Complement System Definition
The complement system, also known as the complement cascade, is a component of the immune system that boosts the ability of antibodies and phagocytic cells to eliminate germs and damaged cells from an organism, stimulate inflammation, and assault the cell membrane of a pathogen.
- The complement system is an incredibly potent system composed of fast acting glycoproteins, numerous proenzymes, and components, and it exists in a state of inactivity in the plasma.
- Normal persons have complement components in their blood at all times.
- The term complement refers to the capacity of a system of nonspecific proteins in normal human serum to enhance the effects of other immune system components, such as antibodies.
- The complement system, a vital component of the human innate host defence system, is comprised of roughly 20 proteins found in normal human serum.
- It is a component of the innate immune system, which is not adaptive and does not change during the course of an individual.
- However, the complement system can be recruited and activated by antibodies produced by the adaptive immune system.
- The complement system is comprised of several tiny proteins generated by the liver and circulating in the blood as inactive precursors.
- Proteases in the system cut certain proteins in response to one of several stimuli, releasing cytokines and initiating a cascade of subsequent cleavages.
- This complement activation or fixation cascade results in the activation of the cell-killing membrane assault complex and the stimulation of phagocytes to remove foreign and damaged material.
- The complement system is composed of around 50 proteins and protein fragments, including serum proteins and cell membrane receptors. About 10% of the globulin fraction of blood serum is composed of these proteins.
- The complement system is triggered by three biochemical pathways: the classical complement pathway, the alternative complement pathway, and the lectin pathway.
- Considering that the alternative pathway is responsible for the majority of terminal pathway activation, therapeutic attempts in disease have centred on its suppression.
Properties of Complement
Complement demonstrates the following attributes:
- It is found in the blood of all mammals, including humans, as well as birds, amphibians, and fishes.
- These compounds are inactivated by heating serum to 56°C for 30 minutes.
- These are glycoproteins that are largely generated by liver cells and to a lesser extent by macrophages and numerous other cell types. The rate of complement glycoprotein production increases when complement is activated and eaten.
- The complement does not often bind to antigens or antibodies, but rather to antigen–antibody complexes.
- The significance of the complement rests in the fact that it contributes to an individual’s acquired and innate immunity.
Nomenclature of Complement
- Components of the complement are indicated by the numerals C1–9. In the form of inactive proenzymes, these components circulate in plasma.
- Activation involves proteolytic cleavage into peptide fragments.
- C3 is divided into two parts, C3a and C3b, for example.
- Typically, the large component is called “b,” whereas the small portion is designated “a.” However, for historical reasons, the larger part of C2 is called C2a and the smaller fragment is designated C2b.
Effects of complement
Four principal impacts of complement:
- It causes cell lysis (such as bacteria, viruses, allografts, and tumour cells).
- It creates mediators that contribute to the activation of certain cellular activities, inflammation, and immunoregulatory molecule secretion.
- It enhances opsonization, the process by which phagocytes more rapidly and effectively ingest germs.
- It results in immunological clearance, in which immune complexes are removed from the circulation and delivered to the spleen and liver.
Activation of Complement
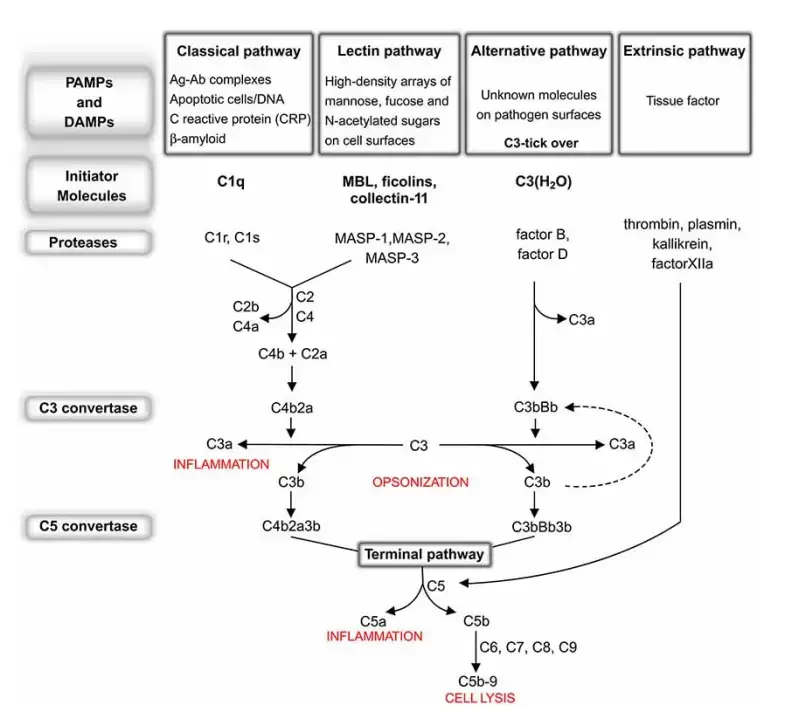
These components must be activated because they are proenzymes that must be cleaved in order to become active enzymes. Complement activation can be started by antigen–antibody complexes or by a number of non-immunologic substances, such as endotoxin (lipopolysachharides). Complements are typically denoted by the letter C followed by a number, such as C1, C2, C3, etc. Some have merely the letters, such as B and D. Some are represented solely by their names, such as homologous restriction factor. The subunits of C1 are C1q, C1r, and C1s. a and b are the two constituents of C2-C5. The larger subunits are marked by the letter b, whereas the smaller ones are denoted by the letter a. (except C2a, which is larger than C2b). Activation of complement and cell lysis.
Complement activation happens via the following three pathways:
- Classical pathway
- Alternative pathway
- Lectin pathway (or mannose binding lectin pathway)
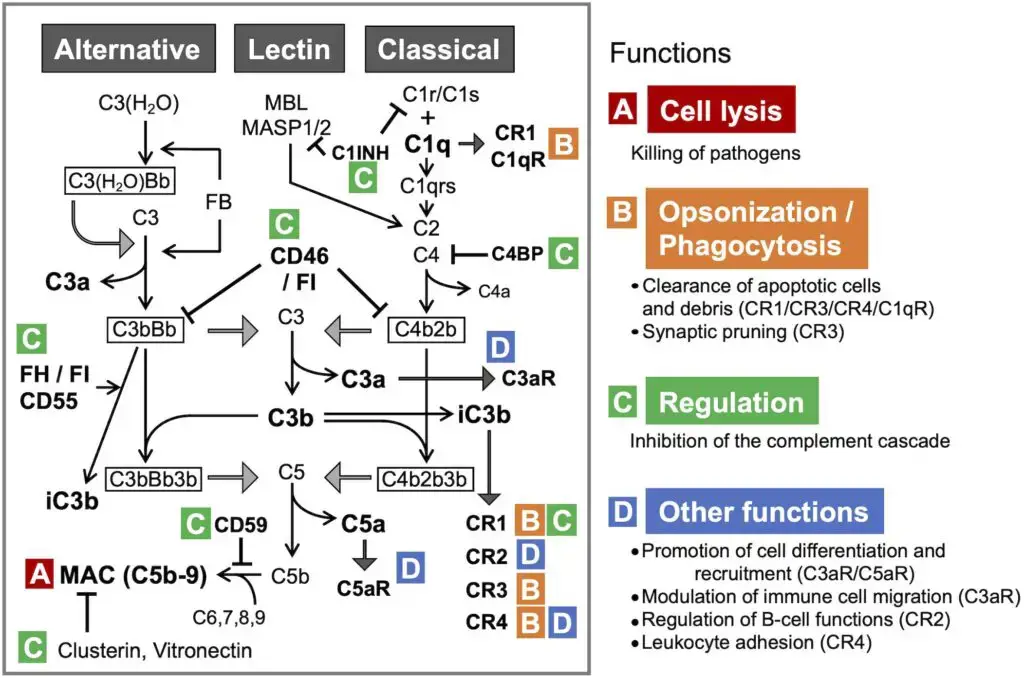
The initial step of the complement system varies between routes. C3 convertase, which cleaves C3 into C3a and C3b, and C5 convertase, which cleaves C5 into C5a and C5b, produce enzyme complexes. Thus, C3b binds C3 convertase to produce C5 convertase. C5 convertase, which is produced via the alternative, classical, or lectin pathway, promotes the activation of late components of the complement system to create the membrane attack complex (MAC), which finally kills the pathogen. This occurs via three pathways: the Classical pathway, which is activated by an antigen-antibody response; the Alternative pathway, which is activated on microbial cell surfaces; and Mannose binding. Activation of the lectin pathway by a plasma lectin that binds to mannose residues on microorganisms.
Function of C3b
The C3b performs two essential functions:
- First, it joins with other complement components to form C5 convertase, the enzyme that leads to the formation of membrane attack complex.
- Second, it opsonizes bacteria due to the presence of C3b receptors on the phagocytes’ surface.
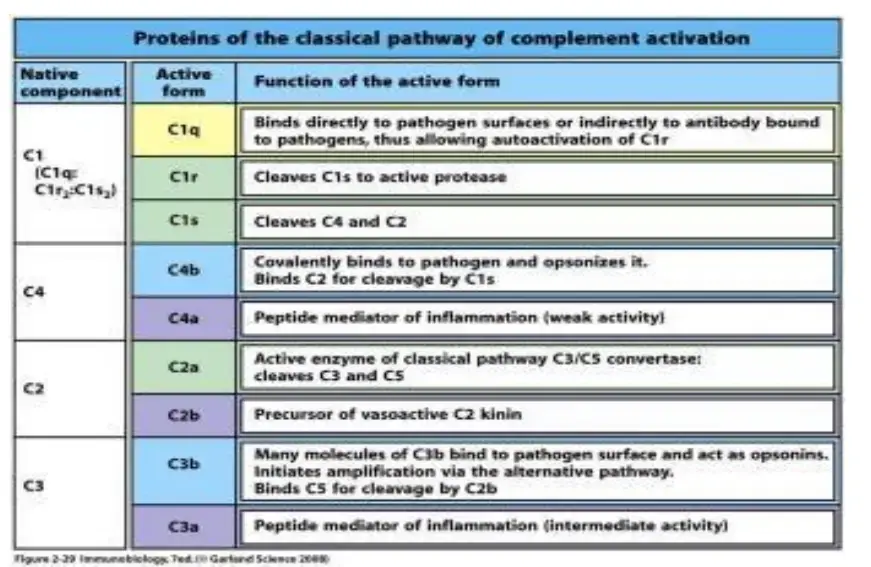
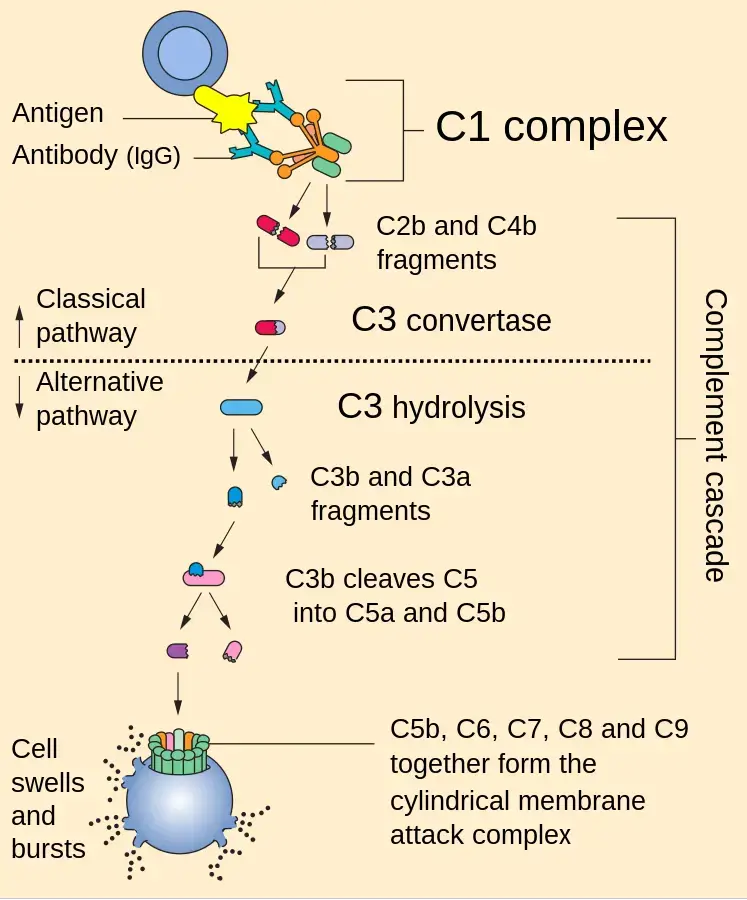
Classical Pathway of Complement Activation
- To begin the classical pathway, antigen-antibody complex formation is required (immune complex).
- The antibody (IgM/IgG) attaches to the antigen when it enters the body. Changes in the Fc region of the antibody are triggered by this process, revealing a region that can bind to C1 protein.
- That’s why the antibody can’t trigger the complement system unless it’s attached to an antigen.
- C1 is a massive multimeric protein complex with one C1q molecule and two C1r and C1s molecules.
- C1q combines with an antibody that has attached to an antigen (Fc portion). Both C1r and C1s are proteases that aid in the cleavage of C4 and C2.
- C4 is a protein called by the immune complex linked to C1. C4 is cleaved into C4a and C4b.
- Activated C4b binds to the target surface in the vicinity of C1q, whereas C4a dissociates. Next, C4b pulls in C2, which is then split into C2a and C2b.
- When C2a binds C4b, the resulting C4b2a complex displaces C4b. For C3, the active C4bC2a is the trigger.
- Since the C4b2a complex is responsible for converting inactive C3 into an active form by cleaving C3a and C3b apart, it is also known by that name.
- C4b2a can cleave several C3 molecules with just one molecule. C3b can interact with the convertase or the microbial surface.
- By binding to C3 convertase, C3b stimulates C5 to be cleaved into C5a and C5b by the enzyme C4bC2aC3b (C5 convertase). However, C5b is held in place by binding C6, whereas C5a is free to diffuse away.
- Later, C5bC6 links to C7. After binding C8, the C5bC6C7 complex is introduced into the phospholipid bilayer of the cell membrane.
- All of these (C5b678) work together with C9 to generate the membrane assault complex (MAC).
- This creates a breach in the bacterial cell wall, allowing for the escape of internal contents and the entry of foreign chemicals. As a result, the cell undergoes lysis due to an input of water and a loss of electrolytes since it is unable to maintain its osmotic stability.
- The outer membrane of Gram-negative bacteria is conducive to MAC production, making this strategy more effective than it would be against Gram-positive bacteria, which have a thick, hard coating of peptidoglycan in their place.
- Some C3b molecules do not interact with C4b2a, but they do serve as opsonins by coating immune complexes or microbial cell surfaces.
- Opsonization is the process by which opsonin molecules connect to the receptor of phagocytic cells (such as neutrophils and macrophages) and facilitate the engulfment of foreign particles (such as bacteria, tumour cells, and RBCs).
- Complement’s smaller subunits diffuse away from the site and can trigger localised inflammatory reactions by binding to certain receptors.
Alternative Pathway
- The Ag-Ab complex is unnecessary for the beginning of the complement pathway in the alternative pathway.
- Foreign components of the cell surface set off the process. This outer layer of molecules could consist of lipopolysaccharides or something similar.
- When inflammation allows bacteria to enter the host body, complements travel to the place where C3 molecules come into contact with antigen and activate.
- Serum C3, which contains an unstable thioester link, undergoes delayed spontaneous hydrolysis to produce C3a and C3b by this mechanism. C3b attaches to the outside of a foreign cell and then joins forces with a serum protein known as factor B.
- Serum protein D, which has catalytic activity, now has its substrate exposed thanks to factor B.
- Next, factor D splits B into Ba and Bb, which assemble into C3 convertase (C3bBb). Similar to the traditional process, C3 convertase gives rise to C5 convertase, which in turn gives rise to a MAC.
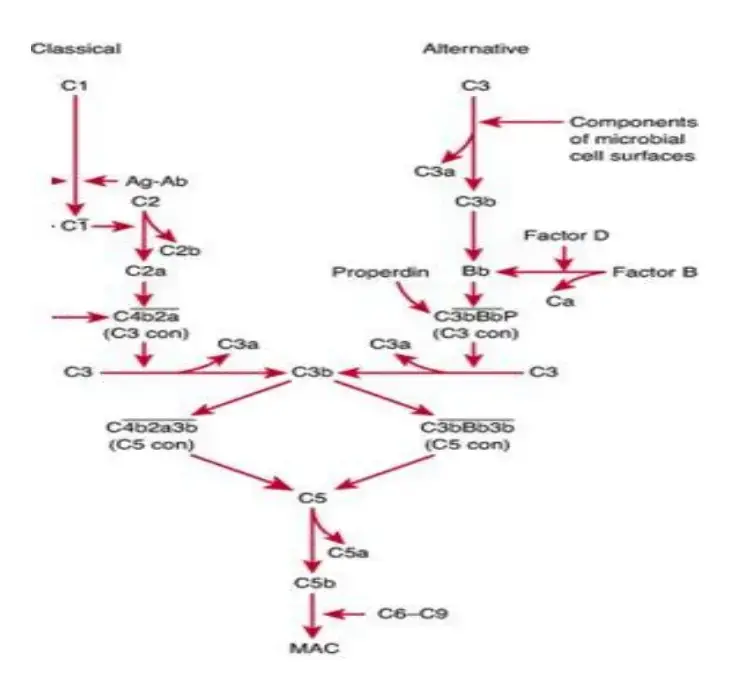
Mannose binding Lectin (MBL) Pathway
- It is possible for some bacteria to trigger the complement system in the absence of both antibodies and endotoxins.
- This is because the binding of circulating lectin (MBL) to mannose residues on glycoproteins or carbohydrates on the surface of bacteria activates the MBL pathway.
- Among the microorganisms that can activate the MBL pathway include pathogenic bacteria like Salmonella, Listeria, and Neisseria strains, as well as fungi and viruses like HIV-1. It is known that MBL, an acute phase protein, is present in higher amounts when inflammation is present.
- It is the carbohydrate on the target cell that the lectin identifies and attaches to, which triggers the complement response.
- The MBL route produces activated complement system proteins via the action of C4 and C2, much like the classical pathway.
- Similar to C1q in structure, MBL has the same functional effect. Two components, MASP-1 and MASP-2, bind to MBL after it has bound to carbohydrate residues on the surface of a cell or pathogen.
- Serine proteases related with MBL. An enzyme pair, analogous to C1r and C1s, forms a tetrameric complex that cleaves C4 and C2 to generate C3 convertase.
- Following this step, the formation of C5 convertase and the MAC proceeds along the traditional pathway.
Regulation of Complement System
Due to the complex nature of the complement system and the large number of biologically active compounds it generates, the human body has developed a wide variety of regulatory mechanisms to limit any unnecessary harm to the host. Several mechanisms control the complement activation cascade by regulating the activity of the various complement components activated at each stage. The complement system is controlled by the following:
1. Level of antibody
- The conventional process begins with the regulation of antibody levels.
- The complement-binding sites on the heavy chains of IgG and IgM are inaccessible to the C1 component of the complement if antigen is not linked to the antibodies.
- This indicates that even if IgM and IgG are always present in the blood, complement is not activated.
- A conformational change occurs upon antigen binding with particular antibodies, allowing the C1 component to bind and kick off the cascade process.
2. C1 inhibitors
- Antibodies like these are essential for keeping complement activation to a minimum. These trigger C1s dissociation from C1qrs, preventing the formation and function of C1qrs complex.
- C1 inhibitors have the potential to aid in the clearance of the complete C1 complex from antigen-antibody complexes.
3. Other inhibitory substances
- Several chemicals can block the classical route at various points in its activation process.
- These are examples of defensive mechanisms employed by the host cell.
- These defence mechanisms probably aid in shielding host cells from any bystander damage that may be caused by activated complement fragments (C3b and C4b) forming on and near the cell surface.
4. Decay-accelerating factor (DAF)
- It is another type of inhibitory chemical found in the membranes of many different kinds of host cells. This is because it has been shown to hasten the dissociation of active C4b2a complexes, hence inhibiting their capacity to activate native C3.
- As an added bonus, DAF sticks around after membrane-bound C4b and C3b are done interacting with C2a and factor B, respectively.
- Host cells are protected from complement-mediated membrane damage because they do not produce the two forms of C3 convertases (C4b2a and C3bBb).
5. Regulation of alternative pathway
- The proteins and processes that control the alternate pathway are distinct from those that control the canonical one.
- Factor H binds to C3b and is cleaved off of the complex by the plasma protease inhibitor factor I.
- Thus, less C5 convertase is accessible.
Biological Effects of Complement
For the most part, complement is used to boost the body’s humoral immune response. The complement through its various products participates in the inflammatory response, opsonization of antigen, viral neutralization, and clearance of immune complexes as follows:
Chemotaxis
- Polymorphonuclear leukocytes and phagocytic cells specifically identify the chemotactic ligand C5a. When an antigen-antibody response is taking place, this substance recruits leukocytes to the area. A phagocytic cell may identify and consume opsonized particles at that location.
- In addition to its chemotactic impact, C5a stimulates neutrophils, leading to the reversible aggregation of these cells and the release of their stored enzymes, including proteases.
- Additionally, neutrophils’ ability to adhere to the endothelium is improved by C5a.
Opsonization
- Opsonization of infectious bacteria and viruses is facilitated by complement. Phagocytic cells have an easy time engulfing bacteria and viruses when the complement component C3b is present.
- As a result, many phagocytes have receptors for the C3b component on their surface.
Hypersensitivity Reactions
- To some extent, complement is involved in both type II (cytotoxic) and type III (immune-complex) hypersensitivity reactions.
- Mast cells’ degranulation and subsequent release of mediators like histamine is prompted by C3a, C4a, and C5a components.
- Vasoactive amines (such as histamine) and heparin are released from storage when the C3a fragments bind to receptors on basophils and mast cells.
- An increase in capillary permeability and smooth muscle contraction follows histamine’s release into the tissues.
- The release of fluid into the tissue leads to edoema and swelling. C3a and C5a may also improve permeability of blood vessels by acting directly on endothelial cells.
- What happens when IgE antibodies attached to the membranes of mast cells and basophils react with their respective antigens is remarkably similar to the typical anaphylactic reaction. This is why C3a and C5a get the name “anaphylatoxins” (allergy toxins).
Cytolysis
- Cytolysis is mediated by complement. C5b-9 complex (MAC) insertion into the cell membrane causes erythrocyte, bacterial, and tumour cell death or lysis.
- The membrane is disrupted by the entrance of the MAC complex, allowing water and electrolytes to enter the cell.
Antibody Production Enhancement
- C3b’s attachment to activated B cell surface receptors greatly boosts antibody production compared to B cells triggered by antigen alone. Therefore, there is a correlation between C3b deficiency and decreased antibody production.
- As a result, severe pyogenic infections occur when there is a shortage of both C3b and antibodies, which compromises the host’s defence.
Deficiency of Complement
- A healthy complement system is crucial to human health. Many diseases have been linked to deficiencies in specific nutrients.
- Angioedema is brought on by a lack of C1 esterase inhibitors, which can be inherited. Overproduction of esterase is caused by a deficiency in C1 esterase inhibitors. Because of this, more anaphylatoxins are released, leading to increased capillary permeability and swelling.
- Increased complement-mediated hemolysis is a side effect of developing a deficit in DAF. Paroxysmal nocturnal hemoglobinuria is the clinical hallmark of this disorder.
- Susceptibility to Neisseria bacteremia and other infections is dramatically increased in people who lack C5-8 components, whether by genetics or through other means. Severe recurring pyogenic sinusitis and respiratory infections result from a lack of C3.
- Severe liver illness, like chronic hepatitis or alcoholic cirrhosis, impairs the body’s ability to produce enough complement to fight infections. Therefore, pyogenic bacterial infections are more dangerous for these people.
Biosynthesis of Complement
- The complement is created in several different organs. The intestinal epithelium produces C1, macrophages produce C2 and C4, the spleen produces C5 and C8, the liver produces C3 and C6, and the intestines produce C9 and C10.
- During the initial stages of inflammation, the levels of complement components 3, 4, 5, and 6 rise.
- Plasma levels of complement and other proteins in the acute phase reactants family rise with a severe inflammatory response.
References
- Textbook of Microbiology & Immunology by Subhash Chandra Parija
- Janeway CA Jr, Travers P, Walport M, et al. Immunobiology: The Immune System in Health and Disease. 5th edition. New York: Garland Science; 2001. The complement system and innate immunity. Available from: https://www.ncbi.nlm.nih.gov/books/NBK27100/
- Trouw, L. A. (2017). Complement System. Kelley and Firestein’s Textbook of Rheumatology, 355–365. doi:10.1016/b978-0-323-31696-5.00023-1
- Jha, P., Bora, P. S., & Bora, N. S. (2010). Role of Complement in Ocular Immune Response. Encyclopedia of the Eye, 160–164. doi:10.1016/b978-0-12-374203-2.00004-x
- https://primaryimmune.org/about-primary-immunodeficiencies/specific-disease-types/complement-deficiencies
- https://courses.lumenlearning.com/wm-biology2/chapter/complement-system/
- https://www.news-medical.net/life-sciences/Complement-System-Overview.aspx
- https://www.vedantu.com/biology/complement-system
- https://www.creative-biolabs.com/complement-therapeutics/complement-system.htm
- https://www.ndvsu.org/images/StudyMaterials/Micro/Complement-System.pdf
- https://www.virology.ws/2009/09/28/the-complement-system/’
- https://new.bhu.ac.in/Content/Syllabus/Syllabus_300620200520103014.pdf
- https://www2.nau.edu/~gaud/bio372/class/immuno/complem.html
- http://www.jiwaji.edu/pdf/ecourse/zoology/THE%20COMPLEMENT%20SYSTEM.pdf
- https://www.physio-pedia.com/Complement_System
- https://en.wikipedia.org/wiki/Complement_system
- Text Highlighting: Select any text in the post content to highlight it
- Text Annotation: Select text and add comments with annotations
- Comment Management: Edit or delete your own comments
- Highlight Management: Remove your own highlights
How to use: Simply select any text in the post content above, and you'll see annotation options. Login here or create an account to get started.