of the antibodies bound to host cells. The binding triggers the release of cytotoxic substances by NK cells, leading to the death of the targeted cells.
These mechanisms collectively contribute to the cytotoxic effects observed in Type II hypersensitivity reactions. The opsonization, complement activation, and ADCC processes can lead to tissue damage and destruction, depending on the target cells involved.
Pathology of Type II Hypersensitivity
- Abs directed against antigens located on cell surfaces or the extracellular matrix (type IIA) or Abs with agonistic/antagonistic characteristics mediate this interaction (type IIB).
- Blood cells are the most common type of involved cells. The result could be:
- hemolytic anemia if RBCs are involved,
- leukopenia involving WBCs, or
- thrombocytopenia involving platelets.
- Under some circumstances, a cytotoxic attack on vascular epithelial cells will cause a vasculitis with local vascular leakage.
- The lesion contains antibody, complement and neutrophils.
Examples of Type II Hypersensitivity
Blood group AB and Rh responses (blood transfusion reactions, erythroblastosis foetalis)
Autoimmune diseases:
- In rheumatic fever, antibodies cause damage to joints and heart valves.
- Idiopathic thrombocytopenia purpura in which antibodies cause platelet breakdown.
- In myasthenia gravis, antibodies attach to acetylcholine receptors on muscle cells, resulting in abnormal muscular enervation.
- In Goodpasture’s syndrome, antibodies cause the death of kidney cells.
- Graves’ disease is characterised by the production of antibodies against thyroidstimulating hormone receptors on thyroid cells, resulting in impaired thyroid function.
- Multiple sclerosis characterised by the production of antibodies against the oligodendroglial cells that produce myelin, the protein that forms the myelin sheath that insulates the nerve fibres of neurons in the brain and spinal cord.
| Alternative Name | Cytotoxic hypersensitivity |
| Principle | Antibody-mediated destruction of healthy cells. |
| Primary Mediator | IgG/IgM |
| Other components as mediators | Complement, Neutrophils |
| Reaction time | 5-8 hours |
| Antigen | Fixed on cells |
| Antigen origin | Exogenous or endogenous |
| Antibody | Free in circulation |
| Mechanism | IgG or IgM antibody binds to cellular antigen, resulting in activation of the complement system and cell lysis. IgG is also capable of facilitating ADCC with cytotoxic T cells, natural killer cells, macrophages, and neutrophils. |
| Complement activation | Yes |
| Appearance | Lysis & necrosis |
| Transfer with serum | Passive transfer |
| Desensitization | Easy but short-lived |
| Examples | Rhesus incompatibility (Rh hemolytic disease), Transfusion Reactions, Cell Destruction due to autoantigens, Drug-Induced Hemolytic Anemia |
3. Type – III Hypersensitivity (Immune complex mediated hypersensitivity)
Type III hypersensitivity, also known as immune complex-mediated hypersensitivity, involves the formation of antibody-antigen complexes (immune complexes) during an immune response. Here is an overview of the characteristics and mechanisms of Type III hypersensitivity:
- Formation of immune complexes: Immune complexes are formed when antibodies, usually of the IgG or IgM class, bind to soluble antigens. These complexes can circulate in the bloodstream or deposit in various tissues, including vessel walls, the basement membrane of the lungs and kidneys, and joint tissues (synovia).
- Localization and tissue damage: Immune complexes can settle in specific tissues, leading to the activation of the complement system and inflammatory processes. The immune complexes can bind to Fc receptors on various cells, inducing degranulation and the release of inflammatory mediators. Complement factors C3a and C5a (anaphylatoxins) can be generated, further promoting inflammation. The presence of immune complexes in tissues triggers an inflammatory reaction that can lead to tissue damage.
- Arthus reaction: The Arthus reaction is a specific type III hypersensitivity reaction that occurs when an antigen enters the skin of an individual who already has preformed IgG antibodies against that antigen. The immune complexes formed in the skin can bind to Fc receptors, leading to degranulation of inflammatory cells and recruitment of more inflammatory cells. Complement activation occurs, resulting in the release of C5a and the induction of local inflammation, platelet accumulation, and eventually blood vessel occlusion with necrosis.
- Systemic and local disease: Type III hypersensitivity reactions can manifest as systemic or local diseases.
- Systemic disease: In systemic diseases like serum sickness and systemic lupus erythematosus (SLE), there is a large excess of antibodies and immune complexes. These complexes are deposited in the vessel walls and other tissues, leading to necrotizing vasculitis and the accumulation of neutrophils. SLE is a representative example of a systemic disease associated with Type III hypersensitivity.
- Local disease: In local diseases such as the Arthus reaction, immune complexes form at the site of antigen exposure, causing local tissue damage and inflammation. For example, intradermal injection of antigens in individuals with preformed antibodies can result in local vasculitis, redness, and swelling. Repeated vaccinations with certain antigens can also rarely lead to local vasculitis.
Other examples of Type III hypersensitivity reactions include poststreptococcal acute glomerulonephritis, where immune complexes deposit in the glomeruli, and hepatitis B virus (HBV) infection, where HBsAg-Ab complexes may cause acute glomerulonephritis.
Overall, Type III hypersensitivity reactions involve the formation and deposition of immune complexes, leading to tissue damage and inflammation in various organs and tissues. Understanding the underlying mechanisms helps in diagnosing and managing immune complex-mediated diseases and developing targeted therapeutic approaches to modulate the immune response.

Characteristics
- In type III hypersensitivity, soluble immune complexes are generated in the blood, deposited in various tissues (usually skin, kidney, and joints), activate the classical complement system, and result in inflammatory damage.
- It is mediated by immunological complexes that are soluble. They are predominantly IgG, however IgM may also be present.
- The reaction takes between three and ten hours to develop.
- Exogenous (chronic bacterial, viral, or parasite infections) or endogenous (self-produced) antigens may be present (non-organ specific autoimmunity: e.g., Systemic Lupus Erythematosus-SLE).
- The antigen is water-soluble and is not linked to the affected organ.
- A sustained presence of soluble antigen and antibody is required for the development of an immune-complex illness.
Mechanism of Type III Hypersensitivity
The mechanism of Type III hypersensitivity, also known as immune complex-mediated hypersensitivity, involves the formation, deposition, and subsequent activation of immune complexes. Here is an overview of the mechanism:
- Formation of immune complexes: Soluble antigens and antibodies, typically of the IgG or IgM class, combine to form immune complexes. These complexes can be cleared by macrophages in the spleen and liver under normal circumstances.
- Lodging and deposition of immune complexes: When excessive amounts or large immune complexes are present, they can become lodged in capillaries and pass between endothelial cells, particularly in the skin, joints, and kidneys. These immune complexes get trapped on the basement membrane surrounding these cells.
- Activation of the complement pathway: The immune complexes activate the classical complement pathway. Complement proteins, including C3a and C5a, are generated, leading to inflammation and the recruitment of inflammatory cells.
- Inflammation and tissue damage: The immune complexes and complement proteins, especially C5a, trigger a cascade of inflammatory events:
- Neutrophil activation: Neutrophils are recruited to the site of immune complex deposition, attracted by C5a. The presence of immune complexes causes neutrophils to release their lysosomes, containing enzymes and toxic substances, leading to tissue damage and additional inflammation.
- Complement-mediated tissue injury: The membrane attack complex (MAC) formed during complement activation can directly cause the death of adjacent tissue cells, contributing to tissue damage.
- Platelet aggregation and microthrombi formation: The activation of platelets by immune complexes leads to platelet aggregation, resulting in increased inflammation and the production of microthrombi (small blood clots). These microthrombi can block capillaries, further impairing blood flow and contributing to tissue damage.
Overall, the formation and deposition of immune complexes, along with the activation of the complement pathway, lead to inflammation, tissue damage, and the characteristic manifestations of Type III hypersensitivity reactions. The excessive immune complex deposition and the subsequent inflammatory response can affect various organs and tissues, resulting in diseases such as serum sickness, systemic lupus erythematosus (SLE), and vasculitis. Understanding the underlying mechanisms helps in diagnosing and managing immune complex-mediated diseases and developing targeted therapeutic approaches to modulate the immune response.
Pathology of Type III Hypersensitivity
- The affinity of antibodies and the size of immune complexes are crucial for the development of disease and identification of the implicated tissue.
- The lesion is predominantly composed of neutrophils, immune complex deposits, and complement.
- Later-stage infiltrating macrophages may have a role in the healing process.
- The route by which antigen enters the body has a significant impact on the placement of immune complexes:
- Antigens inhaled through the lungs cause pneumonitis.
- Antigens that penetrate the skin result in localised skin lesions.
- Antigens that enter the bloodstream generate immune complexes that are deposited in the glomeruli of the kidneys or the joints.
- Variable clinical manifestations may include fever, dermatological manifestations, polyarthritis, ataxia, behavioural changes, or nonspecific manifestations such as vomiting, diarrhoea, or abdominal pain.
| Alternative Name | Immune complex hypersensitivity |
| Principle | Antigen-antibody complex-mediated destruction of cells. |
| Primary Mediator | IgG/IgM |
| Other components as mediators | Complement, phagocytes and K cells |
| Reaction time | 2-8 hours |
| Antigen | Free in circulation ( Soluble) |
| Antigen origin | Exogenous or endogenous |
| Antibody | Free in circulation |
| Mechanism | In tissues, antigen-antibody complexes are deposited. Activation of the complement produces inflammatory mediators and attracts neutrophils. Neutrophil-released enzymes are destructive to tissue. |
| Complement activation | Yes |
| Appearance | Erythema & edema |
| Transfer with serum | Passive transfer |
| Desensitization | Easy but short-lived |
| Examples | Glomerulonephritis, Systemic Lupus Erythematosus, Farmer’s lung arthritis, Vasculitis |
4. Type – IV Hypersensitivity (Cell Mediated Hypersensitivity) (Delayed Type Hypersensitivity)
Type IV hypersensitivity, also known as cell-mediated hypersensitivity or delayed-type hypersensitivity (DTH), is a type of immune reaction that involves T cells. Here is an overview of the characteristics and mechanisms of Type IV hypersensitivity:
- Sensitization phase: Type IV hypersensitivity begins with the sensitization phase. Haptens, small molecules that cannot function as antigens on their own, can penetrate the skin and bind to carrier proteins. Antigen-presenting cells in the skin, such as Langerhans cells, capture the hapten-carrier complexes and migrate to regional lymph nodes. In the lymph nodes, antigen presentation to T cells occurs, leading to T-cell stimulation and activation. This sensitization phase typically lasts around 10-14 days.
- Re-exposure and T-cell activation: Upon re-exposure to the hapten, antigen-specific T cells that were previously sensitized migrate to the skin. These T cells accumulate at the site of exposure and proliferate. The activated T cells release cytokines, which contribute to local inflammation and the recruitment of other immune cells. This response is typically delayed, occurring within 48-72 hours after re-exposure.
- Types of Type IV hypersensitivity reactions:
- a) Acute Type IV hypersensitivity: Mediated by CD4+ T helper cells. Examples include the tuberculin test, used to detect exposure to Mycobacterium tuberculosis, and contact dermatitis caused by allergens such as nickel or chemicals found in rubber. Sensitized CD4+ T cells become activated upon exposure to the specific antigen, leading to the release of cytokines and the resulting inflammatory response.
- b) Chronic Type IV hypersensitivity: Mediated by CD8+ cytotoxic T cells. Examples include granuloma formation and graft rejection. CD8+ T cells and lymphocytes surround epithelioid cells, leading to the formation of granulomas, which are characteristic of chronic inflammation.
- Mechanism: In Type IV hypersensitivity reactions, T cells play a central role. The reaction occurs as follows:
- The body is initially exposed to the antigen, either directly or through sensitization.
- Antigen-presenting cells, such as macrophages, engulf the antigen and present it to T cells.
- T cells become activated and sensitized, leading to the release of cytokines and chemokines.
- The cytokines and chemokines attract and activate other immune cells, leading to tissue damage and inflammation at the site of exposure.
- The delayed reaction is important for the body’s defense against pathogens, such as mycobacteria and certain fungi, as well as tumors.
Type IV hypersensitivity reactions are typically characterized by delayed onset and involve T-cell-mediated immune responses. Understanding the mechanisms and subtypes of Type IV hypersensitivity is important for diagnosing and managing conditions such as contact dermatitis, tuberculosis, and chronic inflammatory diseases.

Characteristics of Type IV Hypersensitivity
- As the reaction takes more than 12 hours to develop, type IV hypersensitivity is also known as delayed type hypersensitivity. The maximal reaction time is typically between 48 and 72 hours.
- It is mediated by cells that induce an inflammatory response to external or endogenous antigens.
- The predominant cells are T lymphocytes and monocytes/macrophages.
- T cells and antigen-presenting cells (APC) release cytokines that trigger a local inflammatory response in a sensitised individual in response to foreign antigens.
- DHR cannot be transmitted via antibodies or serum from one animal to another. However, it is transmissible by T cells, specifically CD4 Th1 cells.
Mechanism of Type IV Hypersensitivity
The mechanism of Type IV hypersensitivity, also known as cell-mediated hypersensitivity or delayed-type hypersensitivity (DTH), involves the activation and response of CD8 cytotoxic T cells and CD4 helper T cells. Here are the key steps involved in the mechanism of Type IV hypersensitivity:
- Antigen presentation: Antigen-presenting cells (APCs), particularly macrophages, play a crucial role in Type IV hypersensitivity. They capture and process antigens associated with type I or type II major histocompatibility complex (MHC) molecules.
- T-cell activation: CD8 cytotoxic T cells and CD4 helper T cells recognize the antigens presented on the MHC molecules by interacting with specific T-cell receptors. CD4 T cells further differentiate into specific subsets, such as Th1 or Th17 cells, which play a significant role in the immune response.
- Release of cytokines: Activated CD4 T cells and other immune cells release various cytokines, including interleukin 1 (IL-1), tumor necrosis factor (TNF), and monocyte chemotactic factor. These cytokines promote the recruitment and activation of other immune cells, such as antigen-nonspecific T cells and macrophages, to the site of inflammation.
- Inflammatory response: Cytokines and chemokines released by activated T cells and other immune cells attract antigen-nonspecific T cells and macrophages to the affected tissue. Keratinocytes, APCs, and T cells also secrete cytokines that contribute to the local inflammatory response.
- Effector cell responses: The activated CD8 cytotoxic T cells directly recognize and destroy target cells that present the specific antigen. Upon contact, CD8 cells release cytotoxic molecules that induce apoptosis in the target cells. On the other hand, activated macrophages produce hydrolytic enzymes and transform into large cells called multinucleated giant cells. These cells contribute to tissue damage and inflammation.
Overall, the mechanism of Type IV hypersensitivity involves the activation of T cells, the release of cytokines, the recruitment of immune cells, and the subsequent inflammatory response. The effector responses of CD8 cytotoxic T cells and activated macrophages play a crucial role in causing tissue damage and inflammation at the site of exposure or sensitization.
Pathology of Type IV Hypersensitivity
- Many autoimmune and infectious disorders (tuberculosis, leprosy, blastomycosis, histoplasmosis, toxoplasmosis, leishmaniasis, etc.) and granulomas caused by infections and foreign antigens are associated with Type IV hypersensitivity.
- There are three types of delayed hypersensitivity, and their maximum reaction time is indicated between brackets:
- Contact (48 to 72 hours)
- Tuberculin (48 to 72 hours)
- Granulomatous (21 to 28 days)
- Lesions of delayed hypersensitivity consist predominantly monocytes and a few T lymphocytes.
- Contact: Infiltrates of mononuclear cells are present in both the dermis and epidermis.
- Tuberculin: leukocyte infiltration of the skin
- Granulomatous: epithelioid-cell granuloma and large cells at the lesion’s centre, surrounded by lymphocytes.
| Alternative Name | Cell-mediated hypersensitivity/ Delayed type of hypersensitivity |
| Principle | T lymphocytes mediated the destruction of cells. |
| Primary Mediator | Specific subsets of CD4+ helper T cells or CD8+ cytotoxic T cells. |
| Other components as mediators | Dendritic cells, macrophages, and cytokines |
| Reaction time | After 24 hours only, mostly 48-72 hours after contact |
| Antigen | Soluble or cell-bound |
| Antigen origin | Exogenous or endogenous |
| Antibody | Not applicable |
| Mechanism | Th2 cells secrete cytokines, which activate macrophages and cytotoxic T cells. |
| Complement activation | No |
| Appearance | Erythema & induration |
| Transfer with serum | Cannot be transferred with serum; but possible with T cells transfer |
| Desensitization | Difficult but long-lived. |
| Examples | The tuberculin reaction, Granuloma formation, Allergic contact dermatitis, Type-1 diabetes |
5. Type V (Stimulatory Type) Hypersensitivity
- In this form of hypersensitive reaction, antibodies bind with antigens on the cell surface, inducing cell proliferation and differentiation and boosting the activity of effector cells.
- The type V hypersensitivity reaction plays a crucial part in the pathophysiology of Graves’ disease, in which excessive thyroid hormones are produced.
- Long-acting thyroid-stimulating antibody, which is an autoantibody to thyroid membrane antigen, is hypothesised to combine with thyroid-stimulating hormone (TSH) receptors on the surface of a thyroid cell.
- Graves’ illness is caused by the interaction between the TSH receptor and TSH, which exerts a similar effect to that of TSH and results in the overproduction and secretion of thyroid hormone.
Diagnosis of Hypersensitivities
The diagnosis of hypersensitivities involves various diagnostic tests and a comprehensive medical history. Here are the key methods used for diagnosing hypersensitivities:
- Type I Hypersensitivity Diagnosis:
- Serum IgE levels: Testing the levels of IgE antibodies in the blood can provide information about allergic sensitization. However, elevated IgE levels do not always indicate an allergy.
- Prick-prick skin test (PPST): Allergens are applied to the patient’s skin through superficial pricks. The skin is observed for any wheal-flare reaction, indicating an allergic response. PPST is considered a practical and cost-effective method for diagnosing allergies.
- Intradermal test: A small amount of allergen is injected into the dermis using a tuberculin needle. The skin is examined for any wheal-flare reaction, similar to the PPST. This test can be more sensitive but also carries a higher risk of false-positive results.
- Type III Hypersensitivity Diagnosis:
- Medical history: A detailed patient history is essential for diagnosing Type III hypersensitivities since the symptoms can overlap with other conditions.
- Observation of symptoms: Type III hypersensitivity reactions often present with visible symptoms. However, these symptoms can be associated with various other conditions, making accurate diagnosis challenging.
- Additional tests: In cases of hypersensitivity pneumonitis, specific tests such as bronchoalveolar lavage (BAL), pulmonary function tests, and high-resolution computed tomography (HRCT) may be performed to establish the diagnosis. These tests help evaluate lung function and identify characteristic patterns of inflammation and fibrosis.
It’s important to note that the diagnosis of hypersensitivities requires a combination of clinical evaluation, laboratory tests, and consideration of the patient’s medical history. Consulting with an allergist or immunologist who specializes in hypersensitivity disorders can help ensure an accurate diagnosis and appropriate management of the condition.
Treatments of Hypersensitivities
The treatment of hypersensitivities depends on the specific type of reaction and underlying condition. Here are the treatments commonly used for different types of hypersensitivities:
- Immediate Hypersensitivity Reactions:
- Anaphylaxis management: Anaphylaxis, a severe and potentially life-threatening allergic reaction, is treated with intramuscular adrenaline (epinephrine) to alleviate symptoms and stabilize blood pressure. Additional measures may include oxygen therapy, intravenous antihistamines, IV fluids to support blood pressure, and avoidance of allergens.
- Allergic bronchial asthma: Treatment options include inhaled short-acting and long-acting bronchodilators, inhaled corticosteroids, leukotriene antagonists, disodium cromoglycate, and environmental control measures. Medications like methotrexate or cyclosporine, and omalizumab (a monoclonal anti-IgE antibody), can be used in certain cases.
- Autoimmune Disorders:
- Treatment of autoimmune disorders like systemic lupus erythematosus (SLE) may involve a combination of nonsteroidal anti-inflammatory drugs (NSAIDs), hydroxychloroquine, azathioprine, methotrexate, mycophenolate, cyclophosphamide, low-dose IL-2, intravenous immunoglobulins, and belimumab.
- Omalizumab: This monoclonal antibody can be used to treat moderate to severe allergic bronchial asthma by targeting the high-affinity IgE receptor on mast cells.
- Delayed Hypersensitivity Reactions:
- Treatment of delayed hypersensitivity reactions focuses on addressing the underlying cause.
- Tuberculosis: Common drugs used for tuberculosis treatment include isoniazid, rifampin, ethambutol, and pyrazinamide. Drug-resistant cases may require a combination of antibiotics like amikacin, kanamycin, or capreomycin.
- Leprosy: Treatment involves a combination of drugs such as rifampicin, clofazimine, and dapsone, tailored based on the type of leprosy (paucibacillary or multibacillary).
- Schistosomiasis: Praziquantel is the drug of choice for treating infections caused by Schistosoma species.
- Sarcoidosis: Hydroxychloroquine and chloroquine can be used in the therapy of sarcoidosis involving the skin, lungs, and nervous system.
- Crohn’s disease: Anti-TNF monoclonal antibodies like adalimumab and certolizumab have been approved for the treatment of Crohn’s disease.
It is important to note that treatment approaches may vary based on individual patient factors, severity of the condition, and the guidance of healthcare professionals. Proper diagnosis and personalized treatment plans are crucial for managing hypersensitivity reactions effectively.
Selected Immunologic Diseases: Pathogenesis And Therapeutic Strategies
In the part that follows, we detail the pathogenesis of certain diseases caused by antibodies and T cells, as well as the application of novel therapeutics to these diseases, to demonstrate the previously described ideas.
Systemic Lupus Erythematosus: The Prototypic Immune Complex–Mediated Disease
- SLE is a chronic, remitting and relapsing, multisystem autoimmune illness that primarily affects women, with a female-to-male ratio of 10:1 and an incidence of 1 in 700 among women between the ages of 20 and 60 (approximately 1 in 250 for black women).
- Principal clinical signs include rashes, arthritis, and glomerulonephritis; however, hemolytic anaemia, thrombocytopenia, and involvement of the central nervous system are also prevalent. Multiple autoantibodies are detected in SLE patients.
- Antinuclear antibodies, especially antiDNA antibodies, are the most common; others include antibodies against ribonucleoproteins, histones, and nucleolar antigens.
- Immune complexes produced from these autoantibodies and their unique antigens are the cause of glomerulonephritis, arthritis, and vasculitis affecting the body’s tiny arteries.
- Hemolytic anaemia and thrombocytopenia are caused by erythrocyte- and platelet-specific autoantibodies, respectively.
- The presence of antinuclear antibodies is the primary diagnostic test for the condition; antibodies against double-stranded native DNA are specific for SLE.
Pathogenesis of Systemic Lupus Erythematosus
- SLE is a complex disease in which environmental and genetic variables contribute to the loss of tolerance in self-reactive B and T cells.
- Among the genetic factors is the transmission of certain HLA alleles. The odds ratio (relative risk) for persons with HLA-DR2 or HLA-DR3 is 2 to 3, and the odds ratio is approximately 5 if both haplotypes are present.
- In around 10% of SLE patients, genetic deficits of classical route complement proteins, specifically C1q, C2, or C4, are observed.
- The deficits in complement may lead to poor clearance of immunological complexes and apoptotic cells, as well as the absence of B cell tolerance.
- Some patients have been shown to have a polymorphism in the inhibitory Fc receptor FcRIIB; this may contribute to insufficient inhibition of B cell activation immunological cells.
- Genome-wide association studies have identified a large number of additional genes, but their roles and contributions to the development of the disease remain unknown. Environmental considerations include exposure to ultraviolet (UV) light.

- It is hypothesised that this results in apoptotic cell death and the release of nuclear antigens.
- Recent observations have led to the development of new hypotheses on the pathophysiology of SLE. First, patient investigations have demonstrated that blood cells exhibit a remarkable molecular signature (pattern of gene expression) that suggests exposure to IFN-, a type I interferon that is mostly produced by plasmacytoid dendritic cells.
- According to a number of studies, plasmacytoid dendritic cells from SLE patients also produce unusually high levels of IFN. Toll-like receptors (TLRs) that identify DNA and RNA, particularly the DNA-recognizing TLR9, play a crucial role in the activation of B lymphocytes specific for self nuclear antigens, according to animal model research.
- A model for the pathogenesis of SLE has been developed on the basis of these investigations.
- UV irradiation and other environmental stressors result in cell death, according to this concept.
- Due in part to abnormalities in clearance systems such as complement proteins and receptors, inadequate clearance of the nucleus of these cells leads in a high load of nuclear antigens.
- Polymorphisms in several lupus susceptibility genes result in impaired ability to maintain self tolerance in B and T cells, resulting in the survival of self-reactive lymphocytes.
- Failure of B cell tolerance may be caused by receptor editing, faulty elimination of immature B cells in the bone marrow, or faulty peripheral tolerance.
- Self-nuclear antigens excite self-reactive B cells that have not been rendered tolerant, resulting in the production of antibodies against the antigens.
- Antigen-antibody complexes can be internalised via binding to Fc receptors on dendritic cells and the antigen receptor on B cells.
- The nucleic acid components activate TLRs and encourage B cells to make autoantibodies, as well as activating dendritic cells, especially plasmacytoid dendritic cells, to create IFN-, which further enhances the immune response and induces additional apoptosis.
- The end result is a cycle of antigen release and immune activation that results in the development of high affinity autoantibodies or a failure to suppress innate inflammatory responses.
New Therapies for Systemic Lupus Erythematosus
- Recent breakthroughs in our understanding of SLE have led to the development of novel therapy strategies.
- The efficacy of anti–IFN- antibodies is being evaluated in clinical studies, and efforts to suppress TLR signals are being examined.
- Using an antibody against the B cell surface protein CD20 to deplete B cells has generated significant interest. For the treatment of SLE, an antibody that inhibits the B cell growth factor BAFF has now been licenced.
Rheumatoid Arthritis
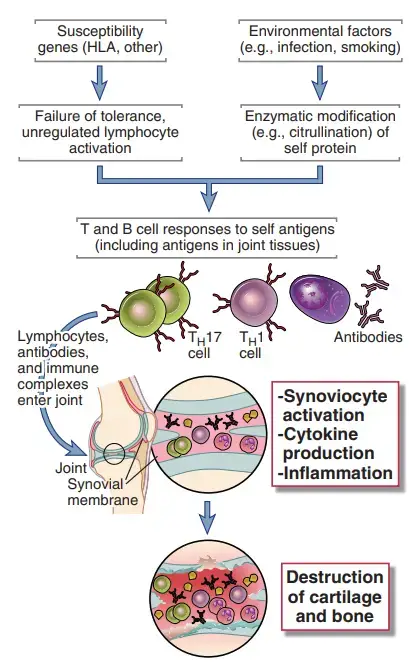
- The inflammatory disease rheumatoid arthritis (RA) affects the small and big joints of the extremities, including the fingers, shoulders, elbows, knees, and ankles.
- The condition is characterised by inflammation of the synovium, loss of cartilage and bone in the joint, and a morphology that suggests a local immune response.
- It is possible that both cell-mediated and humoral immune responses contribute to the development of synovitis. Inflamed synovium contains CD4+ TH1 and TH17 cells, activated B lymphocytes, plasma cells, and macrophages, as well as other inflammatory cells; in severe cases, lymphoid follicles with germinal centres may be present.
- In the synovial (joint) fluid, several cytokines, including IL-1, IL-8, TNF, IL-6, IL-17, and IFN-, have been discovered.
- It is hypothesised that cytokines recruit leukocytes whose products cause tissue damage and stimulate resident synovial cells to create proteolytic enzymes, such as collagenase, that mediate breakdown of cartilage, ligaments, and tendons of the joints.
- The release of the TNF family cytokine RANK (receptor activator of nuclear factor B) ligand by activated T cells may be responsible for the increased osteoclast activity in the joints that contributes to bone loss in RA.
- RANK ligand stimulates the differentiation and activation of osteoclast precursors by binding to RANK, a member of the TNF receptor family expressed on osteoclast precursors.
- Lung damage and vasculitis, probably produced by immune complexes, are systemic consequences of rheumatoid arthritis.
- Although T cells have been the primary focus of research on RA, antibodies may potentially contribute to joint damage.
- Frequently, the synovia of afflicted joints contains activated B cells and plasma cells. Patients commonly have IgM or IgG antibodies that react with the Fc (and infrequently the Fab) regions of their own IgG molecules.
- These autoantibodies are referred to as rheumatoid factors, and their existence is utilised to diagnose RA. Rheumatoid factors may be involved in the production of harmful immune complexes, although their pathogenic role has not been determined.
- Antibodies specific for cyclic citrullinated peptides (CCP), which are produced from particular proteins changed in an inflammatory milieu by the enzymatic conversion of arginine residues to citrulline, have been found in at least 70% of patients.
- These so-called antiCCP antibodies serve as a disease marker and may be involved in tissue damage.
Pathogenesis of Rheumatoid Arthritis
- Similar to other autoimmune illnesses, RA is a complicated disorder in which genetic and environmental variables contribute to the loss of self-antigen tolerance.
- Because the specificity of pathogenic T and B cells is unclear, our understanding of pathophysiology is insufficient.
- The HLA-DR4 haplotype correlates with RA susceptibility. Recent linkage and genome-wide association studies have identified a large number of genes whose polymorphisms are related with rheumatoid arthritis (RA).
- There is a relationship with the PTPN22 gene, which encodes a tyrosine phosphatase, however the function of this enzyme in lymphocyte control remains unclear.
- Anti-CCP immune responses have led to new theories on the pathophysiology of RA.
- According to one theory, environmental insults, such as smoking and some diseases, cause the citrullination of self-proteins, which results in the formation of new antigenic epitopes. Failure of tolerance to these epitopes in genetically vulnerable people results in T cell and antibody responses against the proteins.
- If these altered self-proteins are also present in the joints, T cells and antibodies will target the joints. TH17 and maybe TH1 cells generate cytokines that recruit leukocytes to the joint and stimulate synovial cells to manufacture collagenases and other enzymes.
- The end effect is increasing cartilage and bone degeneration. The immunological response in the joint may be powerful enough to induce the formation of tertiary lymphoid tissues in the synovium, which may maintain and perpetuate the local inflammatory response.
New Therapies for Rheumatoid Arthritis
- Understanding the essential function of T cells and cytokines in the disease has resulted in a tremendous advancement in treatment based on the targeting of specific molecules.
- Principal among these novel medicines are TNF antagonists, which have altered the disease history in many patients from relentless and inexorable joint destruction to smouldering but controlled chronic inflammation.
- An IL-1 antagonist, an antibody against the IL-6 receptor, and a fusion protein consisting of the extracellular domain of CTLA-4 and the Fc part of IgG, which binds to B7 molecules and inhibits B7:CD28 interactions, are all authorised therapy.
- Antibodies that inhibit IL-17 are undergoing clinical testing. In some cases, the B cell-depleting anti-CD20 antibody is beneficial.
- The positive effect of B cell elimination does not appear to be fully attributable to decreased autoantibody formation, suggesting that B cells may play additional functions in the disease, such as presenting antigens to harmful T cells.
Multiple Sclerosis and Experimental Autoimmune Encephalomyelitis
- Multiple sclerosis (MS) is an autoimmune disease of the central nervous system (CNS) in which CD4+ T cells of the TH1 and TH17 subsets react against self myelin antigens, causing inflammation in the CNS with activation of macrophages around nerves in the brain and spinal cord, destruction of the myelin, abnormalities in nerve conduction, and neurologic deficits. It is the most prevalent neurologic condition among young adults.
- On pathologic inspection, the white matter of the CNS is inflamed and demyelinated secondarily. Multiple sclerosis is characterised clinically by weakness, paralysis, and ocular symptoms with exacerbations and remissions; CNS imaging reveals that there is frequent new lesion formation in patients with ongoing illness.
- Experimental autoimmune encephalomyelitis (EAE) in mice, rats, guinea pigs, and nonhuman primates serves as a model for the disease since it is one of the best-characterized experimental models of an organ-specific autoimmune disease mediated mostly by T cells.
- Animals are immunised with antigens normally found in CNS myelin, such as myelin basic protein, proteolipid protein, and myelin oligodendrocyte glycoprotein, together with an adjuvant containing heat-killed mycobacterium, which is required to elicit a robust T cell response.
- Animals develop encephalomyelitis 1 to 2 weeks after vaccination, which is characterised by perivascular infiltrates of lymphocytes and macrophages in the white matter of the CNS, followed by demyelination.
- Lesions of the nervous system can be modest and self-limiting or chronic and recurring. These lesions cause gradual or intermittent and recurrent paralysis.
- Using T cells from infected animals, the illness can also be transmitted to healthy animals. Although antibodies against myelin antigens have been found in both people and animal models, the pathogenic significance of these antibodies has not yet been determined.
Pathogenesis of Multiple Sclerosis
- There is ample evidence that activated CD4+ TH1 and TH17 cells specific for myelin protein antigens cause EAE in mice.
- MS is believed to be caused by myelin-specific TH1 and TH17 cells, as these cells have been discovered in patients and isolated from their blood and CNS.
- The activation of these cells in patients remains a mystery. Molecular mimicry suggests that an infection, most likely a viral infection, triggers self-myelin-reactive T cells.
- It is possible for self-tolerance to fail due to the inheritance of susceptibility genes. Identical twins have a concordance rate of 25% to 40% for the development of multiple sclerosis, but nonidentical twins have a concordance rate of 1%, suggesting genetic influences in the disease’s development.
- MS is connected with genetic variations at the HLA locus, with the HLA-DR2 relationship being the greatest.
- Genome-wide association studies have uncovered a link between a variation in the noncoding region of the CD25 gene encoding the chain of the IL-2 receptor and a disease.
- Expression of CD25 on effector and memory T cells may differ between patients and healthy individuals, although it is unclear how this contributes to disease.
- Some studies have revealed that MS patients have faulty regulatory T cells, however it is unknown how this relates to a lack of self-tolerance.
- Once myelin-specific T cells are activated, they travel into the central nervous system (CNS), where they encounter myelin proteins and produce cytokines that recruit and activate macrophages and more T cells, resulting in the destruction of myelin.
- Epitope spreading appears to be the mechanism through which EAE spreads, according to studies on the disease.
- The disintegration of tissue results in the release of new protein antigens and the development of new, previously sequestered epitopes, which activate additional autoreactive T lymphocytes.
New Therapies for Multiple Sclerosis
- Immunotherapy treatment multiple sclerosis has depended heavily on methods whose scientific basis is not yet fully understood.
- The administration of -interferon, which may affect cytokine responses, and treatment with a random polymer of four amino acids, which is hypothesised to bind to HLA molecules and inhibit antigen presentation, are examples of such interventions.
- Patients have benefited from the use of an antibody against the VLA-4 integrin to inhibit leukocyte migration into the CNS.
- In a tiny proportion of patients, however, this treatment reactivated a latent JC virus infection that produces a severe and sometimes deadly CNS illness.
- Another recently approved treatment for MS inhibits leukocyte migration. The fingolimod (FTY720) medication inhibits the sphingosine 1-phosphate–mediated mechanism of T cell egress from lymphoid organs.
- In a substantial subset of patients, B cell depletion has been proven to be beneficial. These data show a significant role for B cells in the activation of pathogenic T lymphocytes.
- Due to the fact that myelin basic protein is an important self antigen that is the target of the immune response in multiple sclerosis, attempts have been made to inject peptides derived from this antigen into patients in the hopes of inducing tolerance or producing regulatory T cells specific to the relevant antigen.
Type 1 Diabetes Mellitus
- Type 1 diabetes mellitus, formerly known as insulin-dependent diabetes mellitus, is a multisystem metabolic illness caused by decreased insulin production. It affects approximately 0.2% of the U.S. population, with a peak age of start between 11 and 12 years, and its incidence is rising.
- The condition is marked by hyperglycemia and ketoacidosis. Progressive atherosclerosis of arteries, which can lead to ischemia necrosis of limbs and internal organs, and microvascular blockage that damages the retina, renal glomeruli, and peripheral nerves are chronic consequences of type 1 diabetes.
- Insulin insufficiency due to immune-mediated death of the insulin-producing cells of the islets of Langerhans in the pancreas necessitates constant hormone replacement therapy for these individuals.
Pathogenesis of Type 1 Diabetes
- Inflammation driven by CD4+ TH1 cells reactive with islet antigens (including insulin), CTL-induced lysis of islet cells, local production of cytokines (TNF and IL-1) that damage islet cells, and autoantibodies against islet cells may all contribute to cell loss.
- The islets exhibit cellular necrosis and lymphocytic infiltration comprised of both CD4+ and CD8+ T lymphocytes in the rare instances where pancreatic lesions have been studied during the early active phases of the disease.
- This condition is known as insulitis. In addition, autoantibodies against islet cells and insulin are identified in these patients’ blood.
- In vulnerable youngsters (such as relatives of patients) who have not yet developed type 1 diabetes, the existence of antibodies against islet cells is predictive of the development of the disease, suggesting that these antibodies are harmful.
- A useful animal model of the condition is the non-obese diabetic (NOD) mouse, which develops diabetes spontaneously. In this model, there is evidence of poor regulatory T cell survival and function, as well as effector T cell suppression resistance.
- Type 1 diabetes is connected with many genes. Significant focus has been placed on the function of HLA genes.
- 90% to 95% of Caucasians with type 1 diabetes have HLA-DR3, DR4, or both, compared to 40% of normal persons; 40% to 50% of patients are DR3/DR4 heterozygotes, compared to 5% of normal subjects.
- Intriguingly, vulnerability to type 1 diabetes is linked to alleles of DQ2 and DQ8 that are frequently in linkage disequilibrium with DR3 and DR4.
- Several non-HLA genes also play a role in illness development. Insulin is the first to be identified; tandem repetitions in the promoter region are connected with disease vulnerability.
- This association’s mechanism is unknown, although it may be related to insulin expression in the thymus, which controls whether insulin-specific T cells are eliminated (negatively chosen) during maturation.
- Several additional polymorphisms, including those in the IL-2 and CD25 genes, have been discovered in patients and NOD mice. Unknown are the functional effects of these polymorphisms.
- Several studies indicate that viral infections (such as coxsackievirus B4) may precede the onset of type 1 diabetes, possibly by commencing cell injury, generating inflammation and the production of costimulators, and initiating an autoimmune response.
- Nevertheless, epidemiological data imply that repeated infections protect against type 1 diabetes, comparable to the NOD model.
- In fact, it has been hypothesised that the management of infectious diseases contributes to the increased prevalence of type 1 diabetes in affluent nations.
New Therapies for Type 1 Diabetes
- The most promising novel therapy approaches for type 1 diabetes involve establishing tolerance with diabetogenic peptides derived from islet antigens (such as insulin) or creating or giving patients regulatory T cells.
- These clinical trials have just begun.
Inflammatory Bowel Disease
- Inflammation driven by T cells produces intestinal harm in Crohn’s disease and ulcerative colitis, the two diseases comprising inflammatory bowel disease.
- Chronic inflammation and breakdown of the intestinal wall, with frequent fistula formation, characterise Crohn’s disease.
- Lesions in ulcerative colitis are restricted to the mucosa and consist of ulcers with underlying inflammatory foci.
- Antibodies against TNF, IL-17, and the p40 chain of IL-12 and IL-23 are newly developed treatments for these disorders.
FAQ
What is hypersensitivity?
Hypersensitivity refers to an exaggerated or abnormal immune response to harmless substances or self-antigens, resulting in allergic or autoimmune reactions.
What are the different types of hypersensitivity?
Hypersensitivity reactions are classified into four types: Type I (immediate), Type II (antibody-mediated), Type III (immune complex-mediated), and Type IV (cell-mediated or delayed).
What causes hypersensitivity reactions?
Hypersensitivity reactions can be triggered by various factors such as allergens (pollen, dust mites, certain foods), drugs, insect bites, infections, or autoimmune disorders.
What are the symptoms of hypersensitivity?
Symptoms vary depending on the type of hypersensitivity but may include allergic rhinitis, asthma, skin rashes, swelling, hives, itching, difficulty breathing, fever, joint pain, and organ damage in severe cases.
How is hypersensitivity diagnosed?
Diagnosis involves a thorough medical history, physical examination, and sometimes specific tests like skin prick tests, blood tests to measure IgE levels, patch tests, or provocation tests, depending on the suspected type of hypersensitivity.
Can hypersensitivity be prevented?
Some hypersensitivity reactions can be prevented or minimized by avoiding known triggers, practicing good hygiene, maintaining a healthy lifestyle, and following proper safety measures (e.g., using protective equipment).
What are the treatment options for hypersensitivity?
Treatment depends on the type and severity of the hypersensitivity reaction. It may involve allergen avoidance, medications (antihistamines, corticosteroids, immunosuppressants), desensitization therapy, or specific treatments for underlying autoimmune disorders.
Are there any long-term complications associated with hypersensitivity?
Severe or poorly managed hypersensitivity reactions can lead to chronic conditions like asthma, eczema, sinusitis, or recurrent infections. In autoimmune hypersensitivities, organ damage and systemic complications may occur.
Can hypersensitivity reactions be life-threatening?
Yes, certain hypersensitivity reactions, particularly Type I hypersensitivity (anaphylaxis), can be life-threatening if not promptly treated. Anaphylaxis can cause difficulty breathing, a drop in blood pressure, and organ failure.
Can hypersensitivity be cured?
While there is no definitive cure for hypersensitivity, symptoms can be effectively managed and controlled through proper diagnosis, avoidance of triggers, appropriate medications, and lifestyle modifications.
References
- Bagirova, S. F. (2007). Hypersensitivity. Comprehensive and Molecular Phytopathology, 247–263. doi:10.1016/b978-044452132-3/50013-4
- Stetson, S., & Siegel, P. D. (2007). Hypersensitivity. xPharm: The Comprehensive Pharmacology Reference, 1–4. doi:10.1016/b978-008055232-3.60777-9
- Ritzmann, S & Daniels, Jerry. (1982). Immune complexes: Characteristics, clinical correlations, and interpretive approaches in the clinical laboratory. Clinical chemistry. 28. 1259-71. 10.1093/clinchem/28.6.1259.
- Galli SJ, Tsai M. IgE and mast cells in allergic disease. Nat Med. 2012 May 4;18(5):693-704. doi: 10.1038/nm.2755. PMID: 22561833; PMCID: PMC3597223.
- https://www.coursehero.com/study-guides/microbiology/hypersensitivities/
- https://www.slideshare.net/drsomeshwaranamsana/hypersensitivity-reactions-dr-somesh-microbiology
- https://www.yourarticlelibrary.com/immunology/type-iii-hypersensitivity-and-its-mechanism-human-immunology/28081
- https://www.microbiologybook.org/mobile/m.immuno-17.htm
- https://www.biologyonline.com/dictionary/hypersensitivity
- https://teachmephysiology.com/immune-system/immune-responses/hypersensitivity-reactions/
- https://www.ndvsu.org/images/StudyMaterials/Micro/Hypersensitivity.pdf
- https://www.lybrate.com/topic/hypersensitivity
- https://fpnotebook.com/ent/exam/HyprsnstvtyRctn.htm
- https://www.omicsonline.org/open-access/hypersensitivity-an-overview-105371.html
- https://www.amboss.com/us/knowledge/Hypersensitivity_reactions/
- https://semmelweis.hu/mikrobiologia/files/2014/05/hypersensitivity.pdf
- https://basicmedicalkey.com/streptococcus-enterococcus-and-similar-organisms/