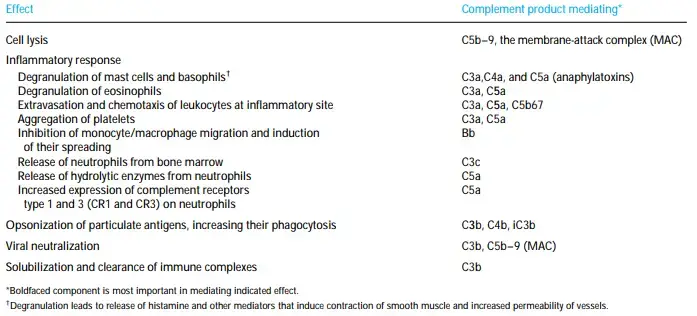
- Through its role as a mediator, complement helps the humoral response become a potent defence mechanism that can eliminate pathogens.
- Other complement components or split products help with the inflammatory response, opsonization of antigen, virus neutralisation, and clearance of immune complexes in addition to the MAC’s role in mediating cell lysis.
- The binding of complement fragments to complement receptors, which are expressed on a wide variety of cells, is essential for many of the complement system’s biological actions.
- Additionally, some complement receptors regulate complement activity by binding biologically active complement components and reducing them into inactive products.
The Membrane-Attack Complex Can Lyse a Broad Spectrum of Cells
- Lysis of gram-negative bacteria, parasites, viruses, erythrocytes, and nucleated cells is possible because to the membrane-attack complex generated by complement activation.
- Important innate immune defences against pathogenic microbes are the alternative and lectin activation pathways, which typically occur without an initial antigen-antibody interaction.
- An additional, more targeted line of defence is provided by the classical pathway’s insistence on an initial antigen-antibody response.
- Antibodies are often a necessary part of the activation event, and so-called natural antibodies, which are produced in the body after being exposed to the ubiquitous components of common microorganisms, may be able to fulfil this need.
- Cell-mediated immunity has been emphasised as a crucial part of the host’s defence against viral infections in earlier chapters.
- Still, antibodies and complement are an important part of the host’s defence against viruses, particularly in limiting viral transmission during an acute infection and preventing reinfection.
- All or almost all enveloped viruses can be killed by a combination of antibodies and the body’s complement system. Pore creation by the membraneattack complex is possible because the viral envelope is predominantly generated from the plasma membrane of infected host cells.
- Herpesviruses, orthomyxoviruses, paramyxoviruses, and retroviruses are all pathogenic viruses that can be killed by the body’s immune system using complement-mediated lysis.
- In most cases, the complement system will efficiently lyse gram-negative bacteria. However, both gram-negative and gram-positive bacteria can avoid being killed by complement by using different strategies.
- Some gram-negative bacteria, for instance, have evolved a resistance to complement-mediated lysis that is directly correlated with their virulence.
- The smooth bacterial phenotype is connected with complement resistance in Escherichia coli and Salmonella. This phenotype is defined by the presence of long polysaccharide side chains in the cell-wall lipopolysaccharide (LPS) component of these bacteria.
- Some researchers believe that the higher levels of LPS in the resistant strains’ cell walls prevent the MAC from inserting into the membrane and, as a result, the complex is expelled from the bacterial cell rather than forming a pore.
- Humans infected with a strain of Neisseria gonorrhoea resistant to complement-mediated death have been diagnosed with a case of disseminated gonococcal infection. There is some proof that membrane proteins of resistant Neisseria strains interact with the MAC in a noncovalent fashion, preventing the MAC from inserting into the bacterial cell’s outer membrane.
- Some gram-negative bacteria are resistant to complement, although they are the exception.
- mediate lysis The strong peptidoglycan layer in the cell wall of Gram-positive bacteria limits insertion of the MAC into the inner membrane, making these bacteria resistant to complement-mediated lysis.
- Even while complement activation can take place on the cell membrane of encapsulated bacteria like Streptococcus pneumoniae, the capsule precludes interaction between C3b deposited on the membrane and the CR1 on phagocytic cells.
- Elastase is a protein found in some bacteria that deactivates C3a and C5a, rendering the shattered proteins incapable of triggering an inflammatory response.
- Besides these defence systems, many pathogens, such as bacteria, viruses, fungi, and protozoa, have surface proteins that can block the complement cascade, thereby imitating the actions of the normally occurring complement regulation proteins C4bBP, CR1, and DAF.
- It takes the production of several membrane attack complexes (MACs) to lyse nucleated cells, while just one MAC is enough to lyse a red blood cell. The MAC can be endocytosed by a wide variety of nucleated cells, including the vast majority of cancer cells.
- The cell can fix any membrane damage and regain osmotic balance if the complex is eliminated quickly after it occurs.
- Endocytosis of the MAC has the unintended result of perhaps neutralising a promising weapon against cancer: complement-mediated lysis by antibodies specific for tumor-cell antigens.
Cleavage Products of Complement Components Mediate Inflammation
- Although the final result of the complement cascade, cell lysis, receives most of the attention, many peptides formed during MAC formation play a crucial part in the establishment of an efficient inflammatory response.
- The cleaved complement fragments C3a, C4a, and C5a are known as anaphylatoxins and they trigger degranulation and the release of histamine and other pharmacologically active mediators by binding to receptors on mast cells and blood basophils.
- Smooth muscle contraction and enhanced vascular permeability are two additional effects of anaphylatoxins. Consequently, complement activation causes fluid influxes that transport antibody and phagocytic cells to the site of antigen entry.
- The serum protease carboxypeptidase N cleaves an Arg residue from the C terminus of these highly reactive anaphylatoxins, generating des-Arg versions, thereby modulating their activity.
- There is no measurable chemotactic activity or induction of smooth muscle contraction in the des-Arg forms of C3a and C4a, but the des-Arg form of C5a retains roughly 10% of both of these properties.
- Each of C3a, C5a, and C5b67 has the ability to stimulate monocytes and neutrophils to attach to vascular endothelial cells, extravasate through the endothelial lining of the capillary, and migrate toward the site of complement activation in the tissues.
- C5a is the most powerful cytokine in mediating these activities, and it is efficacious in picomolar concentrations.
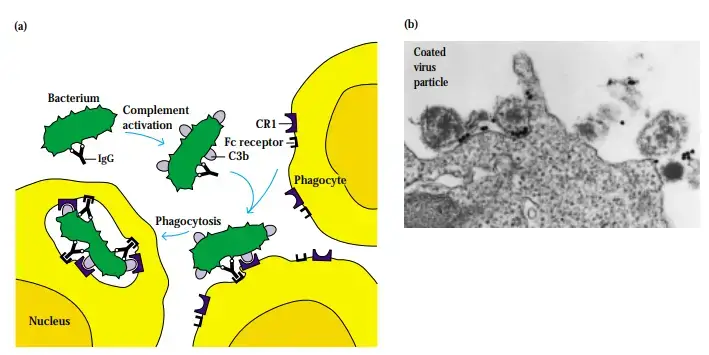
C3b and C4b Binding Facilitates Opsonization
- Although C4b and iC3b also exhibit opsonizing action, C3b is the primary opsonin of the complement system. C3 activation amplifies immunological responses by coating immune complexes and particle-based antigens with C3b.
- To bind C3b, C4b, or iC3b, phagocytic cells and other cells express complement receptors (CR1, CR3, and CR4). C3b-coated antigen is recognised by CR1-expressing cells.
- To the extent that the cell is a phagocyte (such as a neutrophil, monocyte, or macrophage), phagocytosis will be facilitated.
- It has been found that the number of CR1s on activated phagocytes increases from 5000 on resting phagocytes to 50,000 on activated cells, substantially enhancing their phagocytosis of C3b-coated antigen. This increase occurs upon activation of phagocytes by various agents, including C5a anaphylatoxin.
- Recent research indicates that C3b complement fragment, when combined with protein antigens, functions as an adjuvant.
- C3b enhances the commencement of antigen processing and speeds up the generation of particular antibodies by directing the antigen directly to the phagocyte.
The Complement System Also Neutralizes Viral Infectivity
- Serum antibodies attach to the structural proteins of viruses, forming particulate immune complexes that are activated by the conventional complement pathway.
- In the absence of an antibody, several viruses (including retroviruses, Epstein-Barr virus, Newcastle disease virus, and rubella virus) can trigger the alternative, lectin, or classical route.
- Multiple pathways in the complement system mediate viral neutralisation. Due to the reduction in the total number of infectious viral particles caused by the creation of bigger viral aggregates, a degree of neutralisation is accomplished.
- In vitro studies reveal that the C3b component increases aggregate formation with as few as two molecules of antibody per virion, suggesting that antibody is not necessary for the development of viral aggregates.
- For instance, adding activated C3 serum to polyoma virus that has been coated with antibodies renders the virus harmless.
- Proteins can be seen as a thick coating on the surface of a virus particle when an antibody and/or complement bind to it. By preventing viral attachment to susceptible host cells, this coating effectively eliminates viral infection.
- Antibody and complement deposits on virus particles aid in the virus’s ability to connect to cells with Fc or type 1 complement receptors (CR1).
- Binding can be followed by phagocytosis and intracellular destruction of the viral particle in the case of phagocytic cells.
- Finally, complement is able to lyse the majority of enveloped viruses by causing the envelope to split and the nucleocapsid to disintegrate.
The Complement System Clears Immune Complexes from Circulation
- Patients with the autoimmune disease systemic lupus erythematosus provide compelling evidence for the relevance of the complement system in eliminating immunological complexes (SLE).
- Because to complement-mediated lysis and the activation of type II or type III hypersensitivity, these people generate massive amounts of immune complexes and experience tissue damage.
- Paradoxically, defects in C1, C2, C4, and CR1 predispose an individual to SLE; in fact, 90% of individuals who are completely lacking C4 acquire SLE. Complement plays a crucial role in the development of tissue damage in SLE.
- It is believed that the insufficiency of complement prevents immune complexes from being effectively dissolved and cleared, which then causes more tissue damage by the immune system.
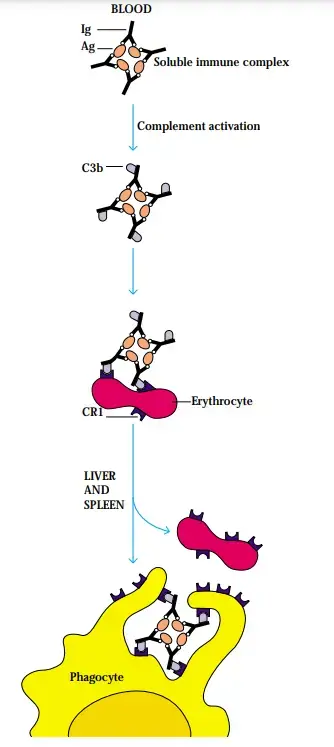
- In order to better bind to CR1 on erythrocytes, soluble immune complexes are considered to be coated with C3b.
- There are around 103 erythrocytes for every white blood cell, hence erythrocytes account for about 90% of the circulating CR1. However, red blood cells express lower quantities of CR1 (5 102 per cell) than granulocytes (5 104 per cell).
- This is why erythrocytes are pivotal in transporting immune complexes coated with C3b to the liver and spleen for processing.
- To prevent immune complexes from being deposited in tissues, these organs phagocytose RBCs and remove the immune complexes.
- Patients with SLE often have low amounts of C3b on immune complexes because of deficits in C1, C2, and C4.
- Patients with SLE may have impaired binding and clearance of immunological complexes due to reduced levels of CR1 expressed on erythrocytes.
Complement Deficiencies
C1q, C1r, C1s, C4, and C2 Deficiencies
- Each component of the complement system has been linked to genetic deficits. The same symptoms, including an increased risk of developing immune-complex illnesses including systemic lupus erythematosus, glomerulonephritis, and vasculitis, are seen in people with homozygous defects in any of the early components of the classical route (C1q, C1r, C1s, C4, and C2).
- These limitations underscore the crucial role of C3b in solubilization and clearance of immune complexes, as well as the necessity of the early complement interactions in producing C3b.
- People with complement deficits are more likely to get recurring infections from pyogenic (pus-forming) bacteria such streptococci and staphylococci, in addition to developing immune-complex illnesses.
- Because they are gram-positive, these organisms are immune to the lysis effects of the MAC. By initiating a limited inflammatory response and opsonizing the bacteria, however, the early complement components typically prevent recurrent infection.
- Lack of factor D and properdin, two early components of the alternative route, appears to be linked to Neisseria infections but not immune-complex illness.
C3 deficiencies
- Due to its essential function in activating C5 and forming the MAC, those deficient in C3 tend to have the most severe clinical symptoms.
- A young child who suffered from recurrent, life-threatening bacterial infections was the first person to be diagnosed with a C3 deficiency.
- Normal levels of immunoglobulin were found, however a deficit in C3 was found. The importance of the complement system in turning a humoral antibody response into a functional defence mechanism is demonstrated here.
- People with C3 deficiency tend to suffer from immunecomplex illnesses and chronic bacterial infections.
Homozygous deficiencies
- Persistent Neisseria meningococcal and gonococcal infections are a result of homozygous defects in components involved in the MAC.
- These gram-negative bacteria are typically eliminated in healthy people due to their susceptibility to complement-mediated lysis or the opsonizing activity of C3b.
- Immune-complex illness is uncommon in MAC-deficient people, suggesting that they make enough C3b to eliminate immune complexes.
- Fascinatingly, a lack of C9 does not cause any outward symptoms, showing that the full MAC is not always required for complement-mediated lysis.
Congenital deficiencies
- Malfunctions in complement regulating proteins have also been documented at birth. By blocking C1 from activating C4 and C2 to high levels, C1 inhibitor (C1Inh) controls classical pathway activity.
- The absence of C1Inh is an autosomal dominant disorder that affects one in every thousand people. Hereditary angioedema results from the deficiency, and it presents clinically as localised edoema of the tissue, most commonly in response to trauma but also in the absence of any obvious trigger.
- Subcutaneous tissues, the bowel, causing abdominal pain, and the upper respiratory tract, causing airway obstruction, are all potential locations for the edoema that produces swelling.
The majority of what is known about the function of individual complement components in immunity comes from studies of humans and experimental animals with homozygous deficits in complement components. Studies utilising knockout mice, in which specific complement components are absent through genetic engineering, have substantially expanded these results. Researchers have been able to deconstruct the complex system of complement proteins and assign exact biological roles to each thanks to in vivo complement activity studies in these animals.
Regulation of the Complement System
- Complex regulatory systems have developed to direct complement activity only toward its intended targets, as many complement system components can also damage host cells in addition to invading cells and microbes.
- Highly labile components that undergo spontaneous inactivation if they are not stabilised by reaction with other components are a common method of control in all complement pathways.
- Additionally, many complement components can be disabled by a cascade of regulatory proteins. For instance, C1r2s2 can be deactivated from C1q and prevented from activating C4 or C2 by forming a complex with the glycoprotein C1 inhibitor (C1Inh).
- Hundreds of C3b molecules are produced by the reaction catalysed by C3 convertase enzymes of the classical, lectin, and alternative routes, making this the key amplification step in complement activation.
- The C3b produced by these enzymes can bind to adjacent cells, facilitating damage to the healthy cells by inducing opsonization by phagocytic cells expressing C3b receptors or the membraneattack complex.
- By the time C3b has diffused 40 nm away from the C4b2a or C3bBb convertase enzymes, it has undergone spontaneous hydrolysis and can no longer attach to its target site, preventing damage to normal host cells.
- C3 convertase activity in both the traditional and alternative pathways is regulated by a family of related proteins, further limiting the potential damage to healthy host cells that C3b could do.
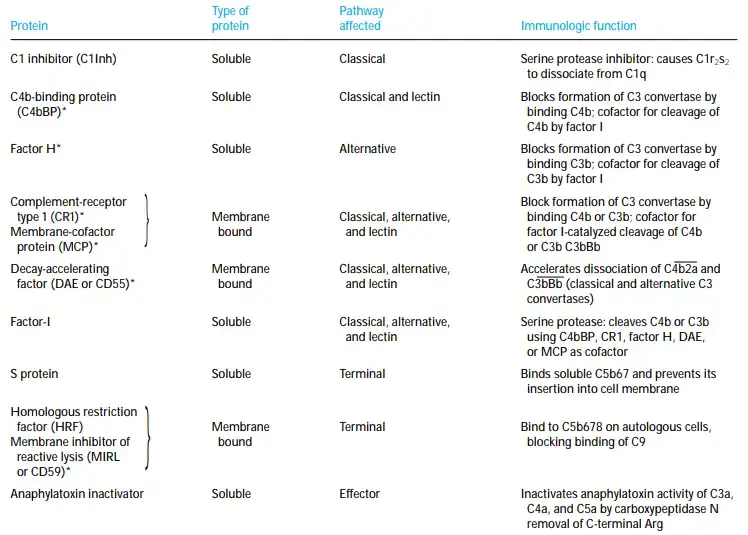
- Short consensus repeats, consisting of roughly 60 amino acid residues, are found repeatedly in all of these regulatory proteins (SCRs). These proteins are encoded by a single gene cluster on human chromosome 1 called RCA (regulators of complement activation).
- Three physically diverse RCA proteins operate similarly to impede C3 convertase assembly in the classical and lectin routes.
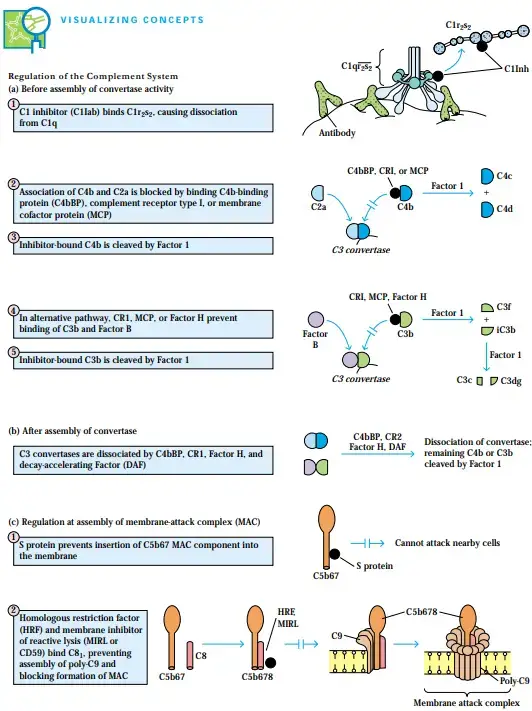
- Soluble C4b-binding protein (C4bBP) and the membrane-bound proteins complement receptor type 1 (CR1) and membrane cofactor protein are among these regulating proteins (MCP).
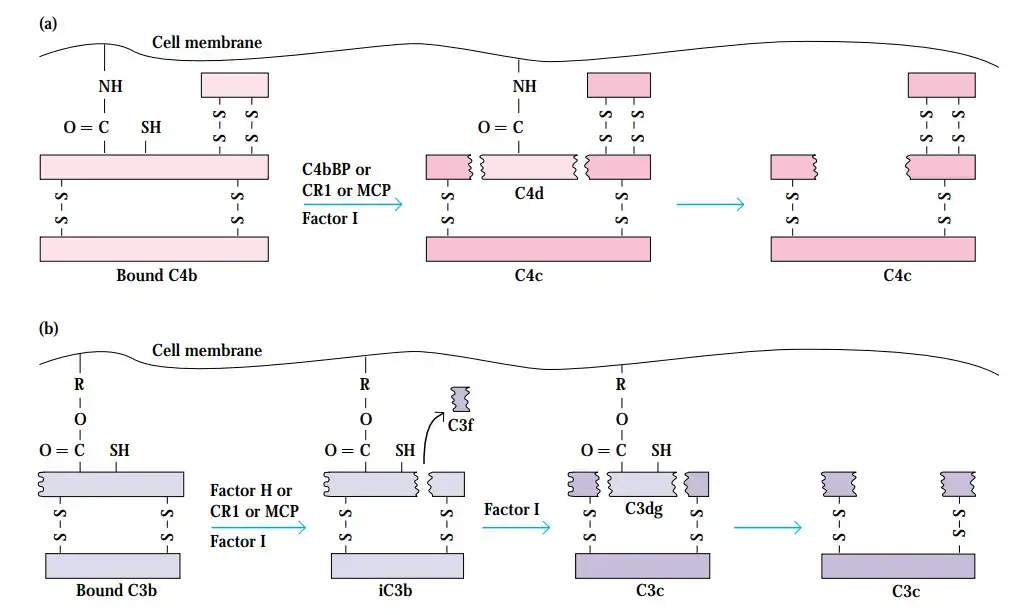
- C4b is prevented from binding to C2a by each of these regulatory proteins. Once a binding protein like C4bBP, CR1, or MCP binds to C4b, another regulatory protein called factor I cleaves the C4b into bound C4d and soluble C4c.
- The assembly of the C3 convertase C3bBb is inhibited by a comparable regulatory region in the alternative pathway. C3b is prevented from binding factor B by the presence of CR1, MCP, or a regulatory component known as factor H.
- As soon as CR1, MCP, or factor H binds to C3b, factor I cleaves C3b into a bound iC3b fragment and a soluble C3f fragment. C3dg remains attached to the membrane after being released from iC3b by factor I cleavage.
- Not only do C4bBP, CR1, and factor H, but also several other RCA proteins, act on the assembled C3 convertase to cause its dissociation. C3 convertase can also be detached by the glycoprotein decayaccelerating factor (DAF or CD55), which is itself covalently attached to a glycophospholipid membrane protein.
- In the Clinical Focus, we discuss the repercussions of a lack of DAF. The enzymatically active subunit (C2a or Bb) of C3 convertase is released from the cell-bound subunit at a faster pace thanks to each of these RCA proteins (C4b or C3b).
- Factor I cleaves the remaining membrane-bound C4b or C3b component after C3 convertase dissociation, rendering the convertase permanently inactive.
- Additionally, regulatory proteins function at the membrane-attack complex. If the C5b67 complex is released, it could cause collateral damage to healthy cells.
- Serum proteins fight back against this danger by attaching to released C5b67 and blocking its entry into the membrane of adjacent cells. Serum protein S binds to C5b67, causing it to undergo a hydrophilic shift and blocking its entry into the membranes of neighbouring cells.
- If the complement is not from the same species as the cells being lysed, the lysis will be more efficient. Two membrane proteins that prevent MAC formation are required for this occurrence.
- Homologous restriction factor (HRF) and membrane inhibitor of reactive lysis (MIRL) are two membrane proteins found in a wide variety of cells (MIRL or CD59).
- Both HRF and MIRL bind to C8, which stops poly-C9 from assembling and membrane insertion, therefore protecting cells from nonspecific complement-mediated lysis.
- But only if the complement components are of the same species as the target cells does this inhibition take place.
- For this reason, the term “homologous restriction” (HRF) is used to describe the relationship between MIRL and HRF.
- Text Highlighting: Select any text in the post content to highlight it
- Text Annotation: Select text and add comments with annotations
- Comment Management: Edit or delete your own comments
- Highlight Management: Remove your own highlights
How to use: Simply select any text in the post content above, and you'll see annotation options. Login here or create an account to get started.