What is Flash chromatography?
- Flash chromatography refer as a rapid form of column chromatography which used for separating mixtures of organic compound mainly.
- It was developed from classical column chromatography but with pressure used (around 10–15 psi) for increase flow of solvent / eluent.
- In the technique, the stationary phase usually consist of silica gel (SiO₂) or sometimes alumina, that packed tightly in a glass column.
- Mobile phase is passed by applying air or nitrogen pressure which make the separation more faster and efficient, although resolution sometime slightly less precise.
- Compounds in sample get separated based on different polarity and their adsorption interaction with silica surface.
- The solvent polarity mostly adjusted stepwise or gradually, that help in eluting the compounds from non-polar to more polar nature.
- Usually, solvent systems like hexane / ethyl acetate or dichloromethane / methanol are used, depending on the compound type.
- The collected fractions are monitored by TLC (Thin Layer Chromatography) to identify which fraction contain the target compound.
- Flash chromatography is favored by chemists since it save time and give good yield, although sometimes small impurities may co-elute.
- It used mostly in organic synthesis labs, for purification of crude product after reaction.
- The method can handle medium pressure and moderate sample load; hence also called as medium pressure chromatography (MPLC) in few context.
- Sometimes gradient elution used for better separation, but the system become little complex and require experience for control.
- Modern flash systems have automated detectors (UV / ELSD) and programmable solvent gradients, making the work more convenience.
- It not suitable for proteins or biological macromolecules because silica surface may cause denaturation or adsorption loss.
- Overall, flash chromatography represent a fast and handy purification tool for chemists, though resolution is lower than HPLC, it’s cheaper and simpler.
Principle of Flash column chromatography
- Separation in flash column chromatography is based on different affinities of compounds for a stationary phase and a mobile phase.
- A solid stationary-phase (often silica gel or alumina) is packed in a column and the mixture is applied on top, then mobile phase (solvent) is pushed through it.
- The mobile phase carries the components down the column, but each component interacts differently with the stationary phase so they travel at different speeds, thus separation occurs.
- The technique is often described as an adsorption chromatography mechanism, meaning that compounds are temporarily adsorbed on the solid surface then eluted by solvent.
- Pressure (gas or pump) is used to increase the flow of solvent through the column (rather than relying purely on gravity) so process is faster.
- The choice of solvent (mobile phase) polarity compared to stationary phase polarity influences how strongly each compound sticks vs how quickly it moves, so solvent gradient or stepwise change can improve separation.
- Sample load and particle size of the packing affect resolution and speed: smaller particles increase surface area so better separation but may increase back-pressure. Chrom Tech
- Essentially, the principle is: If a compound has strong interaction (adsorption) with the stationary phase, it moves slowly; if weak interaction then it moves faster and elutes earlier.
- The goal is to collect separated fractions containing individual compounds after they exit the column.
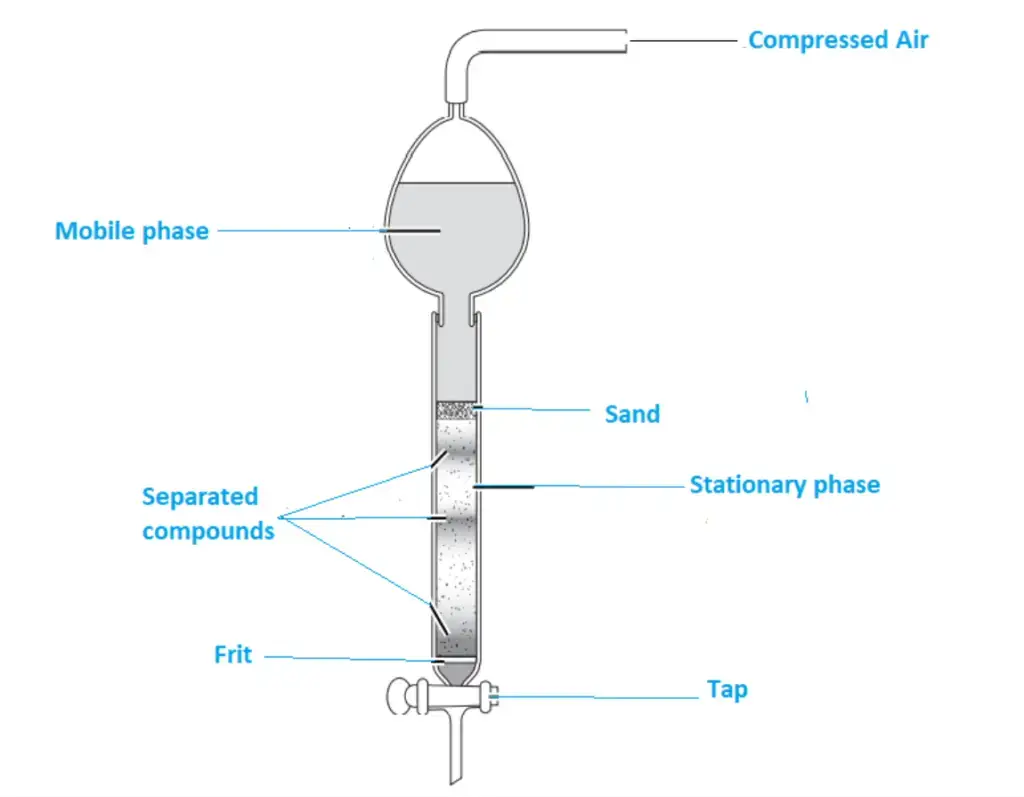
Flash column chromatography equipment
Flash chromatography General consist of following parts
- Pump system
- Mobile phase
- Mobile phase modifier
- Stationary phase
- Columns
- Cartridges
- Detector
1. Pump Systems
- Pump systems play a critical role in flash chromatography, enabling precise control of solvent flow rates and pressure for optimal separation results. Different pump controllers are available to suit various separation requirements and operating conditions.
- The Pump Controller C-610 is designed for isocratic separations and is compatible with the Pump Module C-601. With a pressure range of up to 10 bar, it allows for efficient and reliable separations. The flow rate can be easily adjusted using a knob, and the pump controller features a large illuminated LCD display to indicate the flow rate. Additionally, it is equipped with an overpressure sensor to ensure maximum safety during operation.
- For more versatile applications involving both isocratic and gradient separations at higher pressures, the Pump Manager C-615 is an ideal choice. It offers fast operation, easy programming, and a large graphical display for quick and convenient setup. During a separation, the Pump Manager C-615 provides real-time information on running time, solvent consumption, and actual pressure, allowing for optimization of the separation process. It includes input/output ports for connecting two solvent valves and level sensors, and it comes with a pressure sensor and a mixing chamber.
- To achieve precise control over the entire chromatography system, the Control Unit C-620 in combination with Sepacore Control offers advanced functionality. It can be connected to multiple pump modules (C-601 or C-605), fraction collectors, detectors (such as UV and RI), and sequential modules (C-623 or C-625) for automated sequential chromatography. The Control Unit C-620, included in the Sepacore Control package, ensures precise control and coordination of the chromatography system components, facilitating efficient and reliable separations.
- Overall, the pump systems and controllers mentioned above provide options for controlling flow rates, pressure, and gradient formation, allowing for effective flash chromatography separations. The choice of pump controller depends on the desired separation mode, pressure requirements, and the level of automation desired for the chromatography system.
Type of pump
There are different types of pumps commonly used in flash chromatography systems, each offering specific capabilities and functionality. Here are some notable types of pumps:
- Pump Module C-601 (10 bar): The Pump Module C-601 is designed for fast isocratic flash separations. It operates silently and features a 3-piston design, ensuring a constant and pulse-free flow. It provides a flow rate range of 2.5 to 250 ml/min, allowing for reproducible and rapid separations. With a maximum working pressure of 10 bar (145 psi), it is suitable for sample sizes up to 5 grams. The Pump Module C-601 is compatible with pre-packed polypropylene (PP) cartridges, enabling safe and efficient implementation of both normal phase and reversed phase applications.
- Pump Module C-605 (50 bar): The Pump Module C-605 is similar to the C-601 but offers a higher maximum working pressure of 50 bar (725 psi). It is ideal for performing fast separations with larger sample sizes, up to 100 grams. The C-605 is particularly suitable for reversed phase separations and is compatible with glass and plunger columns, as well as silica gel particle sizes below 40 μm.
- Pump Manager C-615: The Pump Manager C-615 is a versatile pump system designed for both isocratic and gradient flash separations. It can be used with either the Pump Module C-601 or C-605. The Pump Manager C-615 provides advanced features such as solvent selection, timed runs, and solvent level control. It enables efficient solvent mixing under pressure and delivers a pulsation-free solvent flow, ensuring optimal separation performance.
- Vacuum Pump/Peristaltic Pump: In addition to the specialized pump modules and managers, flash chromatography systems may include vacuum pumps or peristaltic pumps. These pumps are typically used to transfer solvents from the mobile phase reservoir to the flash pump. They assist in maintaining a continuous flow of solvents during the separation process.
The selection of a pump type depends on factors such as the desired separation mode (isocratic or gradient), maximum working pressure requirements, sample size, and the level of control and automation desired in the flash chromatography system. Each pump type offers specific advantages and is suited to different separation needs.
Mobile phase
- The mobile phase is a crucial component in chromatography that aids in the separation of mixtures based on their polarity. The choice of the mobile phase depends on the type of stationary phase being used and the polarity of the mixture to be separated.
- When employing normal-phase silica gel as the stationary phase, a mobile phase with lower polarity is preferred. Solvent systems such as dichloromethane/methane, hexane/ethyl acetate, or hexane/ether are commonly used in normal-phase chromatography.
- On the other hand, if reversed-phase silica gel is used as the stationary phase, a mobile phase with higher polarity is required. Solvent systems like water/isopropanol or water/acetonitrile are commonly used in reversed-phase chromatography.
- In addition to considering polarity, the solubility of the mixture being separated is also important. The mobile phase should be capable of completely dissolving the sample components without causing precipitation. In some cases, certain low-polarity mobile phases may result in the formation of oily precipitates. To overcome this issue, solvents with higher polarity should be chosen.
- To determine the optimal mobile phase for separation, chemists perform analytical trials using thin-layer chromatography (TLC). They select solvents that efficiently move the mixture components, ensuring that the Rf value is at least 0.25 and undesired components are sufficiently distanced with an Rf value of at least 0.2.
- Commonly used solvents as mobile phases in chromatography, along with their corresponding polarity values, include hexane (0.06), n-heptane (0.20), toluene (2.40), methyl chloride (DCM) (3.40), tetrahydrofuran (4.20), ethanol (4.30), ethyl acetate (4.30), 1-propanol (4.30), acetonitrile (6.20), methanol (6.60), and water (10.28).
- In some cases, a mixture of two solvents, one with higher polarity and the other with lower polarity, is used as the mobile phase to enhance separation. For example, Hexane/Ethyl acetate (1:1) or dichloromethane/methanol (95:5) can be employed.
- It’s important to note the terms “solvent system strength” and “solvent selectivity.” Solvent system strength refers to the ability of the mobile phase to migrate all compounds simultaneously on the column, ensuring efficient separation. On the other hand, solvent selectivity indicates the mobile phase’s ability to migrate specific compounds differently from others, contributing to the selectivity of the separation process.
Mobile phase modifier
Mobile phase modifiers are chemical reagents that are added to the mobile phase in chromatography to reduce peak tailing and improve the resolution of separations, particularly when dealing with compounds that have acidic or basic groups. These modifiers interact with the residual surface silanol groups on the chromatographic support, mitigating the unwanted interactions that lead to peak tailing.
Mobile phase modifiers are typically added in very low concentrations, usually 1% or less, to avoid excessive changes in the mobile phase composition. By incorporating a mobile phase modifier, peaks become sharper and more symmetrical, resulting in improved separation of acidic or basic compounds.
Several common mobile phase modifiers are frequently utilized in chromatography. These include:
- Triethylamine: This amine-based modifier is often employed to reduce peak tailing for basic compounds. It helps neutralize residual acidic silanol groups on the stationary phase, leading to improved peak shape.
- Acetic Acid: Acetic acid is utilized as a mobile phase modifier to address peak tailing for acidic compounds. It helps neutralize residual basic silanol groups on the stationary phase, enhancing peak symmetry.
- Ammonium Hydroxide: Ammonium hydroxide is an alkaline mobile phase modifier used for the separation of basic compounds. It acts as a base, neutralizing residual acidic silanol groups and minimizing peak tailing.
- Trifluoroacetic Acid: Trifluoroacetic acid is a strong acidic modifier that is commonly employed in reverse-phase chromatography. It helps improve peak shape and resolution for both acidic and basic compounds.
By incorporating these mobile phase modifiers, chromatographers can effectively address peak tailing issues and achieve better separation results, particularly when dealing with compounds containing acidic or basic functional groups. It is important to carefully optimize the concentration of the modifier to ensure the desired improvements in peak shape and resolution without significantly altering the overall mobile phase composition.
Stationary phase
The selection of the stationary phase in chromatography is crucial for achieving effective separation of organic compounds. The choice of the stationary phase is primarily influenced by the polarity of the compounds and the specific functional groups present in them. Various types of stationary phases are available, with silica gel being the initial and commonly used stationary phase in flash chromatography. Other stationary phases, such as reverse phase C18, alumina, and ion exchange resin, have also been employed in chromatographic separations.
Here are some considerations when selecting the stationary phase:
- Low Polarity Samples: For organic compounds with low polarity, normal phases, reverse phases, or neutral alumina can be suitable stationary phases. These phases provide interactions based on differences in polarity to achieve effective separation.
- High Polarity Samples: When dealing with highly polar compounds, C18 or cyano-based stationary phases are often used. These phases offer enhanced interactions with polar functional groups, facilitating separation based on polarity differences.
- Basic Functional Groups: Compounds containing basic functional groups can be effectively separated using C18, normal phases, basic alumina, or strong cation exchange (SCX) stationary phases. These phases provide specific interactions with the basic moieties for improved separation.
- Acidic Functional Groups: For compounds with acidic functional groups, normal phases, C18, acidic alumina, neutral alumina, or strong anion exchangers are commonly utilized as stationary phases. These phases allow for interactions with the acidic functional groups to achieve efficient separation.
- Acid-Sensitive Samples: In the case of acid-sensitive samples, it is advisable to use neutral alumina, diol, or cyano-based stationary phases. These phases provide alternative interactions that are less likely to affect the acid-sensitive compounds.
- Charged Samples: If the compounds in the sample are charged, C18 or cyano-based stationary phases are often employed. These phases can interact with charged species and facilitate their separation based on charge differences.
The selection of the appropriate stationary phase is essential for achieving optimal separation and resolution in chromatography. By considering the polarity of the compounds and the nature of the functional groups, chemists can make informed decisions about the most suitable stationary phase for their specific application.
Types of Elution/Techniques of mobile phase
Elution techniques in chromatography refer to the methods used to vary the composition of the mobile phase during the separation process. The two main types of elution techniques are isocratic elution and gradient elution.
1. Isocratic Elution
In isocratic elution, the composition of the mobile phase remains constant throughout the chromatographic separation. The mobile phase can consist of a single solvent or a mixture of two solvents, but its composition remains unchanged during the entire process. Isocratic elution is commonly used in classical flash chromatography, where the mobile phase composition is optimized for the separation of target compounds.
Key points about isocratic elution:
- Mobile phase composition remains constant.
- Suitable for separating compounds with similar polarities.
- Commonly used in routine separations.
- Relatively simple and straightforward to implement.
2. Gradient Elution
In gradient elution, the composition of the mobile phase is intentionally varied during the separation process. This technique offers several advantages over isocratic elution, including shorter elution times, improved separation efficiency, and higher sample loading capacity. Gradient elution can be further classified into three types: stepped gradient, linear gradient, and mixed gradient.
- Stepped Gradient: In a stepped gradient, the mobile phase composition is changed abruptly at specific time intervals or volume increments. This approach involves discrete steps in which the solvent composition is adjusted, leading to different elution strengths at different stages of the separation.
- Linear Gradient: In a linear gradient, the mobile phase composition changes linearly over time or volume. This gradual change in composition allows for a smoother transition between different elution strengths, providing improved separation and resolution of compounds.
- Mixed Gradient: A mixed gradient combines elements of both stepped and linear gradients. It involves a combination of discrete steps and gradual changes in mobile phase composition. This versatile approach allows for fine-tuning of the elution conditions and is often employed to optimize separations.
Advantages of gradient elution:
- Enables efficient separation of complex mixtures.
- Provides higher resolution and improved peak shape.
- Reduces the time required for separation.
- Increases the sample loading capacity.
- Offers greater control over elution conditions.
The choice between isocratic and gradient elution depends on the specific separation requirements, the complexity of the sample, and the desired level of resolution. Gradient elution is particularly advantageous when separating complex mixtures or compounds with varying polarities, as it allows for better separation and shorter analysis times.
Flash chromatography columns
Flash chromatography columns are essential components of flash chromatography systems used for the purification of organic compounds. There are two main types of columns that are commonly used in flash chromatography: manually-packed columns and pre-packed columns.
- Manually-packed Columns: Manually-packed columns are prepared by loading suitable stationary phases, such as silica gel or other adsorbents, into glass columns. However, the packing process is done manually and may not always result in perfectly packed columns. Imperfect packing can lead to decreased resolution and less efficient separations. Manual packing requires expertise and careful attention to ensure uniform packing and avoid air pockets or channeling within the column.
- Pre-packed Columns: To overcome the limitations of manual packing, pre-packed columns are available commercially in various sizes. These columns come pre-packed with the stationary phase, eliminating the need for users to manually pack the columns themselves. Pre-packed columns offer several advantages over manually-packed columns:
a) Increased Effectiveness: Pre-packed columns are designed to provide optimal packing density and uniformity, ensuring efficient compound purification and improved resolution. The consistent packing quality leads to more reliable and reproducible separations.
b) Time-saving: Using pre-packed columns saves significant time as users do not have to go through the laborious process of manually packing the columns. This allows for quicker set-up and execution of flash chromatography experiments.
c) Safety: Pre-packed columns offer enhanced safety compared to manually-packed columns. When manually packing columns, users may be exposed to silica dust, which can be hazardous. Pre-packed columns eliminate this risk, providing a safer working environment.
d) Productivity and Repeatability: The consistent packing quality of pre-packed columns ensures high productivity and repeatability in flash chromatography. Users can rely on the performance of pre-packed columns for consistent results across different purification runs.
Overall, pre-packed columns in flash chromatography offer convenience, improved performance, and safety benefits. They are widely used in laboratories for their time-saving capabilities, increased productivity, and the assurance of reliable and reproducible separations.
Flash chromatography cartridges
Flash chromatography cartridges are cylindrical pipe-like devices that are used in automated flash chromatography systems to introduce the sample onto the columns. These cartridges are particularly useful for samples with low solubility. There are two main types of cartridges available in the market: empty solid-load cartridges and pre-packed solid-load cartridges.
1. Empty Solid-load Cartridges
Empty solid-load cartridges provide flexibility in choosing the adsorbent material. While various adsorbents can be used, silica gel is commonly employed. The process of preparing and using empty solid-load cartridges involves the following steps:
- a) Dissolving the Sample: The sample to be purified is dissolved in a suitable solvent.
- b) Mixing with Silica Gel: Powdered silica gel is mixed with the sample solution. The sample gets coated with the silica gel particles.
- c) Solvent Removal: The solvent is evaporated using a rotary evaporator, leaving behind the sample coated with silica gel.
- d) Loading the Cartridge: The sample coated with silica gel is poured into an empty cartridge and loaded into the flash chromatography system. The cartridge is directly connected to the column, minimizing the risk of contamination.
In addition to silica gel, other adsorbent materials like Celite, diatomaceous earth, or boiling chips can be used in these empty solid-load cartridges.
2. Pre-packed Solid-load Cartridges
Pre-packed solid-load cartridges are commercially available cartridges that come already packed with a specific adsorbent material. These cartridges are preferred by users who find the process of preparing empty cartridges tedious. The use of pre-packed cartridges offers the advantage of quick and convenient sample loading. The steps involved in using pre-packed solid-load cartridges are as follows:
- a) Sample Dissolution: The sample is dissolved in a suitable solvent.
- b) Application to Cartridge: The dissolved sample is applied directly to the pre-packed cartridge.
- c) Absorption: The adsorbent material in the cartridge absorbs the dissolved sample.
- d) Drying: The wet cartridge, with the absorbed sample, is thoroughly dried using a high vacuum pump before being placed into the flash chromatography system.
Using pre-packed solid-load cartridges ensures efficient sample loading and saves time compared to the process of preparing empty cartridges.
Overall, flash chromatography cartridges, whether empty solid-load or pre-packed, provide a convenient and effective means of introducing samples into the chromatographic system. They facilitate the purification of low-solubility samples and contribute to the efficiency and reliability of flash chromatography processes.
Detection techniques in Flash chromatography
Detection techniques play a crucial role in flash chromatography by allowing chemists to identify and monitor the separated compounds. With the advent of automation, various detectors have been integrated into flash chromatography instruments to streamline the detection process. Here are some commonly used detection techniques in flash chromatography:
- UV-Vis Detector: The UV-Vis detector is one of the most widely employed detectors in flash chromatography. Many organic compounds exhibit absorption in the ultraviolet (UV) or visible (Vis) regions of the electromagnetic spectrum. The UV-Vis detector utilizes this property to measure the absorbance of compounds as they elute from the column. It provides valuable information about the presence and concentration of analytes, allowing for real-time monitoring and fraction collection.
- Refractive Index Detector: The refractive index (RI) detector is another commonly utilized detection technique in flash chromatography. It is particularly useful for compounds that lack significant UV absorption or have low UV absorbance. The RI detector measures changes in the refractive index of the eluting compounds, providing a signal that corresponds to their concentration. This detector is especially effective for detecting non-UV active compounds, such as sugars and polymers.
- Fluorescence Detector: The fluorescence detector is employed when the compounds of interest exhibit fluorescence properties. Certain compounds possess intrinsic fluorescence or can be derivatized to become fluorescent. The fluorescence detector excites the eluted compounds with specific wavelengths of light and measures the fluorescence emitted as a result. This technique offers high sensitivity and selectivity, making it suitable for the analysis of fluorescent compounds.
- Evaporative Light Scattering Detector (ELSD): The evaporative light scattering detector (ELSD) is a universal detection technique that can be used with any compound regardless of its UV absorbance or fluorescence properties. It is particularly useful for detecting non-volatile and thermally unstable compounds. The ELSD operates by nebulizing the eluent from the column and directing it into a flow of dry gas. The sample particles are evaporated, and the remaining solid particles scatter light, which is then detected. The ELSD provides a signal proportional to the concentration of the compounds, allowing for their detection and quantification.
These are just a few examples of detection techniques commonly used in flash chromatography. Other techniques, such as mass spectrometry (MS) and evaporative mass detector (EMD), can also be integrated into flash chromatography systems for enhanced compound identification and characterization. The choice of detection technique depends on the nature of the compounds being analyzed and the specific analytical requirements of the study.
Packing the Column
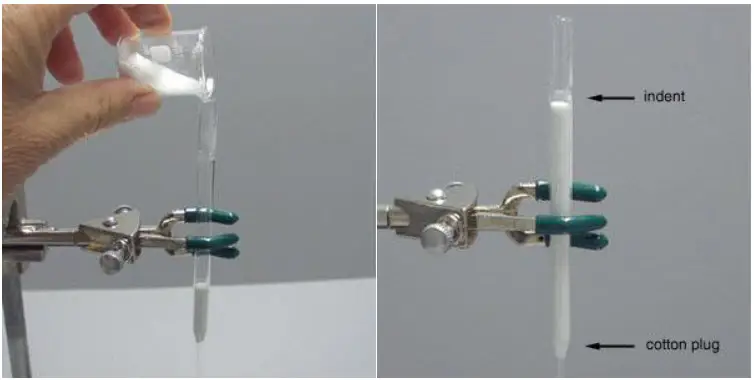
- Prepare the column: Obtain a glass column with a glass frit or a plug of cotton wool positioned directly above the stopcock. This prevents the silica gel from escaping through the stopcock. Ensure that the column is clean and dry before proceeding.
- Add a layer of sand: Place a layer of clean sand, approximately 1/2 inch thick, on top of the glass frit or cotton wool plug. Use only enough sand to create a flat surface with the same diameter as the column body. It is important to achieve a flat surface for effective packing.
- Add the silica gel: Use dry silica gel adsorbent with a mesh size of 230-400, preferably labeled “for flash chromatography.” There are two common methods to add the silica gel:a. Scoop and tamp method: Invert the column into the jar of silica gel and scoop out the gel. Tamp down the gel inside the column using a tool or by tapping it gently on a bench top. Repeat this process until the column is filled with silica gel.b. Pouring method: Use a 10 mL beaker to pour the silica gel into the column. Gradually add the gel while ensuring an even distribution throughout the column. Tamp down the gel using a tool or by tapping it gently on a bench top to achieve proper packing.
- Pack the silica gel: Regardless of the method used to fill the column, the silica gel needs to be tightly packed. There are a few techniques to accomplish this:a. Tamping: Use a tool to press down and compact the silica gel within the column. Tamp it down firmly, but avoid excessive force that could damage the column.b. Air pressure: Attach a pipette bulb to the top of the column and force air into it. This helps to pack the silica gel more tightly.Properly packed silica gel should fill the column up to just below the indent on the pipette, leaving approximately 4-5 cm of empty space above the adsorbent for the addition of solvent.
- Secure the column: Clamp the packed column securely to a ring stand using a small 3-pronged clamp. Ensure that the column is stable and will not tip over during the chromatographic process.
- By following these steps, you can effectively pack a column for flash chromatography, ensuring optimal separation and efficient sample purification.
Solvating the Silica Gel Column
- Settle the silica gel: After packing the column with silica gel, tap gently and evenly on the sides of the column using a piece of rubber tubing. This helps to settle the silica gel and ensure an even distribution within the column.
- Add elution solvent: Pour a sufficient amount of elution solvent onto the silica gel. The solvent can be added directly to the top of the column. Make sure to use enough solvent to cover the silica gel bed.
- Use pressurized gas: To enhance the solvation process, use pressurized gas (e.g., compressed air or nitrogen) to force the solvent through the silica gel. Connect the gas source to the top of the column, allowing the gas to push the solvent through the column and silica gel bed.
- Remove trapped air: As the solvent passes through the silica gel, it displaces and removes any trapped air within the gel. This step is important to ensure efficient and homogeneous flow of the solvent through the column.
- Flush solvent through the column: Continuously flush the solvent through the column until the entire silica plug becomes homogeneous in appearance. You may need to recycle the solvent by collecting it as it comes out of the column and pouring it back onto the top of the column. This helps to ensure complete solvation of the silica gel.
- Repeat if necessary: Depending on the initial state of the silica gel and the quality of solvation achieved, you may need to repeat the solvent flushing process multiple times. This ensures that all the silica gel is properly solvated.
By following these steps, you can effectively solvate the silica gel column for flash chromatography. This process removes air pockets, allows for uniform flow of the elution solvent, and prepares the column for successful separation of compounds.
Pre-elute the column
- Add pre-elution solvent: Determine the appropriate pre-elution solvent based on the specific procedure or separation requirements. Typically, hexanes or another specified solvent is used. Pour the pre-elution solvent onto the top of the silica gel column.
- Observe solvent flow: As the solvent is added, it will begin to flow slowly down the column. Monitor the solvent level as it progresses through the silica gel bed. It is important to observe both the solvent level within the column and at the top.
- Follow the solvent flow: Watch as the solvent flows down the column. You can visually track its progress by identifying a point marked by an arrow on the column above. The solvent should gradually move down the column at a controlled pace.
- Monitor solvent levels: Pay attention to the solvent level within the column as well as the level at the top. The solvent level within the column should gradually decrease as it flows downward. Ensure that the solvent level at the top of the column remains consistent and does not overflow.
- Determine completion of pre-elution: The pre-elution process is considered complete when the bottom solvent level reaches the bottom of the column. At this point, the column is ready to be loaded with the sample for chromatographic separation.
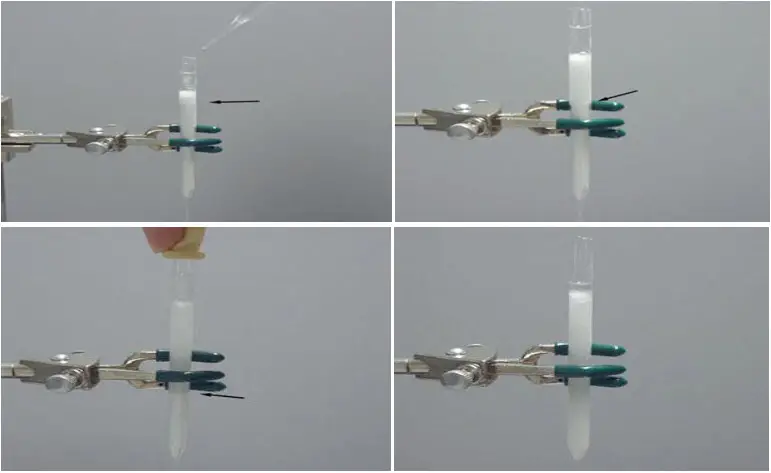
By pre-eluting the column, you ensure that any impurities or residual compounds are flushed out before loading the sample. This step helps to optimize the separation and improve the quality of the chromatographic results. It is essential to follow the specified solvent and monitoring instructions provided in the procedure to achieve the desired pre-elution.
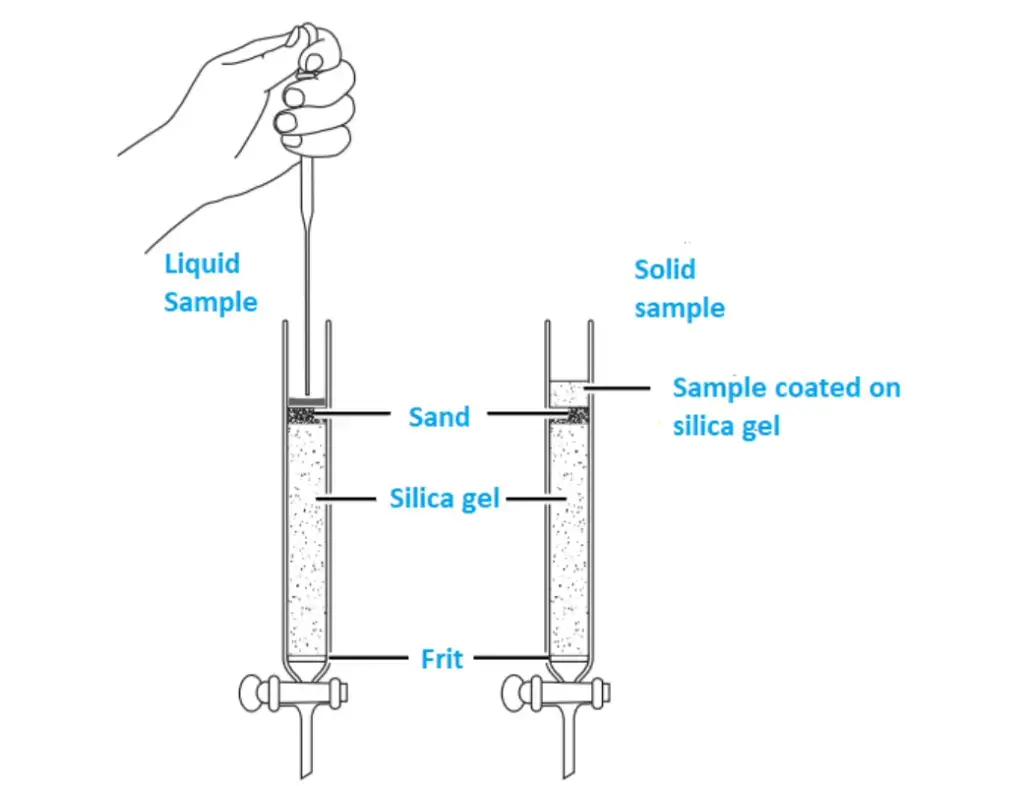
Load the sample onto the silica gel column
To load the sample onto a silica gel column in flash chromatography, there are two methods commonly used: the wet loading method and the dry loading method. Here are the steps for each method:
Wet Loading Method:
- Prepare the sample solution: Dissolve the sample to be purified or separated into components in a small amount of a suitable solvent, such as hexanes, acetone, or another specified solvent. Ensure that the sample is completely dissolved.
- Apply the sample solution: Carefully load the prepared sample solution onto the top of the silica gel column. You can use a pipette or syringe to deliver the sample solution onto the column. Take caution not to disturb the packed silica gel bed while applying the sample.
- Control the volume: For samples dissolved in more polar solvents than the eluting solvents, it is crucial to use only a few drops of the sample solution. Using a minimal volume helps prevent interference with the elution process and ensures proper purification or separation.
Dry Loading Method:
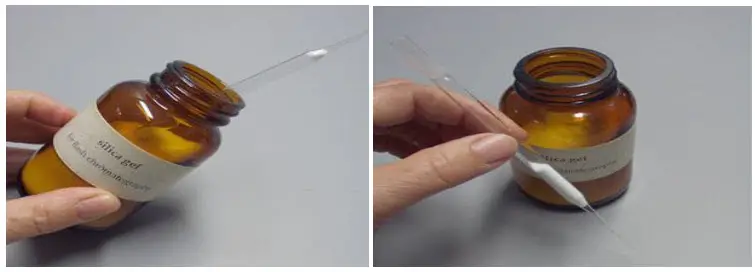
- Dissolve the sample: Dissolve the sample to be analyzed in the smallest possible amount of a suitable solvent. Ensure that the sample is fully dissolved and forms a homogeneous solution.
- Add silica gel: Once the sample is dissolved, add approximately 100 mg of silica gel to the solution. Swirl the mixture gently to allow the solvent to evaporate, leaving behind a dry powder containing the sample and silica gel.
- Transfer the dry powder: Place a folded piece of weighing paper on a flat surface and transfer the dry powder onto it. Ensure that the dry powder is evenly spread on the paper.
- Load the sample onto the column: Take the prepared silica gel column and carefully transfer the dry powder sample from the weighing paper to the top of the column. Ensure that the sample is evenly distributed across the column’s diameter.
- Add eluting solvent: Once the sample is loaded, add fresh eluting solvent to the top of the column. This solvent will initiate the elution process, separating the components of the sample.
With the sample loaded onto the silica gel column, the elution process can begin to separate the components of the sample based on their affinities for the stationary phase. It is important to follow the specific loading instructions provided in the procedure to ensure accurate and effective chromatographic separation.
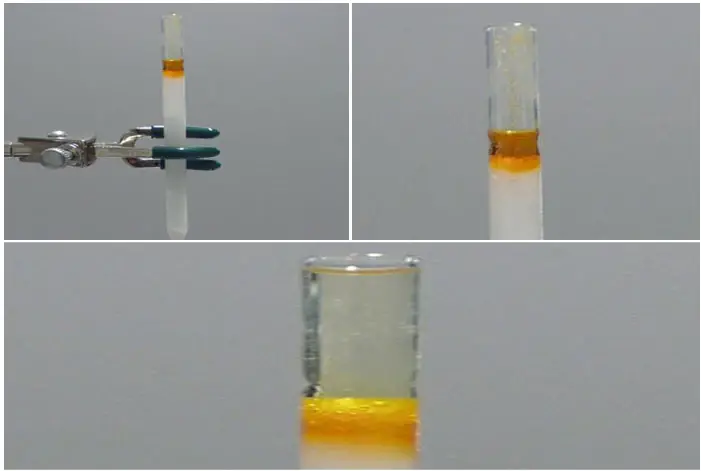
Elute the column
To elute the column in flash chromatography and separate the components of the sample, follow these steps:
- Add elution solvent: Pour a significant portion of the elution solvent onto the top of the silica gel column. The elution solvent should be chosen based on the polarity requirements of the separation and the nature of the sample being analyzed.
- Apply pressure: Apply gentle pressure to force the elution solvent through the column. This can be done by pressing on the top of the Pasteur pipette or by using a pipette bulb. The pressure should be sufficient to maintain a steady flow of solvent through the column, but avoid forcing the solvent too quickly or letting the silica go dry. It is crucial to control the flow rate to ensure effective separation.
- Monitor the elution process: Observe the movement of the solvent through the column. As the elution solvent progresses down the column, it carries the separated components of the sample with it. If the compounds being separated are colored, you can visually track their movement. The colored compound(s) will elute from the column and be visible as they reach the collection point.
- Collect the eluted fractions: As the colored compound begins to elute, change the collection beaker immediately to collect the eluted fraction. If the compound(s) of interest are not colored, it becomes more challenging to determine their elution point. In such cases, equal-sized fractions can be collected sequentially and carefully labeled for later analysis.
- Repeat as needed: Depending on the complexity of the sample and the desired level of separation, you may need to repeat the elution process multiple times with fresh elution solvent to ensure complete separation and collection of all desired fractions.

It is important to carefully control the elution process, maintaining steady solvent flow and ensuring the appropriate collection of fractions. By monitoring the elution and collecting the eluted fractions appropriately, you can obtain purified components or separated fractions for further analysis or use.
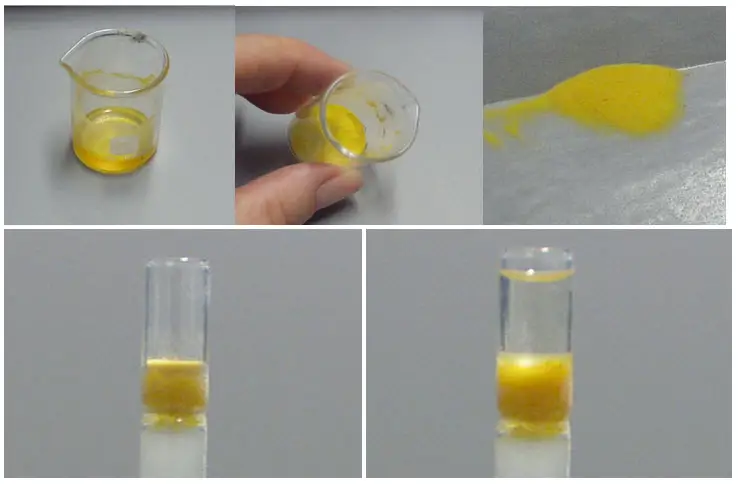
Analyze the fractions
After collecting the eluted fractions from the flash chromatography column, the next step is to analyze these fractions to determine the composition and identify the desired compound(s). The analysis can be performed using TLC (thin-layer chromatography) or other suitable analytical techniques. Here’s how to analyze the fractions:
- Colored fractions: If the eluted fractions are visibly colored, it simplifies the analysis process. Similar-colored fractions can be combined directly, as they likely contain the same or closely related compounds. However, it is still advisable to perform TLC before combining the fractions to ensure accurate identification and confirmation of compound purity.
- Non-colored fractions: For fractions that do not exhibit any visible color, TLC is usually the preferred method for analysis. TLC involves applying a small portion of each fraction onto a TLC plate, which is then developed in a solvent system. The separated spots on the TLC plate can provide valuable information about the composition of the fractions.
- Prepare a TLC plate by marking a baseline and applying spots of each fraction along the baseline.
- Develop the TLC plate in a suitable solvent system, allowing the solvent to move up the plate and separate the components in each fraction.
- Visualize the separated spots using appropriate detection methods, such as UV light or staining reagents.
- Compare the resulting TLC plate to reference standards or known compounds to identify the compounds present in each fraction.
- Once the composition of each fraction is known, the fractions containing the desired compound(s) can be determined.
- Combining fractions: Based on the analysis results, fractions containing the desired compound(s) can be combined to concentrate the target compound(s) and remove impurities. Care should be taken to combine only the fractions that contain the desired compound(s) and exclude any unwanted impurities or interfering substances.
- Combine the identified fractions containing the desired compound(s) into a single vial or container.
- Keep a record of the combined fractions, labeling them appropriately for future reference.
- If necessary, additional purification steps may be performed on the combined fractions, such as solvent evaporation, crystallization, or further chromatographic techniques, to isolate and obtain a pure sample of the desired compound(s).
By analyzing the fractions using TLC or other suitable methods, you can determine the composition of each fraction and selectively combine the fractions that contain the desired compound(s). This allows for the concentration and purification of the target compound(s) for further characterization or subsequent applications.
Cleaning the Column
Cleaning the column is an essential step in flash chromatography to ensure its proper maintenance and prevent cross-contamination between different samples. Here’s how to clean the column effectively:
- Flush out remaining solvent: Start by flushing all the remaining solvent from the column using pressurized gas, such as compressed air or nitrogen. This step helps remove any residual solvent and dry the column. Allow the gas to flow through the column for approximately 2 hours to ensure thorough drying of the silica gel.
- Dispose of silica waste: Carefully pour out the contents of the column, including the silica gel, into a designated silica waste container. It is important to properly dispose of the used silica gel to prevent any environmental contamination or safety hazards.
- Wash the column: Washing the column with appropriate solvents helps remove any remaining impurities or contaminants. The most commonly used solvents for column cleaning are water and acetone. Pour a sufficient amount of water into the column, allowing it to flow through the silica gel. Repeat this step with acetone. This process helps remove residual compounds and ensures a clean column for subsequent use.
- Optional: Use liquid soap: If necessary, a small amount of liquid soap can be used during the cleaning process to enhance the removal of stubborn contaminants. However, it is important to be cautious and avoid abrasive brushes or soaps that may scratch the column.
- Remove remaining solvent: Once all liquid solvents have been drained from the column, use a vacuum source, such as an aspirator, to remove any last remnants of solvent from the column. Applying a vacuum to the bottom of the column helps draw out the remaining solvent effectively.
It is crucial to handle the column with care during the cleaning process to avoid any damage or scratching. Harsh cleaning methods or abrasive materials should be avoided to maintain the integrity and performance of the column.
By following these steps, you can effectively clean the flash chromatography column, ensuring its optimal performance and preventing cross-contamination between different samples. Regular cleaning and maintenance of the column help maintain the accuracy and reliability of the chromatographic separations.
Modern Flash Chromatographic Techniques
Pre-packed plastic cartridges
Pre-packed plastic cartridges have become a popular choice in modern Flash Chromatography systems due to their safety, reproducibility, and convenience. These cartridges offer several advantages over traditional glass columns. Here are some key characteristics and benefits of pre-packed plastic cartridges:
- Disposable plastic cartridges: Unlike glass columns that require packing and unpacking of stationary phases, pre-packed plastic cartridges are ready-to-use and eliminate the need for manual packing. This saves time and ensures consistent packing quality, leading to increased reproducibility in chromatographic separations.
- Cartridges of different sizes: Plastic cartridges are available in various sizes, allowing for easy scale-up or down of chromatographic processes. This flexibility enables researchers to adapt their separations to different sample sizes and throughput requirements.
- Solid sample module and injection valve: Pre-packed plastic cartridges often come equipped with a solid sample module and an injection valve. These features simplify sample loading, especially for solid samples, and enable precise injection of the sample onto the stationary phase. It enhances the overall efficiency and accuracy of the chromatographic process.
- Higher pressure capability: Plastic cartridges can withstand higher pressure, typically up to 100 psi, during the separation process. This higher pressure allows for faster elution and shorter separation times, leading to increased productivity in the laboratory.
- Narrow particle distribution: The stationary phase packed in pre-packed plastic cartridges exhibits a narrow particle distribution. This characteristic results in lower backpressure and higher separation efficiency, allowing for improved resolution and better separation of target compounds.
In addition to these characteristics, modern Flash Chromatography systems can be equipped with detectors and fraction collectors, offering automation capabilities. This integration enables real-time monitoring of separations, automatic fraction collection, and precise control over elution parameters.
Furthermore, the introduction of gradient pumps in Flash Chromatography systems has revolutionized the technique. Gradient pumps allow for the precise control of solvent composition over time, leading to quicker separations, reduced solvent usage, and enhanced flexibility in method development.
Overall, pre-packed plastic cartridges in Flash Chromatography systems provide a safe, reproducible, and efficient platform for chromatographic separations. They offer convenience, scalability, and compatibility with modern automation features, making them a valuable tool in the purification and separation of organic compounds.
Advanced Detection Techniques for Flash Chromatography
Advanced detection techniques have expanded the capabilities of Flash chromatography, particularly for compounds that cannot be easily detected using traditional UV detection methods. Here are a couple of advanced detection techniques used in Flash chromatography:
- Evaporative Light Scattering Detection (ELSD): Originally used in High Performance Liquid Chromatography (HPLC), ELSD has now been adapted for Flash chromatography. ELSD provides a universal detection method that is not reliant on the presence of chromophores in the compounds being separated. It works by evaporating the solvent from the eluted compounds and measuring the scattered light intensity. ELSD allows for the detection of non-UV-absorbing compounds, making it a valuable tool for the purification of compounds with unknown or sub-optimal UV absorption properties. It also enables the detection of compounds even in the presence of interfering solvent absorbance. By using ELSD, users can accurately monitor and fractionate compounds without the need for follow-up TLC (Thin-Layer Chromatography) or subsequent staining techniques.
- All-Wavelength Collection: This advanced detection technique addresses the challenge of collecting compounds with unknown absorbance or in the presence of interfering solvent absorbance. In traditional UV detection, compounds need to have known absorption spectra and be detectable at a specific wavelength. However, in cases where the absorption spectrum of the compound of interest or co-eluting impurities is unknown, detection becomes challenging. All-Wavelength Collection allows the collection of compounds throughout the entire wavelength range, regardless of their absorption properties. This technique ensures that compounds are captured and fractionated accurately, even when their absorption spectra are not well-characterized.
By incorporating these advanced detection techniques into Flash chromatography, researchers can overcome the limitations associated with UV detection for compounds lacking chromophores. ELSD and All-Wavelength Collection provide greater flexibility, improved detection sensitivity, and the ability to fractionate compounds without the need for additional post-chromatographic analysis. These advancements enhance the efficiency and reliability of Flash chromatography as a purification tool for a wider range of compounds.
Green Flash Chromatography
Green Flash Chromatography represents a significant advancement in flash chromatographic technology, aiming to achieve highly efficient sample purification while minimizing environmental impact. This approach focuses on optimizing the purification process by reducing solvent usage, run time, and waste generation. Here are some key features of Green Flash Chromatography:
- Efficient Sample Purification: Green Flash Chromatography utilizes the minimum eluting volume required for sample separation, ensuring that the purification process is carried out with maximum efficiency. By minimizing solvent usage and run time, it offers a more sustainable and eco-friendly approach to flash chromatography.
- Automated Method Development: The Green Flash software incorporates the true theory of flash chromatography and allows for the automatic development of optimized purification methods. By inputting TLC (Thin-Layer Chromatography) results, the software calculates and sets the optimal parameters for flow rate, run time, fraction volume, and other relevant factors. This automated method setup simplifies the purification process and improves the ease of sample purification.
- Maximum Sample Load Information: The software provides information on the maximum sample load that can be accommodated by the selected column. This feature helps users determine the appropriate sample size and optimize the purification process accordingly.
- State-of-the-Art Software: Green Flash Chromatography utilizes advanced software based on the true theory of chromatography. This ensures accurate calculations and efficient method development for achieving high-resolution separations.
- Flexible Control: The system allows for precise control over the sample eluting position and resolution. Users can tailor the purification process according to their specific requirements and obtain the desired results.
- Parallel Detection: Green Flash Chromatography systems support parallel detection using multiple detectors such as UV (Ultraviolet), RI (Refractive Index), or ELSD (Evaporative Light Scattering Detector). This allows for comprehensive analysis and improved detection capabilities during the purification process.
By incorporating these features, Green Flash Chromatography offers a more sustainable and efficient approach to flash chromatography. It enables users to achieve optimal separations while minimizing solvent usage, reducing run time, and maximizing sample purification.
Flash Cromatographywith Tlc Image Reader
Flash Chromatography with TLC Image Reader is a powerful system that combines the capabilities of TLC (Thin-Layer Chromatography) analysis and flash chromatography. It incorporates a built-in UV light source and a camera to capture images of TLC plates, allowing for automated method development and improved sample purification. Here are some key features and advantages of Flash Chromatography with TLC Image Reader:
- Automated Method Development: The system utilizes the captured images of TLC plates to calculate the Rf (retention factor) value of the target compound. By selecting the target compound on the TLC plate, the system automatically develops an optimized chromatography method. This streamlines the method development process and ensures efficient separations.
- Real-time Visualization: The TLC plate is displayed on the system’s screen during the purification process. This allows chemists to monitor the progress of the separation and observe the compound spots on the TLC plate. Additionally, the system displays the compound peaks obtained during flash chromatography, providing real-time feedback on the separation.
- Data Storage: Both the photographic image of the TLC plate and the purification data are saved as data files. This enables easy reference and documentation of the purification process, facilitating record-keeping and analysis.
- Maximum Sample Load Calculation: By selecting the target compound and the nearest impurity on the TLC plate, the system automatically calculates the maximum sample load for each column. This information helps chemists choose the most suitable column for their sample, ensuring optimal purification results.
Advantages
- Fast and Economic: Flash Chromatography with TLC Image Reader offers fast and cost-effective methods for the synthesis laboratory. It allows for efficient purification of compounds up to gram quantities, reducing time and resources required for purification.
- Seamless Transition from TLC to Flash Chromatography: The system facilitates the transfer of results from TLC analysis to flash chromatography. By utilizing the TLC images and automated method development, chemists can easily translate their TLC findings into flash chromatography separations.
- No Expensive Equipment Required: Flash Chromatography with TLC Image Reader eliminates the need for additional expensive equipment. It combines the functionalities of TLC and flash chromatography into a single system, providing a cost-effective solution for sample purification.
- Automated Switching Between Normal Phase and Reversed Phase Chromatography: The system enables automated switching between different chromatography modes, such as normal phase and reversed phase. This enhances the versatility and flexibility of the purification process, accommodating different separation requirements.
In summary, Flash Chromatography with TLC Image Reader offers an efficient and user-friendly approach to sample purification. By integrating TLC analysis with flash chromatography, it enables automated method development, real-time visualization, and seamless transfer of results, leading to improved purification outcomes in a cost-effective manner.
What is Automated flash chromatography
Automated flash chromatography has revolutionized the field of purification by streamlining the process and offering several advantages over manual glass-column flash chromatography. Some of the key advantages include:
- Full Automation: Automated flash chromatography systems provide complete automation of the purification process, eliminating the need for manual intervention at various stages. From sample injection to compound collection, the entire process is controlled and executed by the automated system. This not only saves time but also reduces the need for additional manpower and minimizes human errors.
- Time Savings: One of the significant advantages of automated flash chromatography is the significant reduction in separation time. By employing optimized methods, efficient flow rates, and advanced fraction collection techniques, automated systems can achieve faster separations compared to manual methods. This time-saving aspect is particularly beneficial for high-throughput applications or when dealing with large sample volumes.
- Wide Range of Sample Sizes: Automated flash chromatography systems are versatile and can accommodate a wide range of sample sizes. Whether you have milligram quantities or larger-scale purification needs, the system can be easily adjusted to handle the desired sample size. This scalability is particularly advantageous in research and industrial settings where purification requirements can vary.
- Solvent and Time Efficiency: Automation in flash chromatography enables precise control over the mobile phase flow rates and elution conditions, resulting in more efficient solvent usage and reduced purification time. Automated systems can optimize the elution process, reducing the amount of solvent required for purification without compromising the separation resolution. This not only contributes to cost savings but also promotes environmentally friendly practices.
- Enhanced Method Development: Automated flash chromatography systems often come equipped with advanced software that aids in method development. These software platforms allow chemists to input parameters such as the desired resolution, flow rates, and fraction collection parameters. The system then automatically suggests optimized methods, helping to streamline the method development process and improve purification outcomes.
In summary, automated flash chromatography offers significant advantages over manual glass-column flash chromatography. It provides full automation, reduces separation time, accommodates a wide range of sample sizes, improves solvent and time efficiency, and facilitates method development. These advancements contribute to enhanced productivity, cost savings, and improved purification outcomes in various scientific and industrial applications.
Applications Of Flash Chromatography
- It is widely used for purification of organic compounds in synthetic chemistry, where reaction mixtures are cleaned up from by-products and unreacted reagents.
- In pharmaceutical industry the method is applied for purification of active pharmaceutical ingredients (APIs) and removal of impurities during drug-discovery and lead generation.
- Natural product isolation is a major use: plant extracts (like flavonoids, ginsenosides, catechins) are separated by this technique to get the target bioactive compound.
- Analytical chemistry tasks are facilitated by it, because separated fractions are fed into downstream tools (NMR, MS, IR) once purification is done.
- It is used in research & development labs for quick method development, testing of new compounds and scale-up from mg to gram levels.
- In extraction and formulation industries (for example of botanical extracts, nutraceuticals) the technique is used to remove unwanted components and ensure purity of final product.
- Environmental / sample-cleanup applications: separation of contaminants or unwanted materials in complex mixtures is possible with the flash technique.
Advantages of Flash chromatography
- A major advantage is that separation time is greatly reduced, because the solvent is pushed through by pressure so runs that once took hours might finish in 10-30 minutes.
- It is less expensive / lower cost to set up and operate compared with more sophisticated methods, so laboratories with tighter budgets can use it.
- Larger sample loads are possible meaning more material can be purified in one go which helps when you have grams not just milligrams of product.
- The method is fairly simple and straightforward (in packing, operation) so less technical expertise is strictly required compared to ultra-high performance setups.
- Solvent consumption is often reduced (or at least more efficient) because the run is faster and more optimized, which helps from viewpoint of waste and cost.
- It is versatile for a range of compounds (organic, natural-products, intermediates) so many different purification tasks can be handled with this one technique.
- The equipment is usually more flexible (you can operate manually or automated) so you can scale up or customise the run depending on your lab’s needs.
Disadvantages of Flash chromatography
- It is often observed that the resolution of compounds is lower compared to methods like HPLC (High Performance Liquid Chromatography), so very similar compounds may co-elute or overlap.
- The purity of final product may not reach the high levels required for some applications (like final drug substance analysis) because the separation power is limited.
- Large amounts of solvent may still be used and waste generated, especially when sample loads are high or gradient elution is used, which reduces the environmental / cost advantage.
- When manual-columns are used, the packing and monitoring demand much operator skill and time, and inconsistencies in packing can degrade performance.
- The technique is less suitable for very complex mixtures where extremely high separation efficiency is needed, because the short column lengths / relatively large particle size limit plate number.
- Some automated flash systems incur significant upfront cost (equipment, pre-packed cartridges), and maintenance / consumables may reduce cost-benefit in smaller labs.
Flash chromatography video
- https://www.news-medical.net/life-sciences/What-is-Flash-Column-Chromatography.aspx
- https://www.biotage.com/blog/what-is-flash-chromatography-and-why-should-i-do-it
- https://www.pharmatutor.org/articles/flash-chromatography-area-applicationshttps://chemistnotes.com/organic/flash-chromatography-principle/
- https://www.chromatographytoday.com/news/flash/60/breaking-news/how-does-flash-column-chromatography-work/53862
- https://phenomenex.blog/2018/07/19/flash-chromatography/
- https://pubs.acs.org/doi/10.1021/acs.jchemed.9b00929
- https://kinglab.chemistry.wfu.edu/wp-content/uploads/2020/01/flash_chromatography.pdf
- https://www.rsc.org/publishing/journals/prospect/ontology.asp?id=CMO:0002582&MSID=B514937A
- https://labs.chem.ucsb.edu/zakarian/armen/how-to-do-flash-column-3.pdf
- https://www.chromatographyonline.com/view/flash-chromatography-3
- Text Highlighting: Select any text in the post content to highlight it
- Text Annotation: Select text and add comments with annotations
- Comment Management: Edit or delete your own comments
- Highlight Management: Remove your own highlights
How to use: Simply select any text in the post content above, and you'll see annotation options. Login here or create an account to get started.