A bioreactor is the vessel in which biological reactions is carried out in controlled conditions. It is the process where microorganisms, plant cells or animal cells are allowed to grow and produce useful products. It is designed as a closed container in which temperature, pH, dissolved oxygen (DO) and nutrient level is maintained with the help of sensors and control devices. It is the environment that helps the cells to multiply and convert the substrate into different metabolites. This is referred to as the controlled system for large-scale cultivation of biological materials.
It is the process that developed from early fermentation practices where reactions was not regulated properly. Modern bioreactors now use different designs like stirred tank, air-lift or single-use systems. It is used in industries for the production of vaccines, antibiotics, enzymes, biofuels and many food products. The major source of efficiency in bioreactors is the continuous monitoring of internal conditions which keeps the culture stable throughout the reaction period.
Definition of Bioreactor
A bioreactor is a device or system used to cultivate and grow biological cells, tissues, or organisms under controlled conditions.
Principle of Bioreactor
The principle of a bioreactor is based on maintaining a favourable and uniform environment in which biological cells can grow and carry out their metabolic activities. It is the process where different control systems are used to regulate temperature, pH, dissolved oxygen (DO) and nutrient level so that the culture remains stable throughout the operation. It is designed to keep the medium homogeneous, because uneven distribution of nutrients or gases can reduce the activity of the cells. The reaction is carried out in a vessel where sensors continuously check the internal conditions and these readings are used to adjust the parameters when required.
It is the process in which mixing has an important role because the mixing system helps in equal distribution of oxygen, nutrients and other components. In stirred tank types, impellers break the gas bubbles and this increases the contact between gas and liquid phase. Airlift types use gas movement to circulate the medium. It is regulated so that the shear force is not very high, because high shear may damage sensitive cells. These are the conditions that decide how effectively mass transfer occurs in the culture.
The bioreactor can work in different modes depending on the requirement. In batch mode the medium and nutrients are added once at the beginning and the system is closed till the end. In fed-batch mode nutrients is supplied slowly so that the cells can continue their growth for a longer time. In continuous or perfusion mode, fresh medium is added while the used medium is removed at the same time so that the cells can remain in a steady state. These are some of the main ideas behind the working principle of a bioreactor.
An ideal Bioreactor Should Have Following Qualities
An ideal bioreactor is the vessel in which different microorganisms or cells are allowed to grow under controlled conditions, and it is the system that maintains a uniform environment needed for maximum metabolic activity. It is the process in which physical and chemical factors is regulated for getting high productivity.
Main Qualities–
Some of the main features of an ideal bioreactor are–
- Proper Control of Temperature
It is important that the system maintain a constant temperature because most bioreactions are heat releasing. The vessel is designed with coils or jackets for removing excess heat, and the temperature is monitored continuously for supporting cell growth. - Regulation of pH and Dissolved Oxygen (DO)
The pH and dissolved oxygen is controlled properly because any change in these factors can affect enzyme activity and the growth of organisms. It is the process in which sensors are used for measuring these parameters inside the vessel. The in-line measurement helps in maintaining stable conditions. - Nutrient and Substrate Management
In this step the nutrients and substrates is supplied in proper concentration to avoid limitation. The system allows continuous checking of nutrient level so that the cells can grow without inhibition. - Good Mixing and Mass Transfer
The major source of uniformity inside the bioreactor is proper mixing. The impellers or spargers create fine bubbles to increase surface area for gas exchange. It is the process that maintains homogeneity of oxygen, nutrients, and cells. - Low Shear Stress
Some cells are very sensitive to mechanical forces. So the ideal bioreactor should reduce shear stress. Airlift bioreactors and packed bed reactors is used for shear-sensitive cultures because they can produce smooth flow. - Sterility and Contamination Control
The vessel is made of non-corrosive and non-toxic material. It should tolerate steam sterilization (SIP) and allow easy cleaning. Single-use bioreactors is preferred now because they come pre-sterilized and reduce the risk of contamination. - Scalability and Flexibility
It is the process which allows the system to be used from laboratory level to industrial level without major changes. The same design should support different organisms and different operating conditions. - Automation and Monitoring
Modern reactors have automated control for sensing any change in the process. It is used for regulating temperature, pH, DO, and nutrient flow. Some systems also use advanced software and prediction tools for maintaining product consistency. - High Productivity and Energy Efficiency
The ideal bioreactor increases product yield per unit volume. Techniques like perfusion help in long term cultivation. Systems with low mechanical parts like airlift reactors requires less energy and low maintenance.
An ideal bioreactor is important because it gives maximum product, ensures safety from contamination, and provides a stable environment for different biological processes. It is helpful for research, industrial fermentation, enzyme production, and cell-based systems.
Bioreactor Design
- It is based on the type of organism used, the optimum condition required for formation of desired product, the product value and also the scale at which production is carried out.
- It is the design which help to improve productivity and give better quality products at lower price.
- A bioreactor is a device that is made with different features. Some of the main features are–
– Agitator system
– Oxygen delivery system
– Foam control system
– Temperature and pH control system
– Sampling ports
– Cleaning and sterilization system
– Lines for charging and emptying - The material used for construction of bioreactor must have some important properties. These are–
– It should not be corrosive.
– It should not add toxic substances to fermentation media.
– It should tolerate steam sterilization process.
– It should tolerate high pressure and resist pH changes. - The sizes of bioreactor is different depending on application. It is seen from microbial cell level (few mm3) to shake flask (100–1000 ml) to laboratory scale fermenter (1–50 L) to pilot level (0.3–10 m3) and to plant scale (2–500 m3) for large volume production.
Important factors need to be consider in designing Bioreactors
- The growth requirement of organism must match with the reactor configuration, and it is seen that each microbe have different optimum temperature and pH range which often shift during fermentation.
- The material used should not be corrosive and should not release toxic compounds into the medium, and it is noted that some alloys behave differently under repeated steam sterilization cycles (121°C).
- It is essential that proper oxygen transfer capability is maintained because oxygen limitation disturb the metabolic pathway, and mixing pattern also change when the scale is increased.
- The agitation system is required to give uniform mixing without causing excess shear on sensitive cells, and in many cases dead spaces is formed behind baffles which is corrected by changing impeller designs.
- The reactor need temperature and pH control units which have fast responding sensors, but these sensors sometimes show drift or noise which affect the control loop.
- Heat removal efficiency should be considered since metabolic heat accumulates quickly. Cooling jackets or coils must have enough surface area, but still localized hot spots is formed.
- Sterility maintenance is important, and CIP/SIP (clean-in-place / steam-in-place) systems is required even though their valve clusters make cleaning more difficult.
- Foam control mechanisms is necessary because uncontrolled foam block gas exchange and disturb sampling, and antifoam dosing need proper calibration.
- Sampling and monitoring ports should be placed suitably. Poorly placed sampling ports cause mixing delay or contamination risk, and too many ports reduce vessel strength.
- Scale-up feasibility must be considered so that hydrodynamic pattern remain consistent from laboratory to large volume, even though these pattern rarely stay identical.
- The design need proper pressure tolerance specially under high aeration or gas back pressure, and some structural welds produce weak points.
- Substrate distribution inside reactor should remain uniform. Patchy feeding create metabolic oscillation, and feed line angles sometimes make unwanted gradients.
- Automation and control strategy also influence reactor design because actuator speed, sensor position and signal noise decide how stable the environment is maintained.
Diagram of a bioreactor
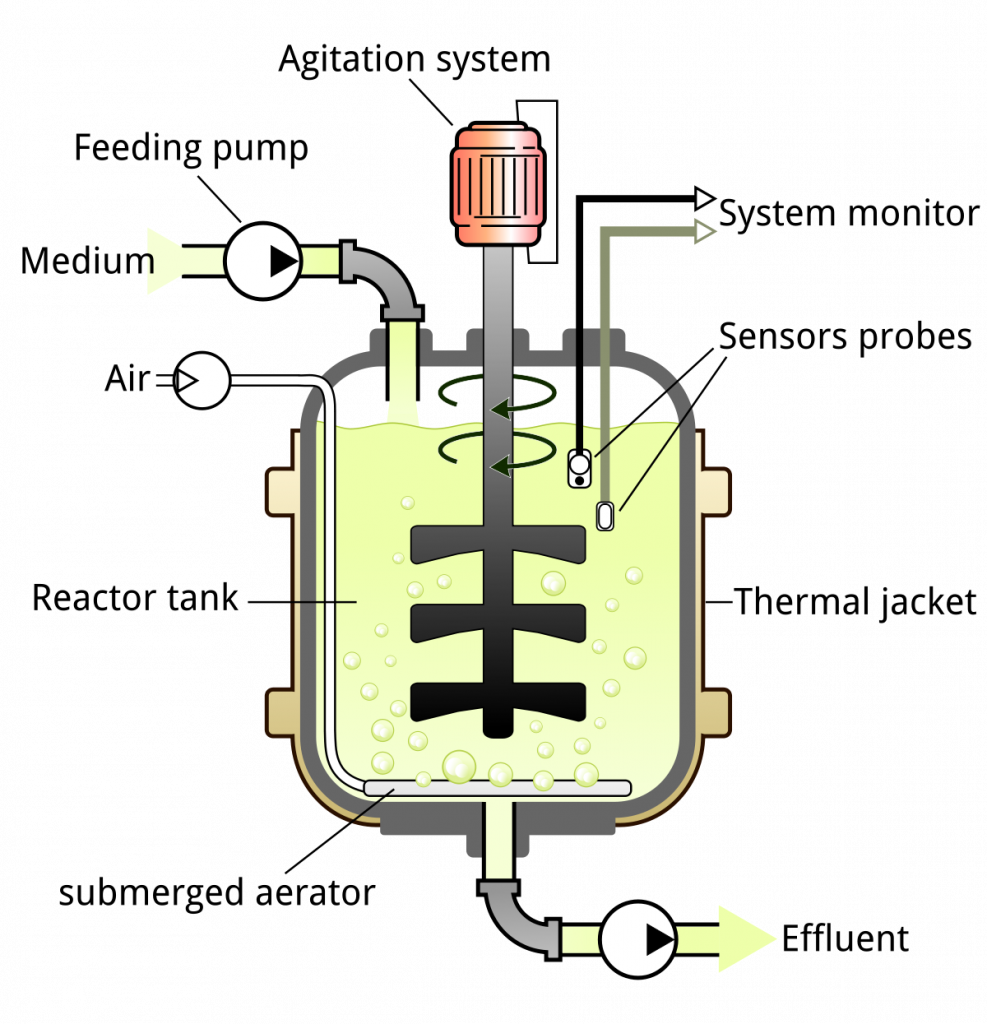
Parts of the bioreactor and their function
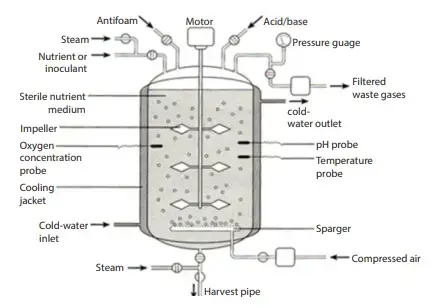
- Fermenter Vessel– It is a large cylindrical vessel closed at top and bottom with different pipes and valves. The vessel is designed so that the process can run under controlled conditions. Glass vessel is mostly used in small scale because it is non-toxic and corrosion proof. Stainless steel vessel is used in large scale and it can tolerate pressure and corrosion.
- Heating and Cooling Apparatus– The outer surface of vessel is fitted with a cooling jacket which carry cooling water. Internal coils or thermostatic baths is used for heating while silicone jackets help to remove excess heat. It is the system needed for sterilizing the medium and removing metabolic heat during fermentation.
- Aeration System– It is the important part for supplying air and oxygen to culture. It usually have sparger and impeller which together maintain proper aeration. Stirring help to mix gas bubbles in the medium and also mix microbial cells so that all cells get uniform access to nutrients.
- Sealing Assembly– It is used to seal the stirrer shaft so proper agitation is maintained. The types commonly used are packed gland seal, mechanical seal and magnetic drives.
- Baffles– These are metal strips attached radially on the vessel wall. It is used to prevent vortex formation and improve aeration inside the fermenter.
- Impeller– Impellers provide uniform suspension of microbial cells in the nutrient medium. They are fixed with impeller blades connected to a motor. The blades help to break air bubbles into small size and distribute them uniformly. Disc turbines and variable pitch turbines is generally used.
- Sparger– It is the system which introduce sterile air into the vessel. The sparger pipe have small holes (5–10 mm) that release pressurized air. The types are porous sparger, nozzle sparger and combined sparger–agitator.
- Feed Ports– These ports are used for adding nutrients or acid and alkali. They are silicone tubes and are sterilized in-situ before adding or removing materials.
- Foam Control– Foam must be controlled to avoid contamination and blocking of gas exchange. A foam sensing unit and a control unit is used. A foam controlling device is usually fixed on top with an inlet into the vessel.
- Valves– Valves regulate the movement of liquid in the vessel. Different valves used are globe valve, butterfly valve, ball valve and diaphragm valve. A safety valve is also present for working under pressure.
- Controlling Devices for Environmental Factors– Different devices is used to monitor and control temperature, pH, oxygen concentration, cell mass, nutrient level and product concentration.
- Use of Computer in Fermenter– Modern fermenters is linked with automated or semi-automated computer systems for monitoring, control and data collection so the process run efficiently.
Bioreactor Types
The different types of fermentors such as;
- Continuous stirred tank fermentor
- Bubble column bioreactors
- Air-lift bioreactors
- Packed Bed Reactors
- Fluidized Bed Bioreactor
- Photobioreactor
- Membrane Bioreactor
- Rotary Drum Bioreactor
- Mist Bioreactor
- Immobilized cell bioreactor
- Activated sludge bioreactor
- Immersed membrane bioreactor
- Reverse membrane bioreactor
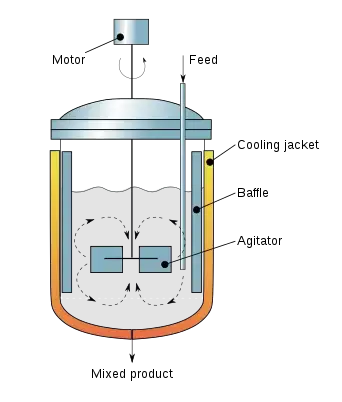
1. Continuous stirred tank fermentor
- Continuous Stirred Tank Bioreactor’s refers as a vessel where the culture medium is added continuously, And the product outflow also removed at same rate so volume stays constant.
- It is important to note that these reactor’s are usually equipped with a mechanical agitator, baffles, and sometimes sparger’s, which keeps the cells or microbe’s in a uniform suspension, creating a quite stable Environment for biochemical process.
- In the field of bioprocessing, a CSTR can be defined as a well-mixed bioreactor, where substrate concentration stays almost same everywhere in tank, although tiny gradients appear sometimes due to mixing inefficiencies, this is normal.
- Nowadays, there is increasing interest in CSTR because the steady-state operation gives predictable kinetics, however—and this is important— the system may drift if inflow composition change’s slightly, it can cause unexpected metabolite shifts.
- The reactor operates with continuous inflow/outflow, and this design gives a look into how cells respond to constant nutrient supply, but also makes them sensitive to wash-out when dilution rate exceed the growth rate.
- A number of studies have been conducted on mixing pattern, still, the agitation in to the vessel often produces shear forces that are problematic for plant cell’s, Nevertheless, microbial culture’s typically tolerate it better.
- This type of bioreactor is characterized by constant stirring, steady concentration profile’s, and easy control of pH / temperature (sometimes written 28 °C or 28°C inconsistently), creating conditions useful for enzyme production etc.
- Interestingly enough, the CSTR hold’s advantage in kinetic study because steady-state allow’s modelers to simplify Monod-type calculations, but practical issues like foaming, oxygen limitation, or contamination may prevail consistent output.
- The dilution rate (D=F/V) is one of the key parameter’s, It regulate’s how fast medium enters/leaves the tank, In practice, small error’s in flow rate measurement cause unstable biomass trends, this happens often.
- Overall, it can be stated that this reactor type provides a continuous and controlled Environment, yet its performance depends strongly on mixing efficiency, nutrient uniformity, and how the microbial population adapt or sometimes fail to adapt.
2. Bubble column bioreactors
- Bubble column bioreactor’s are vertical cylindrical vessels where gas is introduced from the bottom, And the rising bubbles create mixing without any mechanical agitation.
- It is important to note that these reactor’s rely heavily on gas hold-up differences, which produce circulation pattern’s inside the column, sometimes forming uneven flow region’s that researcher’s try to interpret.
- In the field of biochemical engineering, a bubble column can be defined as a pneumatically mixed bioreactor, where the liquid phase is stirred by bubble motion, although small stagnant zone’s may appear near the wall’s.
- Over the past years many studies focused on these systems because they provide low shear environment for fragile biomass, nevertheless, gas distribution plates may clog, And this reduces oxygen transfer unexpectedly.
- The design is characterized by simple construction, typically without internal draft tubes, but hydrodynamic’s become quite complex when superficial gas velocity increase’s, it changes bubble size distribution in to a wide range.
- Interestingly enough, bubble column’s allow large gas–liquid interfacial area, so oxygen transfer coefficient (kLa) stay relatively high, However—and this is important— superficial mixing might still remain inadequate for dense cell suspension’s.
- The air or O₂ sparged (written sometimes 2 L/min, 2L / min, or 2 L /min inconsistently) enters through the bottom distributor, and the bubbles rise due to buoyancy, forming characteristic churn-turbulent regime at high flow.
- In practice, these bioreactor’s also support aerobic fermentation’s, enzyme production, and waste-treatment applications, but foam formation prevail stable operation when surfactant-producing microbe’s are used.
- The column height-to-diameter ratio influence’s circulation loop’s, and slight change in geometry often cause unexpected shift’s in mixing time, this is a common observation in scale-up.
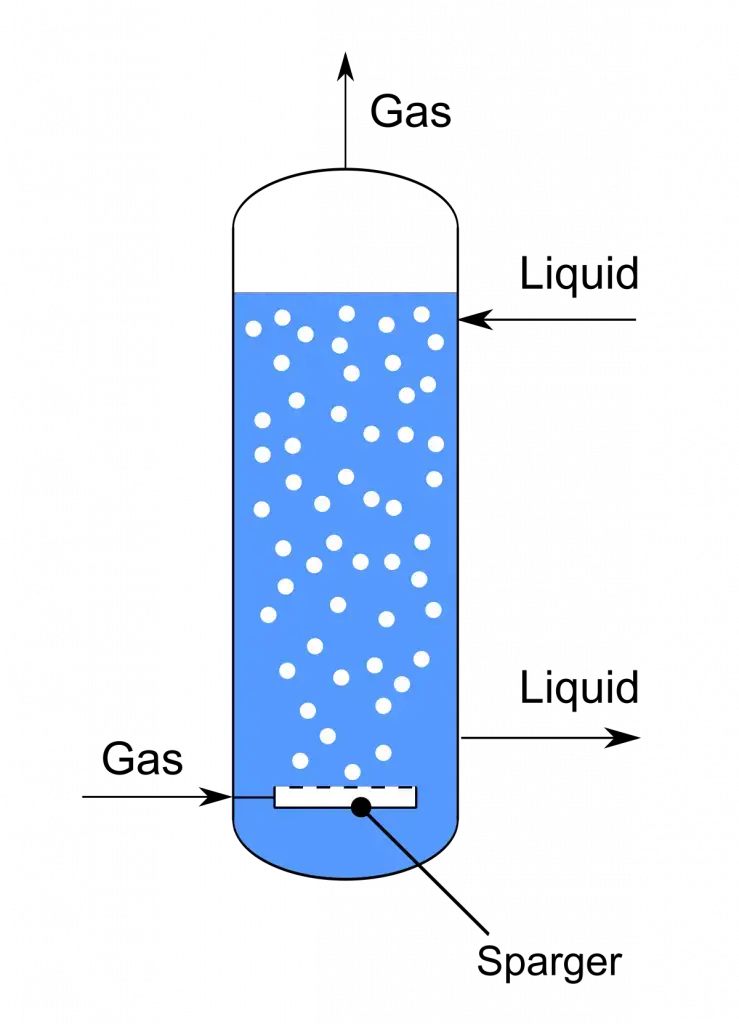
3. Air-lift bioreactors
- Airlift fermentor’s are vessels where circulation of the culture fluid is driven mainly by air injection, And this reduces need for mechanical agitation which often cause’s shear damage.
- It is important to note that these reactor’s typically consist of a riser and downcomer region’s, separated by an internal or external draft tube, creating a loop flow that maintain’s mixing.
- In bioprocess engineering, an airlift device can be defined as a pneumatically mixed fermentor, where gas hold-up differences between riser/downcomer pushes the liquid upward and then downward in to a gentle cycle.
- Over the past few decades, many researcher’s focused on this system because it gives uniform oxygen transfer, nevertheless, foam formation or unstable bubble pattern’s sometimes disturb mass-transfer efficiency.
- The design is characterized by low shear stresses, which is valuable for plant cell’s, fungal pellets, or fragile biomass, but also the hydrodynamic pattern fluctuates when air-flow rate’s are slightly altered, it happens often.
- Interestingly enough, no mechanical impeller is used, so energy consumption becomes lower, However—and this is important— scale-up of airlift fermentor’s may show complex flow regimes; They complicate prediction of mixing time.
- The gas sparging inlet (sometimes written 1.5 L/min or 1.5L / min inconsistently) introduce’s sterile air, this air generate’s buoyancy in the riser, and the liquid returns by gravity through the downcomer, forming a stable circulation loop.
- In the field of biotechnology, these fermentor’s play a vital role in aerobic culture’s because oxygen transfer coefficient (kLa) remain’s relatively high, although measurement error’s frequently distort the calculated value.
- In practice, an airlift system provide’s good temperature control, easy sterilization, and simplified construction, but the internal draft tube thickness or angle sometimes prevail the proper flow distribution.
- Overall, it can be stated that airlift fermentor’s combine gentle mixing, efficient aeration, and lower power requirement’s, yet their performance depends strongly on air-flow uniformity, bubble behavior, and how the microorganism adapt to the hydrodynamic pattern.


4. Packed Bed Reactors

- Packed bed reactor’s are vessels filled with solid support material (like beads, fibers, or porous pellets), And the liquid or gas phase is passed through this packed zone to promote biochemical reaction’s.
- It is important to note that these reactor’s usually operate in plug-flow style, although channeling and dead-zone formation sometimes distort the actual flow pattern quite noticeably.
- In bioprocess engineering, a packed bed can be defined as a fixed-film bioreactor, where cells or enzyme’s remain attached onto the packing surface, creating a high-density catalytic Environment with low shear.
- Over the past years, many researcher’s focused on these systems because immobilized biomass provide’s stability, Nevertheless, clogging or biomass overgrowth may reduce permeability in to the packed region.
- The design is characterized by a cylindrical column that holds the packing, but pressure drop increase’s sharply as flow rate rises, it produce’s operational challenges especially in large scale.
- Interestingly enough, packing materials (sometimes written 8 mm, 8mm, or 8 mm / pellet inconsistently) determine’s the surface area available for attachment, However—and this is important— wrong choice of packing may prevail uniform distribution.
- In practice these reactor’s provide high reaction rate’s due to immobilized cell layer’s, and the substrate travels along the bed length, forming concentration gradient’s that researcher’s use to model kinetics.
- The flow can be upward or downward, and small variation’s in viscosity or particle size cause abrupt shift’s in residence time, this often leads to unexpected fluctuations in product yield.
- In the field of industrial biotechnology, packed bed reactor’s also find application in wastewater treatment, enzyme biotransformation, and continuous fermentation’s etc., but controlling temperature profile along the bed sometimes become complicated.
5. Fluidized Bed Bioreactor
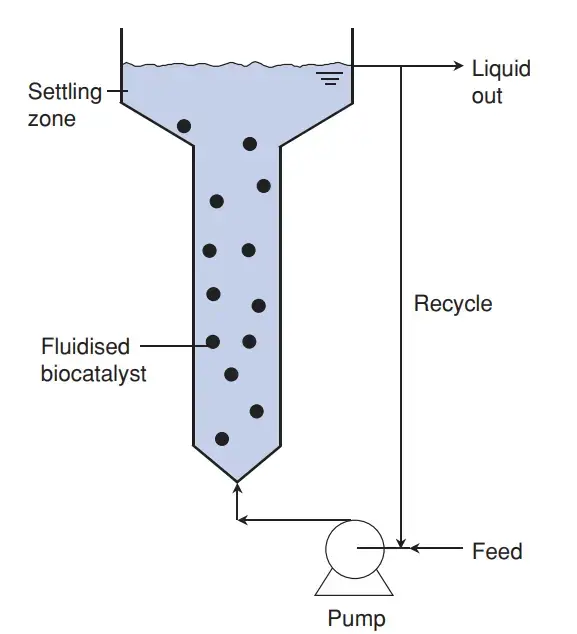
Fluidized bed bioreactor’s are systems where solid particle’s (often carrying immobilized cell’s or enzyme’s) are suspended by upward liquid or gas flow, And this expansion of the bed creates intense mixing with low shear.
It is important to note that the particles remain in a “fluidized state”, meaning the bed height increase’s as flow velocity rises, although channel formation and partial aggregation sometimes distort the expected uniformity.
In bioprocess engineering, a fluidized bed can be defined as a three-phase bioreactor (solid–liquid–gas), where the substrate stream lifts the particles in to circulation, producing high mass transfer and efficient contact between phases.
Over the past few decades many researcher’s studied these reactors because they give high surface area for immobilized biomass, nevertheless, excessive fluidization velocity may cause particle attrition, And this alters the biological performance.
The design is characterized by a column that expands during operation, but bed stability depend’s strongly on particle density variation’s, so slight deviation’s often change the operating regime unexpectedly.
Interestingly enough, fluidized bed bioreactor’s provide excellent oxygen transfer due to continuous movement of particles, However—and this is important— gas distribution plates may clog, prevails inconsistent bubble formation.
The liquid/gas is pumped upward (sometimes 1.2 L/min, 1.2L / min or 1.2 L /min inconsistently), creating buoyancy that expands the bed, and the particles settle back when flow reduces, forming a dynamic mixing cycle.
In practice these reactor’s support continuous fermentation, wastewater treatment, and enzyme biotransformation’s etc., but temperature gradient’s along the expanding bed sometimes remain difficult to control.
The hydrodynamic’s of fluidized beds depend on particle size, flow velocity, and density difference’s, and small change’s in these parameter’s often lead to abrupt shift’s in residence time, this is a common challenge during scale-up.

6. Photobioreactor
A Photobioreactor (PBR) can define as a enclosed system where microalgae’ or other photosynthetic Organism’s are cultivated under controlled light, temperature 25°C/30 °C, and nutrient conditions.
It is considered an apparatus that uses artificial or natural Light to drive the photosynthesis Process, and the illumination often placed in to the reactor in uneven ways that affect growth.
In practice, the PBR systems are designed in tubes, flat-panels, bags—or Hybrid types—so they provides higher biomass productivity compared with open pond’s, which is well known issue in algae biotechnology.
There is no doubt that the internal environment is kept closed so contamination risks are prevail (I mean, prevented), although sometimes the CO₂/air ratio fluctuate’s due to mixing inefficiency.
The algae cells’ receive continuous exposure to photons, And this light distribution is characterized by patches of high/low irradiance which influence cellular metabolism, sometimes creating awkward shading zones.
Nowadays, there is an increasing interest in PBR because they allow precise control of pH (6.8–8.5), nutrient levels, and gas exchange, however the operating cost’s are usually high, it is important to note that.
A PBR system also combines pumps, aerators, and monitoring sensors, and these component’s are assembled in a way that give a look into the growth dynamics even if spacing/noise occur in data sets.

7. Membrane Bioreactor
A Membrane Bioreactor (MBR) can define as a wastewater treatment system where biological degradation is combined with membrane’ filtration in the same integrated unit.
It is important to note that the process relies on activated sludge mixed with microfiltration/ultrafiltration module’s, and this configuration produce’s a clarified effluent with very low solids even when the biomass concentration shift’s.
In practice, the membrane units are immersed in the aeration tank or placed externally—this arrangement often chosen based on space, energy load, And the fouling behavior of the sludge.
The system is characterized by a semi-permeable barrier that allows water to pass while retaining microbial floc’s, although pressure variations and pore-blocking sometimes create operational inconsistencies.
Nowadays, many researcher’s emphasize that MBR offer higher treatment efficiency than conventional activated-sludge, however the energy demand and membrane-cleaning cycles are quite intensive, it should be pointed out that.
The biological reactions occur in the bioreactor chamber, and permeate is drawn through the membrane modules which are fabricated in flat-sheet, hollow fiber, or spiral geometries with inconsistent spacing / flux distribution.
Interestingly enough, the MBR technology also supports stable removal of BOD, nutrients, and pathogens, And this makes the treated water suitable for reuse in some Regions, but the cost’s and membrane lifetime still a concern.

8. Rotary Drum Bioreactor
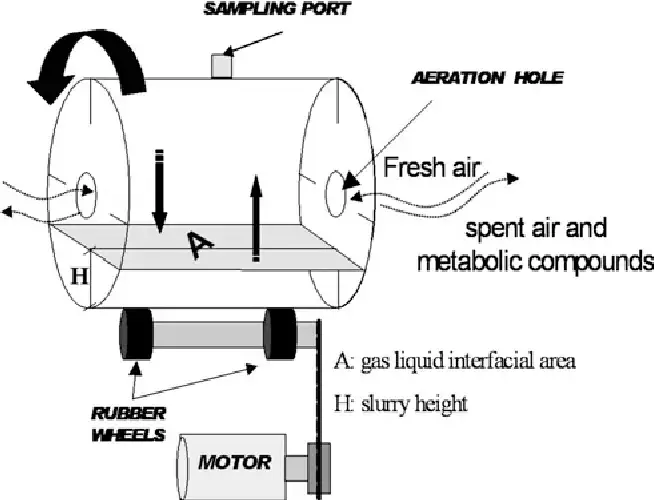
A Rotary Drum Bioreactor (RDB) can define as a cylindrical vessel that rotate’s slowly so the biomass or solid substrate become mixed continuously with air/nutrient’s for microbial processing.
It is worth mentioning that the system is basically a horizontal drum equipped with internal baffles, and the rotation creates periodic lifting of the material which improves oxygen transfer, although the moisture distribution sometimes get’s uneven.
In practice the RDB configuration is chosen for solid-state fermentation (SSF) because it provides gentle agitation, And this slow tumbling action prevent’s compacting of the substrate while also enhancing heat dispersion.
The reactor is characterized by controlled aeration in to the drum, temperature regulation around 28°C–35 °C, and intermittent mixing cycles, however the operational noise and mechanical wear are common issue’s, it should be pointed out that.
Nowadays, many researcher’s consider RDB units useful for cultivating fungi, enzyme production, and biodegradation processes, but the scale-up remain’s challenging due to inconsistent shear zones that develop along the rotating wall.
The drum rotation speed is typically low (1–12 rpm); They depend on substrate type, moisture %, and metabolic heat, and these parameter’s strongly influence microbial growth even when the mass transfer coefficient fluctuate’s.
Interestingly enough the RDB design also supports semi-continuous loading/unloading, And this give a look into efficient processing for compost-like materials or polymer degrading microbes, though fouling and clogging may occur in narrow sections / openings.
9. Mist Bioreactor

A Mist Bioreactor can define as a cultivation system where cells’ or plant tissues are grown over a chamber while a fine nutrient mist/cloud is delivered in to the space instead of traditional liquid immersion.
It is important to note that the reactor generate’s tiny droplets (10–50 µm), and these aerosolized nutrient’s reach the explant surfaces evenly, although the humidity sometimes drift’s unpredictably due to nozzle pressure fluctuation.
The system is characterized by suspended scaffolds, And the root or tissue mass stay’s partially aerated which improves oxygen transfer compared with submerged bioreactors, however evaporation losses can be high.
In practice, the nutrient solution is atomized using ultrasonic, pressure-based, or spinning-disk nozzles, and this mist pattern produce’s a thin film that support’s metabolic activities even when the droplet distribution is awkward in some region’s.
Nowadays, many researcher’s consider Mist reactors useful for aeroponic-type culture, micropropagation, and delicate cell lines like plant protoplast’s, but the clogging of the spray system remain’s a recurrent issue.
The internal chamber usually kept at 22°C–28 °C with air/nutrient ratio’s adjusted through valves; They allow rapid gas exchange, yet control of the mist density become challenging when airflow increase’s suddenly.
10. Immobilized cell bioreactor

An Immobilized Cell Bioreactor (ICB) can define as a reactor system where microbial’ or plant/animal cell’s are fixed onto a solid Matrix so the biocatalytic activity is carried out without cells freely suspended in liquid.
It is well known that the immobilization occur’s on carriers like alginate beads, porous gels, fiber matrices, or synthetic polymers—and this fixed arrangement improves stability of the bioprocess even though diffusion resistance sometimes increase’s.
In practice the cells are entrapped, adsorbed, or covalently bound to the support, And the flowing substrate solution passes across the immobilized layer which create’s distinct reaction zones inside the reactor chamber.
The system is characterized by higher cell density, reduced washout risk, and steady product formation, however channeling and mass-transfer limitations are common issue’s when pore sizes become inconsistent.
Nowadays, researcher’s often select ICB units for continuous bioconversion, fermentation, and wastewater treatment, but the regeneration of the carrier material remain’s challenging especially when mechanical erosion happen’s.
The operational conditions usually maintained at 20°C–37 °C with substrate/product ratio’s adjusted in to the inflow line; They help keep reaction rate stable, although viscosity or pH drift may disrupt uniform contact.
Interestingly enough the immobilized configuration also give a look into long-term reuse of biocatalyst’s, And this reduce’s overall process cost even if the initial preparation workload is quite hardy and tedious / time-consuming.
11. Activated sludge bioreactor
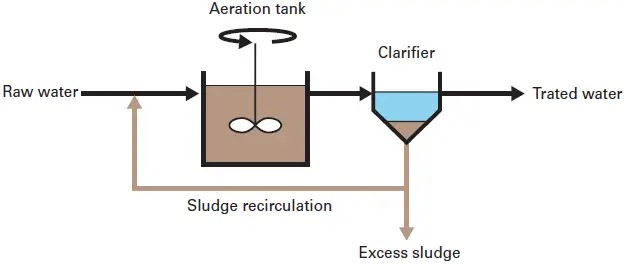
An Activated Sludge Bioreactor (ASB) can define as a wastewater treatment system where microbial’ floc’s suspended in aerated mixed-liquor degrade organic pollutant’s through biochemical oxidation.
It is important to note that the reactor rely’s on continuous aeration and mixing so oxygen is supplied to the biomass, And the microorganisms form aggregates that carry out BOD/COD removal even when the hydraulic load fluctuate’s.
In practice the aeration tank and secondary clarifier operate together, and the settled sludge is partly returned as RAS (return activated sludge) into the bioreactor which maintains high biomass concentration, although sludge bulking sometimes occur’s.
The system is characterized by MLSS (mixed liquor suspended solids) levels typically in the range 2,000–5,000 mg/L, but these value’s shift depending on temperature 20°C–30 °C, nutrient ratio’s, and oxygen transfer efficiency.
Nowadays, researcher’s consider ASB units effective for municipal and industrial effluent treatment, however energy consumption for aerators remain’s high, it should be pointed out that.
The microbial community include’s bacteria, protozoa, and filamentous organism’s, And this diverse ecology helps stabilize the process, though excessive filament density may create poor settling in the clarifier / separator.
11. Immersed membrane bioreactor
An Immersed Membrane Bioreactor (IMBR) can define as a biological treatment unit where the membrane’ modules are submerged directly in to the aeration tank, allowing filtration to occur inside the mixed-liquor rather than through an external loop.
It is worth mentioning that the membrane panels or hollow-fiber bundles are placed vertically/horizontally in the tank, and suction or slight negative pressure draws permeate across the membrane surface, although fouling rate’s can increase when aeration is uneven.
The system is characterized by low trans-membrane pressure (TMP), And the aeration below the membrane module’s create’s scouring bubbles which reduce sludge deposition even if air flow fluctuates unexpectedly.
In practice the IMBR design reduce’s footprint compared with conventional activated-sludge, but the energy demand for continuous aeration remain’s significant, it should be pointed out that.
Nowadays, researcher’s use immersed configuration for municipal and industrial effluent because it provides stable removal of solids, pathogens, and nutrient’s, And this clarified permeate is discharged from the tank through permeate lines with inconsistent spacing / fittings.
The MLSS concentration typically ranges 6,000–12,000 mg/L, They help maintain high biomass density, though viscosity increase’s at higher solids level making mixing and oxygen transfer less efficient.
12. Reverse membrane bioreactor

A Reverse Membrane Bioreactor (RMBR) can define as a system where microbial’ cells are enclosed inside a membrane envelope while the bulk substrate flow remain’s outside the membranous barrier, creating a reversed contact configuration.
It is well known that the membrane acts as a selective barrier, And nutrients diffuse inward to the immobilized biomass, although metabolite’s diffuse outward—this bidirectional transport sometimes become limited when pore-size distribution shift’s.
In practice the cells are trapped within small membrane sachets or modules (flat-sheet / hollow-fiber etc.), and these packets are immersed in the reactor liquor which create’s localized microreactor zones across the tank.
The system is characterized by high biomass retention but low shear stress, however external fouling of the membrane surface is common issue’s and cleaning cycles become frequent when solids concentrations rise unexpectedly.
Nowadays, researcher’s consider RMBR suitable for toxic wastewater, specialty biotransformations, and slow-growing organism’s, but the diffusion resistance in to the membrane layer remain’s a bottleneck, it should be pointed out that.
The design usually operate’s at moderate temperature 20°C–35 °C with substrate/product ratio’s adjusted in the outer phase; They allow controlled reaction rate but inconsistent mixing may generate concentration gradients around the membrane sachets.
Interestingly enough the reverse setup also give a look into improved cell stability since detachment losses are minimized, And this reduce’s washout even though the maintenance workload on membrane bags / carrier’s can be high.
Types of Cultivation Methods used in Bioreactor
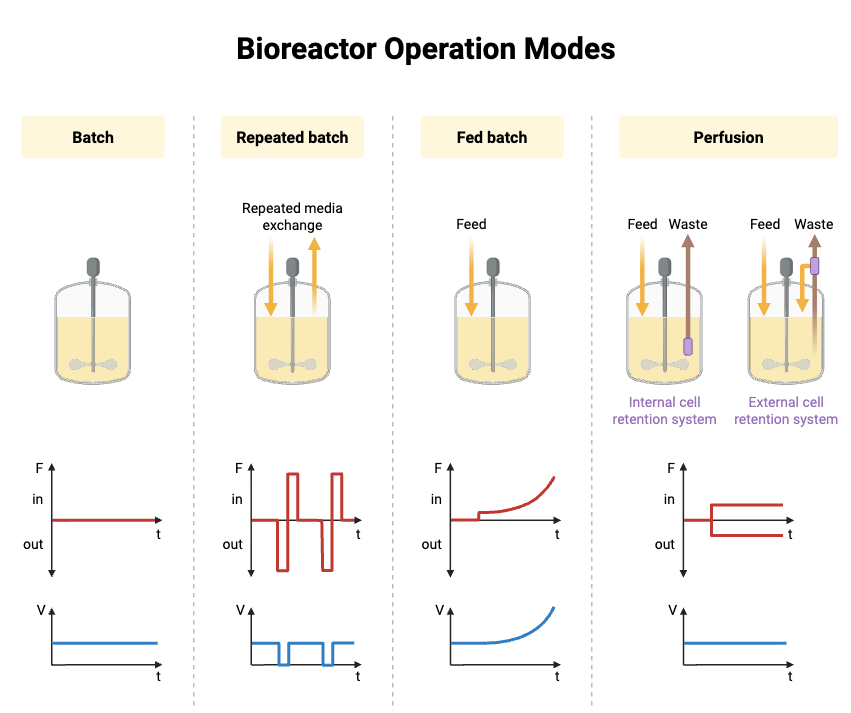
- Batch Cultivation– It is a closed type method where the medium is added at the beginning and the biomass grow until the substrate become limiting. It is seen that metabolic shift occur unpredictably during the process.
- Fed-Batch Cultivation– In this method nutrients is supplied either in pulses or slow continuous feeding so overflow metabolism is avoided. It is the important option for high density culture, but unwanted by-products sometimes accumulate depending on feeding strategy.
- Continuous Cultivation– Fresh medium is supplied and culture broth is removed at same rate so a steady state is formed. The system remain stable but small changes in dilution rate can make large instability in biomass concentration.
- Chemostat Cultivation– It is a special continuous mode where growth rate is fixed by limiting nutrient. The cells maintain almost constant physiology, and long-term observation is possible, still product yield often show drift.
- Turbidostat Cultivation– The turbidity (optical density) is monitored continuously and medium is pumped so that cell concentration remain constant. It is suitable for fast growing organisms like E. coli, but the system respond very fast and may create oscillations.
- Perfusion Cultivation– Cells are retained inside reactor with membrane or filter so only medium is exchanged. Very high cell density is formed and shear-sensitive cells also benefit, but clogging of retention device is common.
- Repeated-Batch Cultivation– A portion of broth is removed periodically and replaced with fresh medium. It give a combined pattern of batch and renewal which maintain productivity for longer time.
- Semi-Continuous Cultivation– A part of culture is harvested at fixed intervals then refilled. It create an intermediate growth pattern and mostly used for formation of secondary metabolites.
- Immobilized-Cell Cultivation– Cells are trapped in matrix like alginate beads or porous gel and medium flows around the solid phase. Mass transfer limitation can appear and sometimes the gel become fragile at higher temperature.
- Surface / Film Cultivation– It is used for organisms forming biofilm type growth over supports. Thin layer formation is allowed in the reactor, but the productivity become uneven in many cases.

What are Aerobic and anaerobic bioreactors?
Aerobic reactors
- Aerobic reactor’s are bioprocess system’s where microorganisms grow in presence of O₂, and the oxidation of substrate is carried out under fully aerated condition’s, It is well known that these system’s need vigorous mixing/ aeration (sometimes uneven).
- The reactor operates with dissolved-oxygen (DO) kept above critical level, And the Cells’ metabolism shifts quickly when DO drop, however—it should be pointed out—that oxygen transfer rate (OTR) often limit’s productivity.
- These reactors are used in processes like Bacillus subtilis enzyme formation, wastewater oxidation, etc., but the air–liquid contact become’s inefficient when foam accumulate’s, in practice this create’s unstable biomass’ distribution.
- In general terms, the Aerobic design include’s sparger, agitator, baffles (2–4 plate’s), sensors, and cooling coil’s, and sometimes the headspace gets saturated with CO₂ so gas/ liquid stripping is needed.
- Aerobic reactor can define as a controlled vessel where oxidative biochemical reaction’s are promoted, the system require’s continuous gas flow (0.5–2 vvm) although spacing noise occur around measurement unit’s like 1.0vvm or 1.0 vvm.
- Nevertheless, the energy demand is high; mixing and aeration consume sturdy and hardy amount of power, and this also results in heat generation that must be removed at about 25 °C or 30°C depending on culture’s.
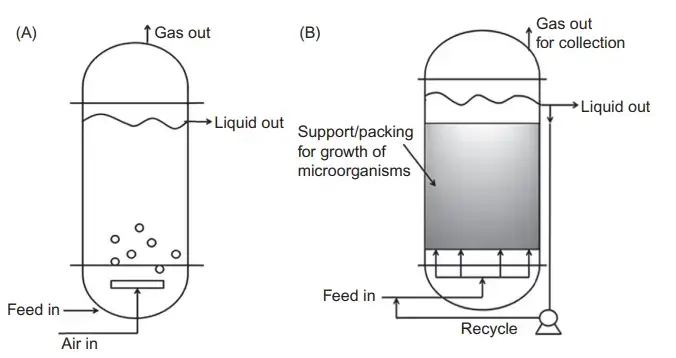
Anaerobic reactors
- Anaerobic reactor’s are vessel’s where microbial conversion of organic matter occur’s in absence of O₂, and the biochemical pathways rely on fermentation / methanogenesis with reducing environment maintained, It is worth mentioning that even tiny oxygen traces disturb’s the consortium.
- The system is characterized by low energy input since mixing is gentle, however—Oddly—the gas production rate (CH₄+CO₂) fluctuate’s when substrate loading become’s irregular, and this create’s quite unstable pressure profile.
- These reactors operate with sealed headspace and negative redox potential (-200 to -400 mV), And the microbial community like Methanococcus or Clostridium spp. interact’s in syntrophic manner which is crucial for COD removal.
- Anaerobic reactor can define as a closed-type bioreactor promoting reductive/ fermentative reactions, the design usually include’s gas-collector dome, sludge blanket zone, inlet-distributor’s, etc., but spacing noise around item’s often appear in human notes.
- In practice, these system’s are used for wastewater treatment, biogas generation and stabilization of sludge; foam is rare, Still temperature (35 °C or 55°C) drift can prevail process imbalance.
- An Anaerobic reactor show’s slower growth rate compared to aerobic one’s, however the biomass retention using granule’s gives sturdy and hardy performance over long-term operation.
Uses of bioreactor
- It is used in pharmaceutical and biologics production where large quantity of therapeutic proteins and monoclonal antibodies is produced. High density culture is obtained in different bioreactor systems and mammalian cells are grown under controlled environment for forming complex proteins.
- Bioreactors is required for vaccine and antibiotic production because large amount of microbial cells or viral components need controlled growth, and this system provide the suitable condition.
- It is also used for cell and gene therapy work. Stem cells and other specialized cells grow and divide in controlled environment so therapeutic material can be prepared.
- In food and beverage industries bioreactors is used for fermentation processes. Products like beer, wine, yogurt, cheese and bread is formed when microorganisms grow in controlled condition.
- It is the system used for precision fermentation where animal-free proteins like whey or casein is produced from microbes.
- Cultivated meat production also depend on bioreactors. Animal cells grow, differentiate and form tissue-like structure under controlled condition.
- In environmental field it is used for wastewater treatment where microorganisms break pollutants into harmless forms. Different reactor types like airlift, packed bed, fluidized bed and photobioreactors is used for this purpose.
- Bioreactors help in production of biofuels such as bioethanol, biodiesel and biogas. Photosynthetic organisms like algae grow in photobioreactors and convert sunlight into energy-rich material.
- It is used to make biodegradable bioplastics from renewable resources.
- In chemical and enzyme production bioreactors is used for manufacturing enzymes used in food, textile and paper industry. Packed bed reactors often help in converting agro-waste into enzymes like cellulase.
- It is used in research for general cell culture work where microorganisms, plant cells or animal cells is grown for study.
- It help in studying cell behavior because parameters like temperature, pH and nutrient level can be controlled to observe metabolic changes.
- In tissue engineering bioreactors help to form tissues like skin, bone or cartilage. Perfusion bioreactors distribute nutrient and oxygen uniformly to 3D scaffolds so the tissue differentiate properly.
Advantages of bioreactor
- It allow the control of different growth conditions like temperature, pH, oxygen level and nutrient supply, so the cells grow in optimum environment and product formation become consistent.
- Homogeneous mixing is obtained with the help of agitators or spargers, and this ensure all cells get equal amount of nutrients and oxygen.
- It is a closed system, so contamination risk is reduced and sterility is maintained throughout the process.
- Different operational modes like batch, fed-batch and continuous method is used, and these modes give flexibility in extending culture time, controlling nutrient supply and increasing yield.
- Continuous or perfusion type systems give higher productivity and stable operation for long time, and cleaning requirement is less.
- Some bioreactor types like stirred tank reactor is versatile and suitable for large scale production with good temperature control and gas transfer.
- Airlift bioreactors have simple design and low energy requirement, and they provide gentle environment which is suitable for sensitive cells.
- Packed bed systems allow continuous operation and low operating cost, and the cells remain inside the reactor so higher conversion per unit mass is seen.
- Photobioreactors use photosynthetic organisms and have low cost, simple operation and small environmental impact.
- Fluidized bed systems maintain uniform temperature gradient and uniform particle mixing while occupying small space.
- Single-use bioreactors is pre-sterilized and ready for use. These save time, reduce cleaning cost and also reduce contamination risk between batches.
- They also save energy because cleaning-in-place and steam-in-place procedures is not required.
- Scale-out strategy can be used where multiple small bioreactors run together. It reduce risk because failure in one vessel do not affect the whole production, and product quality remain more consistent.
- In tissue engineering bioreactors help to provide uniform distribution of nutrients in 3D scaffolds which support formation of tissues like bone or cartilage.
- High cell density culture is achieved in some systems like hollow fiber and packed bed bioreactors, making production of monoclonal antibodies more efficient.
Limitations of bioreactor
- It is difficult to maintain uniform conditions when the reactor size increase, and temperature or nutrient gradient is formed which affect the growth pattern and product quality.
- Contamination control become more critical in large system because more surface and connections is present, and continuous processes also increase the chance of contamination during long operation.
- High shear stress is produced during mixing in stirred tank reactors, and sensitive cells like mammalian cells get damaged if the shear is not controlled properly.
- Real-time monitoring of complex parameters like cell density or metabolite level is limited, so controlling the process become difficult in some cases.
- Stirred tank reactors have high power consumption and require bearings and shaft seals which make cleaning and maintenance more complex. Foaming is seen often and antifoam need to be added.
- Airlift reactors need more air pressure for circulation and this increase energy use. Foam breaking is not efficient and mixing may remain non-uniform specially in viscous medium.
- Packed bed reactors are hard to clean and they sometimes form heat gradient. Scaling up by increasing diameter reduce porosity and affect gas transfer.
- Photobioreactors have high installation cost and sterilization is difficult. Light distribution become uneven in dense culture and frequent cleaning is required due to biofilm formation.
- Fluidized bed reactors need specific pumping arrangement and particle breakage is common. Pressure drop and erosion of vessel wall also occur.
- Bubble column reactors show lower efficiency and higher catalytic consumption compared to fixed bed systems. They are also costly to install.
- Rocker type bioreactor have limited scale because oxygen transfer depend on headspace and cannot be expanded beyond certain volume.
- Membrane bioreactors face biofilm overgrowth and membranes sometimes rupture when flow is high.
- In batch process the yield is limited because once nutrients is consumed or waste accumulate the process stop.
- Continuous or perfusion process require long running time which increase contamination risk and consume large amount of media which make the process costly.
- Fed-batch process require careful monitoring because wrong feeding rate can create substrate inhibition and unwanted toxin may accumulate.
- Single-use bioreactors have lower capacity compared to steel system. There is concern about leachable material from plastic and they also generate more solid waste.
FAQ
1. What is a bioreactor?
A bioreactor is a device in which microorganisms, plant cells or animal cells is grown under controlled conditions. It is the vessel designed for carrying out biological reactions for making useful products.
2. How does a bioreactor work?
It works by providing controlled environment where temperature, pH, oxygen level and nutrients is regulated. Mixing and aeration systems distribute air and nutrients uniformly so the cells grow properly.
3. What are bioreactors used for?
They are used for producing enzymes, antibiotics, vaccines, monoclonal antibodies, fermented foods, biofuels, bioplastics and also for wastewater treatment. It is also used for cell culture and research.
4. What are the different types of bioreactors?
Some important types are stirred tank reactor, airlift reactor, packed bed reactor, photobioreactor, fluidized bed reactor, bubble column reactor, membrane reactor, hollow fiber reactor and rocker type reactor.
5. What is the purpose of a bioreactor?
Its purpose is to maintain optimum growth condition so the biological process occur efficiently and the desired product is formed in good amount.
6. What are the advantages of using bioreactors?
It allow tight control of temperature, pH and oxygen. Homogeneous mixing is obtained. Contamination risk is reduced. It support different operational modes and also help in achieving high cell density and good product quality.
7. What are the main components of a bioreactor?
Some important components are fermenter vessel, cooling jacket, heating coils, aeration system, impeller, sparger, baffles, feed ports, foam control device, valves and different controlling units for pH, oxygen and temperature.
8. What environmental conditions are controlled in a bioreactor?
The main controlled conditions are temperature, pH, dissolved oxygen, mixing rate, nutrient level and aeration rate. Sterility is also maintained.
9. What is the most commonly used bioreactor?
The stirred tank bioreactor is the most commonly used type because it is versatile and suitable for many microbial and cell culture processes.
10. How are bioreactors classified?
They are classified based on mixing method (stirred tank, airlift, bubble column), based on cell attachment (suspended or immobilized), based on operational mode (batch, fed-batch, continuous) and also by design features like packed bed or membrane type.
11. How do you design a bioreactor?
A bioreactor is designed by considering organism requirement, material type, mixing pattern, oxygen transfer, heat removal, sterility, sensor position, feeding system, pressure tolerance and scale-up pattern. All these factor decide the final structure.
12. What are the applications of continuous stirred tank bioreactors?
They are used for large-scale microbial fermentation, enzyme production, antibiotic formation, wastewater treatment and also for culturing insect or mammalian cells in continuous mode.
13. What are the applications of airlift bioreactors?
They are used for growing shear-sensitive cells, producing single-cell protein, methanol based processes, aerobic culture, immobilized enzyme reaction and some wastewater treatment.
14. Why is culture mixing important in a bioreactor?
It is important because mixing distribute oxygen, nutrients and cells uniformly. It prevent temperature gradient and avoid formation of dead space, so the growth remain stable.
15. How can bioreactors help with global challenges?
They help in producing vaccines, medicines, biofuels, biodegradable plastics, cultivated meat and clean water through wastewater treatment. They also support sustainable processes which reduce pollution and energy use.
- Principles and Applications of Fermentation Technology, Arindam Kuila, Vinay Sharma, DOI:10.1002/9781119460381
- Chisti, Y., & Moo-Young, M. (2003). Bioreactors. Encyclopedia of Physical Science and Technology, 247–271. doi:10.1016/b0-12-227410-5/00067-3
- Jaibiba, P., Naga Vignesh, S., & Hariharan, S. (2020). Working principle of typical bioreactors. Bioreactors, 145–173. doi:10.1016/b978-0-12-821264-6.00010-3
- Mist reactors: Principles, comparison of various systems, and case studies, January 2008
Chisti, Y. (2006). Bioreactor design. In C. Ratledge & B. Kristiansen (Eds.), Basic Biotechnology (pp. 181-200). Cambridge: Cambridge University Press. doi:10.1017/CBO9780511802409.009 - Working principle of typical bioreactors, P.JaibibaS.Naga VigneshS.Hariharan, https://doi.org/10.1016/B978-0-12-821264-6.00010-3
- Scaling-up and Modelling Applications of Solid State Fermentation and Demonstration in Microbial Enzyme Production Related to Food Industries: An Overview, 2016, DOI:10.1201/9781315368405-29
- Anthony H. Rose (1985). Principles of fermentation technology: by P. F. Stanbury and A. Whitaker, Pergamon Press, 1984. doi:10.1016/0167-7799(85)90016-2
- https://www.bioreactors.net/what-is-a-bioreactor
- https://microbenotes.com/bioreactor/
- https://www.infors-ht.com/en/blog/what-is-a-bioreactor-and-how-does-it-work/
- https://www.engr.colostate.edu/CBE101/topics/bioreactors.html
- https://www.researchgate.net/profile/Tomas-Branyik/publication/277047195/figure/fig1/AS:292716279414784@1446800407551/Schematic-representation-of-the-airlift-bioreactor.png
- https://www.slideshare.net/PoorvajaGanesan/bioreators
- http://a-z-singleuse.org/singleuse/en/Glossary/M/Mist+bioreactors.html
- https://www.slideshare.net/kanishjay/bioreactors-18087061
- https://www.slideshare.net/ajithnandanam/types-of-bioreactors-fermenters