Algal morphology is defined as the study of body structure and organization of algal cells and their thalli.
Algae are observed in a diversity of sizes and shapes, from microscopic single-celled forms to large seaweeds several meters long.
The science concerned with algae is termed Phycology, and a specialist is called an Algologist or Phycologist.
The body of an alga is referred to as thallus, since true roots, stems, leaves are not present.
In unicellular algae the thallus consists of a single cell only; internal compartments (nucleus, plastids, vacuoles) may be developed.
When a cell divides and daughter cells remain together embedded in mucilage, colonial thalli are produced.
If repeated cell divisions occur in a single plane and daughter cells remain attached in a row, a filamentous thallus is formed.
In some filamentous algae branching is developed: cells divide by vertical planes in addition to transverse ones, yielding branched filaments.
When cell divisions occur in both cross (transverse) and vertical planes, a sheet-like multicellular thallus may be produced (one or more cells thick).
In higher algae (seaweeds) further morphological differentiation may occur over that basic multicellular form.
Morphological categories of algae are recognized: unicellular, colonial, filamentous, heterotrichous, thalloid, and polysiphonoid forms.
In many algal groups transitions between these morphological forms are possible during life cycle or development.
Convergent morphological traits are often seen: unrelated algae may evolve similar thallus forms because of ecological / environmental constraints.
In multicellular algae the thallus organization (sheet, filament, parenchymatous) is crucial in classification and establishing relationships.
In marine lineages especially (reds, browns, some chlorophytes) three-dimensional multicellular thalli are possible.
Mechanical strength, nutrient transport, light capture, and exposure to water movement are factors influencing morphological design of thalli.
In summary, algal morphology describes how algae are built structurally, how their forms vary, and how those variations relate to physiological / ecological function.
Size of Algal Cell
- The size of algal cells is extremely variable, and great differences are encountered among taxa.
- In many unicellular microalgae, diameters of 1.5 to 10 µm have been recorded (for example in Chlorella)
- In a study of Auxenochlorella cells, the majority of cells ranged from about 3 to 5 µm, whereas larger cells in that culture reached 6 to 10 µm
- In marine microalgae in a fluorescence-based cell counter measurement, it was found that “all algae were smaller than 10 µm in size”, with many sea species being ~2 µm diameter.
- Among diatoms, extreme small sizes exist: e.g. Mediolabrus (Minidiscus) comicus has diameters of ~1.9 µm to ~6.0 µm
- In colonial or multicellular/colonial algae, the individual cell size may be similar to microalgae, but the overall colony or thallus dimension becomes much larger (tens to hundreds of µm or more).
- With respect to very large algal cells, exceptions exist: certain characean algae (though not strictly microalgae) can produce cells that exceed 100 µm or more, sometimes even centimeters in length (for example in Chara).
- The cell size is constrained by factors such as diffusion rates, surface-to-volume ratio, metabolic demands, and nutrient transport limitations.
- The scaling relationships between cell size and metabolic rate or growth are studied in green algae, showing that increases in size may impose diffusion constraints, which may be overcome by morphological adaptation (flattening, fractality).
- The variation of size is also influenced by environmental factors (light, nutrients, salinity) such that in stressful or limiting environments smaller cells are favored.
- It is concluded that algal cell size is not uniform, rather a continuous spectrum from ~1–2 µm to tens or even hundreds of µm (in certain large cells) is exhibited, with functional and ecological implications.30
Shape of Algal Cell
- The shape of algal cells is highly variable, and many morphologies are observed among different taxa.
- In many unicellular green algae (e.g. Chlorella), cells are spherical, subspherical or ellipsoidal (rounded / slightly elongated).
- In motile algae such as Dunaliella, cells are ellipsoid / ovoid / cylindrical, sometimes tapering at one end.
- In diatoms, shapes like linear, oblong, spindle, rectangular or cylindrical are common; many species show bilateral symmetry or elongation.
- In filamentous algae (e.g. Oedogonium), individual cells are narrow, cylindrical and arranged end-to-end in unbranched threads.
- In colonial algae, cells are embedded in mucilage and may be spherical, clustered, or dispersed, giving the colony an overall shape (e.g. Sphaerocystis).
- In genera with distinctive projections, cells may show horns / conical projections (e.g. Brachiomonas) with the main cell body having a somewhat rounded form
- From a general perspective, the common microalgal cell shapes include spherical / circular, ellipsoidal / oval, triangular / pyramidal, helical / spiral, sigmoidal / curved, rectangular / box-like.
- With respect to functional constraints, shapes are influenced by surface-to-volume ratio, nutrient uptake, light capture, buoyancy, and hydrodynamic forces
- Because of evolutionary adaptation, similar shapes sometimes evolve independently (convergence), so unrelated algal groups may share morphologies.
- It should be recognized that shape is not a fixed trait; life cycle stage, nutritional state, environmental stress may induce shape changes
- Overall, the shape of algal cell is not uniform, rather a spectrum of morphological forms is exhibited, which is adaptive to ecological / physiological demands29
Morphology of Algae
Morphologically algae can be differentiated as unicellular, colonial, filamentous, heterotrichous, thalloid and polysiphonoid forms.
1. Unicellular Algae
Unicellular Algae are algae whose thallus is composed of a single cell only, even though that cell may show internal compartmentalization (e.g. nucleus, plastids, vacuoles).
In many cases motility is exhibited by them via flagella or similar structures, though non-motile forms also exist.
A wide taxonomic diversity is represented among unicellular algae: green (Chlorophyta), diatoms (Bacillariophyceae), dinoflagellates, chrysophytes, euglenophytes, among others are included.
Characteristics
- A thin cell wall is frequently present, composed of cellulose, silica, or other polysaccharides depending on group, which provides structure and protection.
- A single (or sometimes multiple) chloroplast(s) is (are) contained in the cell, bearing pigments (chlorophylls, carotenoids etc.) adapted to light conditions.
- Storage products (e.g. starch, oils) are deposited in cytoplasm or specialized structures (pyrenoid in many green algae).
- Contractile vacuoles may be developed in freshwater species to regulate osmotic pressure.
- In motile species a stigma or eyespot may be present, enabling phototactic responses.
- Rapid division (binary, multiple fission) is often used as mode of reproduction under favorable conditions.
Types / Forms
- Motile unicellular algae — those which bear one or more flagella (for example, Chlamydomonas).
- Non-motile unicellular algae — lacking locomotive organelles, e.g. many diatoms or Chlorella.
- Some unicellular algae may show pseudopodia, gliding, or mucilage secretion for movement or anchorage under certain conditions.
Classification / Divisions
- In traditional schemes unicellular algae are placed in divisions like Chlorophyta, Euglenophyta, Chrysophyta, Pyrrophyta, etc.
- In modern classification molecular criteria supplement morphological features, causing reassignments.
- For diatoms, silicified cell walls (frustules) are a key taxonomic trait.
- Dinoflagellates are distinguished by their cellulose thecae (plates) and flagellar arrangement.
- In green algal unicells, features like flagellar number, chloroplast shape, pyrenoid presence are used.
Examples
- Chlamydomonas — a biflagellate unicellular green alga, with cup-shaped chloroplast, eyespot, contractile vacuoles.
- Chlorella — a non-motile, spherical unicellular alga lacking flagella, often enclosed in mucilage.
- Diatoms (e.g. Navicula, Cyclotella) — unicellular, siliceous frustules, may move by secretion of mucilage via raphe.
- Dinoflagellates (e.g. Peridinium, Ceratium) — motile, with two dissimilar flagella and thecal plates.
- Extremophilic red alga Galdieria partita — unicellular, sometimes wall-less, capable of switching nutrition modes.
Functional / Ecological Aspects
- Unicellular algae are primary producers in aquatic ecosystems, forming phytoplankton, thus contributing to oxygen generation and carbon fixation.
- In variable environment (nutrients, light) flexibility in reproduction or morphological adjustments may be exhibited.
- Some unicellular algae engage in symbiotic relationships (e.g. with fungi in lichens) or live as endosymbionts.
Limits / Special Cases
- Some “unicellular” algae are actually coenocytic (multinucleate) but not partitioned by septa; such forms blur line between unicellular and multicellular (these are often considered under macroalgal morphology).
- In adverse conditions cysts or resting spores may be produced, in which metabolic activity is very low.12
2. Colonial Algae
Colonial Algae are algae in which individual cells remain attached after division, forming a group or aggregate, rather than existing truly single and independent.
In many instances, a gelatinous matrix or mucilaginous envelope is produced, in which cells are embedded and held in position.
A variety of forms (spherical, plate-like, net, disc, hollow) are displayed, depending on taxon, habitat, and developmental program.
In colonial forms, cells often retain considerable autonomy; metabolic functions (photosynthesis, respiration, excretion) are often performed by each cell independently, though coordination may occur.
Through evolutionary time, colonial algae have been thought to represent transitional states between unicellularity and true multicellularity.
Characteristics
- Cell Arrangement – Cells are arranged in definite patterns (for example, spherical shell, flat plate) with somewhat regular spacing.
- Intercellular Connections – Cytoplasmic strands or bridges sometimes connect adjacent cells, permitting limited communication or transport.
- Extracellular Matrix – A gelatinous sheath, often glycoprotein or polysaccharide based, is secreted, enveloping the colony and giving structural cohesion.
- Differentiation – In some colonies weak differentiation is present, with some cells specializing in reproduction (germ cells) and others in motility or support (somatic cells).
- Motility – Often flagella are borne by peripheral cells, enabling the entire colony to swim; coordination of flagellar beating is needed.
- Size Limits – Colony size is constrained by diffusion of nutrients, light penetration, waste removal; very large sizes demand additional morphological innovations.
- Reproduction – Asexual reproduction via internal colony formation, fragmentation or daughter colony release is common; sexual reproduction (isogamy, anisogamy, oogamy) also occurs in many colonial algae.
- Ecological Role – Colonial algae often form part of phytoplankton or periphyton assemblages, contributing to productivity, oxygen evolution, carbon fixation, and acting as food for microfauna.
Examples / Forms
- Volvox is a well-known colonial green alga forming hollow spherical colonies of many thousands of cells; somatic vs germ cell differentiation is observed.
- Gonium forms flat plate colonies (4–16 or more cells) with no strong differentiation among cells.
- Pleodorina shows partial differentiation; vegetative (somatic) cells and reproductive cells exist, arranged in a hollow colony.
- Yamagishiella forms colonies (16 or 32 cells) arranged in a gelatinous sphere, with isogamous reproduction, and with little somatic/germ distinction.
- Tetrabaena socialis is a colonial alga with four cells attached in a square pattern; motile, with flagella, and minimal integration.
- Prasinoderma coloniale forms loose, sticky colonies of non-flagellated spherical cells in mucilage.
Evolutionary / Developmental Aspects
- Colonial volvocine algae (lineage that includes Chlamydomonas → Gonium → Volvox) have been used as models for evolution of multicellularity and cellular differentiation.
- In some lineages external fertilization (gamete union outside parental colonies) is observed, whereas in more derived colonies internal fertilization or oogamy evolves.
- Developmental processes like colony inversion (turning inside-out of cell layer so that flagella face outward) have evolved in some genera (e.g. Volvox) to allow motile function.
- Transitional genera (e.g. Colemanosphaera) have been discovered that bridge morphological traits between flattened and spheroidal colony forms, helping to resolve evolutionary steps.43
3. Filamentous Algae
Filamentous Algae are algae in which cells are joined end-to-end forming threadlike chains (filaments), rather than being free or loosely aggregated.
In many taxa the filaments may be simple (unbranched) or branched, sometimes forming a network or mat-like structure.
Through filament formation a greater length is attained, which helps in light harvesting and substrate attachment, though diffusion limits impose constraints.
Characteristics
- Cell Arrangement – Cells are aligned linearly in one or more rows, with end walls adhered, sometimes with connecting cytoplasmic bridges.
- Branching – Lateral branches may emerge from main filaments; branching pattern (dichotomous, lateral) is taxonomically informative.
- Mucilaginous Sheath / Matrix – A gelatinous sheath or mucilage envelope may surround filaments, affording protection and cohesion.
- Reproductive Cells / Specialized Cells – In some filaments certain cells transform into reproductive structures (e.g. sporangia, gametangia), others remain vegetative.
- Motility / Gliding – Some filamentous cyanobacteria or algae exhibit gliding movement over substrata, often via slime extrusion or adhesion mechanisms. For example filamentous cyanobacteria show gliding motility tied to mechanical forces.
- Pigments / Plastids – Chloroplast(s) are present in each cell, often with pyrenoids (in green lineages), shape of chloroplast being diagnostic in many genera.
- Physiological Constraints – Filament length is limited by diffusion of nutrients, gases; structural support is needed in longer filaments.
- Attachment / Anchoring – Basal cells or rhizoids may anchor the filament to substrate or other surfaces.
Types / Forms
- Unbranched filaments — e.g. Spirogyra, Ulothrix.
- Branched filaments — e.g. Cladophora.
- Heterotrichous filaments — having both prostrate (creeping) and erect parts.
- Coenocytic filaments — continuous multinucleate filaments without septa (in certain groups; sometimes considered borderline).
Examples / Representative Genera
- Spirogyra — unbranched filament, spiral chloroplasts, joined by conjugation during sexual reproduction.
- Ulothrix speciosa — marine brackish filament, unbranched, with basal cell attachment, cellular chloroplasts and pyrenoids.
- Cladophora — branched filamentous green alga forming dense mats.
- Vaucheria litorea — a filamentous yellow-green alga (Xanthophyceae) with siphonous filaments, branching, lacking transverse cell walls.
- Rhodochorton — filamentous red alga with upright threads, infrequent branching, adapted to low light, often epiphytic or encrusting.
Ecological / Functional Roles
- Filamentous algae contribute significantly to primary production in freshwater, marine, and damp terrestrial habitats.
- In favorable conditions, long filaments form mats that trap gases, float to surface, block light to lower layers, and influence oxygen dynamics (hypoxia risk).
- They may outcompete submerged plants by shading, and interfere with recreational uses (swimming, fishing) when dense mats form.
- Some filamentous forms are used as indicators of nutrient enrichment (eutrophication) because filamentous growth is favored by high N, P levels.
Limitations / Special Cases
- Very long filaments face internal diffusion limits; cells in the interior may suffer low access to nutrients or CO₂.
- In extreme environments some filaments become hollow, or branching increases surface area, or symbiotic associations appear.
- In some lineages, filaments are partially or wholly coenocytic, thus blurring the boundary between filamentous and siphonous forms.5
4. Heterotrichous Forms of Algae
- Heterotrichous Forms of Algae are those in which the thallus is differentiated into two distinct filament systems — a prostrate (or basal) system and an erect (or projecting) system — rather than a uniform filamentous net.
- In such forms, the prostrate filaments often grow over substrate surfaces, and from them the erect filaments arise upward (or outward), frequently branching, which gives a “dual habit” appearance.
- In many cases the erect system is more exposed to light and supports reproductive structures, whereas the prostrate system ensures anchorage and resource absorption.
Characteristics
- Differentiation – Two morphologically distinct filament systems (prostrate + erect) are present.
- Branching – Erect filaments are often branched (primary, secondary, sometimes tertiary), while prostrate system may be more flattened or spreading.
- Attachment – Prostrate system attaches to substrate (rock, plants, sediment) maintaining stability.
- Surface area / light capture – The erect system increases exposure to light, improving photosynthetic efficiency in crowded, low-light zones.
- Reproductive specialization – Reproductive organs (sporangia, gametangia) are frequently borne on erect branches, freeing prostrate parts from direct reproductive roles.
- Structural support / resources – The integration (via cytoplasmic strands or supportive sheaths) between systems may allow transport of nutrients from base to distal parts, and mechanical support in erect filaments.
- Taxonomic relevance – The pattern of differentiation (length of erect vs prostrate, degree of branching) is often used in classification within filamentous green algae, and some brown or red algae.
Examples / Occurrence
- Stigeoclonium is cited often as a classic heterotrichous green alga, with prostrate system and erect branches.
- Genera like Draparnaldia and Fritschiella (in Chlorophyta) also exhibit heterotrichous habit.
- In brown algae (Phaeophyceae), the heterotrichous filamentous condition is described in simpler forms such as Ectocarpus, where basal and erect filaments coexist.
- Some red algae (e.g. Erythrotrichia) also show heterotrichous organization in their thalli.
Evolutionary / Functional Significance
- The heterotrichous form is considered more advanced among filamentous habits, because differentiation between systems allows specialization (e.g. anchoring, light harvesting, reproduction).
- In transitional evolutionary lineages, heterotrichy may represent a step toward more complex thallus architectures (e.g., pseudoparenchymatous or parenchymatous forms).
- Efficiency in resource use is improved: prostrate parts draw nutrients/water, erect parts capture light & disperse spores; thus the internal tradeoffs are balanced.6
5. Thalloid Forms of algae
- Thalloid Forms of Algae are those in which the algal body (thallus) is organized as a flat, expanded sheet or plate, or as a more complex three-dimensional mass, rather than linear filaments or discrete cells.
- In many thalloid algae divisions occur in multiple planes (transverse + longitudinal), giving a laminar or foliose appearance, which increases surface area for light absorption and exchange.
- Through thalloid organization, more compact growth is achieved, which helps resist mechanical stress (waves, current) in aquatic habitats.
Characteristics
- Multiple planes of cell division – Cells divide in two or more directions (not just in one plane), so that the thallus expands laterally as well as in thickness.
- Thallus thickness / layering – The thallus may consist of single cell layer (monostromatic) or multiple layers (multistromatic), sometimes with inner medullary and outer cortical zones.
- Cellular differentiation / specialization – Some cells in thalloid algae may specialize as reproductive units, supportive cells, or protective cells (e.g. surface cells may be thicker).
- Attachment structures – A holdfast, rhizoids, or basal attachment region is often present to anchor the thallus to substratum (rock, other surface).
- Morphological complexity – Lobes, ruffles, lobate edges, branching expansions may be evolved to maximize light interception.
- Pigment & plastid layout – Chloroplasts are arranged in cells so as to optimize light capture; pigmentation may vary across layers, affecting internal shading.
- Transport / internal connectivity – Cytoplasmic strands or pit connections may link cells across layers or zones for movement of nutrients, metabolites.
Types / Variants
- Foliose / leaf-like thalli – Flat sheets with lobes and margins (e.g. some red or brown algae).
- Crustose (crust-forming) thalli – Adherent, low profile thallus that grows tightly on surfaces, often forming a crust (e.g. Hildenbrandia).
- Cushion / mat forms – Thallus is slightly elevated but compact, forming cushiony structures.
- Frondose / erect lobed thalli – Thallus is expanded with upright lobes or fronds emerging from basal sheet or crust (e.g. Apophlaea).
Examples / Representative Genera
- Hildenbrandia is a red alga with a crustose thallus adhering to rock, with vertical cell rows in its pseudoparenchymous layer.
- Apophlaea is a thalloid alga in which a crustose base gives rise to upright branching lobes.
- Cephaleuros is a thalloid green alga (parasitic on plants) in which a prostrate thallus and erect filaments combine (so it is intermediate between thalloid and filamentous).
- Thalloid brown seaweeds (e.g. Lobophora) show foliose/laminar form, with fronds and plate-like structures.
Ecological / Functional Significance
- In habitats with strong currents or waves, the compactness of thalloid forms reduces drag and damage, compared to long filaments.
- Thalloid morphology allows better coverage of substrate and competition for space (light, nutrients) over surfaces.
- A larger surface area relative to volume is achieved, enhancing gas exchange, nutrient uptake, photosynthesis.
- In intertidal or exposed zones, crustose thalli resist desiccation better, and can regrow from basal cells after damage.7
6. Polysiphonoid Forms of algae
- Polysiphonoid Forms of Algae are those in which thallus construction is “polysiphonous” — i.e. a central axial filament is surrounded by several pericentral siphons / tubes (cells), giving a multiseriate cylindrical structure.
- In many red algae this kind of form is exhibited, and the body is called polysiphonous (many tubes) rather than monosiphonous (single tube).
- Through polysiphonoid organization greater mechanical strength and multiple cell rows are achieved, which allows more branching, elevation, and support in marine environments.
Characteristics
- Central axis + pericentral cells – A central row (axial cell chain) is enveloped by a series of pericentral cells which run parallel and of similar length, giving the name “polysiphonous.”
- Cortication – Outer cortical or cover cells may develop over the pericentral cells by periclinal or anticlinal divisions, forming a cortex.
- Pit connections / cytoplasmic continuity – Cells are connected by pit connections that permit transport or communication between axial, pericentral, and cortical cells.
- Differential branching – Branching may occur from pericentral or axial cells; branch bases often show constriction or special morphology.
- Reproductive specialization – Trichoblasts (monosiphonous branchlets) may develop as reproductive branches, distinct from main polysiphonous parts.
- Attachment / anchorage – Rhizoids or haptera may arise from basal cells or prostrate parts to affix the alga to substrate.
Examples / Representative Genera
- Polysiphonia is a classical example: its thallus is composed of a central axial filament with typically 4–20 pericentral cells; branching, cortication, and reproductive structures are common.
- Polysiphonia elongella displays a polysiphonous axis with 4 periaxial cells around a central filament; lateral branching, cortication, and holdfast attachment are present.
- Some species within the Polysiphonia sensu lato group are ecorticate (lacking cortex) or have fewer pericentral cells (5-6) in specific taxa.
Functional / Ecological Significance
- In marine intertidal / subtidal zones, polysiphonoid forms withstand shear stress and water movement better than simple filaments, because of mechanical support from multiple cell rows.
- The geometry (cylindrical, multiseriate) allows increased thickness without sacrificing diffusion too much, enabling vertical growth to reach light levels.
- The combination of axial + pericentral + cortical cells gives flexibility: damage to outer cells may be repaired by inner ones, improving resilience.
- Polysiphonoid species often dominate in epiphytic or lithophytic niches on rocks or on other algae, expanding 3D structure over substrates.
Thallus Organization in Multicellular Algae
The concept of thallus organization in multicellular algae is concerned with how multicellular algal bodies (thalli) are structured, how cells are organized, and how differentiation is achieved.
In many algae structural complexity is built progressively; simple filamentous forms give rise (by further divisions / modifications) to more complex sheet-like, parenchymatous, or siphonous organizations.
The structural schemes recognized include filamentous (branched / unbranched / heterotrichous), pseudoparenchymatous, siphonaceous (coenocytic), and true parenchymatous (multiplanar cell division) types.
Key Features / Components
- Cell Division Planes – In multicellular thalli cells divide not only in one plane (as in simple filaments) but often in two or three planes (transverse, longitudinal, periclinal), allowing lateral extension (sheet, foliose) and thickness.
- Differentiation – Some cells become reproductive (gametangia, sporangia), others remain vegetative or supportive. In more complex thalli internal zones (medulla, cortex) may be differentiated.
- Intercellular Connectivity – Pit-connections, cytoplasmic strands or plasmodesmata (or analogous structures) may link cells for transport of nutrients, metabolites and signals.
- Support / Rigidity – In thicker thalli mechanical strength is required; cortication (outer cell layers), secondary filaments, or reinforcement structures help maintain integrity under water movement.
- Attachment Structures – A holdfast, haptera, or rhizoids may arise from basal thallus cells to anchor the alga to substratum.
Types / Patterns of Organization
- Filamentous Type – Cells arranged end-to-end in one plane (uniseriate), sometimes branched. From this base more complex forms derive.
- Pseudoparenchymatous Type – Filaments (or multiple filaments) are interwoven and closely associated so that the structure looks like parenchyma (tissue), but origin is from filamentous growth.
- Siphonaceous (Coenocytic) Type – Thallus is composed of a large multinucleate cytoplasm without regular cell walls (or septa), forming large tubes or networks.
- Parenchymatous Type – True three-dimensional organization by cell divisions in multiple planes, forming foliose / tubular / blade like structures.
Developmental / Evolutionary Considerations
- Multicellularity in algae is believed to have evolved from simpler filamentous or colonial ancestors by stepwise increases in cell connectivity and division control.
- Many advanced seaweeds (red, brown, green macroalgae) show parenchymatous or pseudoparenchymatous organization, and their thallus architecture is central to classification and functional adaptation.
- Variation in thallus organization reflects adaptation to environmental stresses: light gradients, water motion, nutrient diffusion constraints.
Cell Structures in Algae
1. Cell Wall Composition in Algae
The cell wall in algae is composed of fibrillar polymers, matrix polysaccharides / glycoproteins, and in some cases inorganic / mineral deposits.
The fibrillar framework is often built from cellulose microfibrils, which are semi-crystalline and provide tensile strength.
The matrix polysaccharides (non-fibrillar) fill the spaces between microfibrils and vary widely among algal taxa.
Sulfated polysaccharides are commonly encountered, especially in marine algae:
• Agar, carrageenans are deposited in red algae walls.
• Fucose-containing sulfated polysaccharides (FCSPs) and alginates occur in brown algae.
• Ulvans (a class of sulfated heteropolysaccharides) are found in some green ulvophyte algae.
In certain green algae, mannans or mannans + cellulose combinations are used as structural elements.
Xylans (β-D xylosyl polymers) are also sometimes incorporated, especially in walls with branched architecture.
In some taxa a layered architecture is observed: alternating microfibrillar and amorphous matrix layers
The wall is often impregnated or reinforced by inorganic / mineral components:
• Silica (SiO₂) is used in diatoms, forming rigid frustules (shells) coated with organic polysaccharides.
• Calcium carbonate (CaCO₃) is deposited in some red (coralline) algae and in certain green / brown algae.
In single-celled algae (e.g. many Chlorophyta), walls may be dominated by glycoproteins, especially hydroxyproline-rich glycoproteins (HRGPs), sometimes with weak fibrillar carbohydrate support.
By environmental influence (e.g. marine vs freshwater), wall composition is modified: marine algae tend to incorporate more sulfated polysaccharides and mineral deposits, whereas freshwater species often have more mucilage / simpler polysaccharides.
During growth and development phases in microalgae, the relative proportions of wall components shift (e.g. between polysaccharide & protein fractions), and secondary layers (such as mannan layers) may be formed.
The diversity in cell wall composition is great even within same algal class; interspecific variation is common.
The cell wall is thus a dynamic, chemically complex structure which is adapted to ecological, evolutionary, and functional pressures.25262728

2. The Protoplast in Algae
- The Protoplast in Algae is defined as the living cell content (cytoplasm + nucleus + organelles) which remains when the cell wall is removed (or absent).
- In algae, protoplasts are generated by removal of cell walls either enzymatically, chemically / mechanically, depending on taxon and wall composition.
- The plasma membrane encloses the protoplast, and its integrity must be preserved during wall removal, otherwise rupture would occur.
- A proper osmotic medium is employed so that protoplasts are stabilized and lysis is avoided; isotonic solutions are used to prevent bursting or shrinkage.
- Enzymes such as cellulases, pectinases, and other wall-degrading enzymes are used to digest polysaccharide components of the cell wall, and protoplasts are liberated.
- The yield and viability of protoplasts are influenced by species, age / developmental stage of the algal cell, and effectiveness of enzymatic digestion.
- Applications / Uses
- In physiological studies, membrane properties, transport, and ion flux are measured using algal protoplasts.
- In genetic manipulation, DNA or other macromolecules are introduced into protoplasts, because the absence of cell wall removes a major barrier.
- Protoplast fusion is performed, so somatic hybrids or novel recombinants may be generated among algal species.
- In culture, protoplasts can regenerate their cell wall under favorable conditions, and may divide and develop into new individuals (in some algae).
- Regeneration of protoplasts into whole algal cells depends on suitable culture media, growth regulators (if required), and proper environmental parameters (light, nutrients, osmotic support).
- In green seaweeds, protoplast isolation protocols are more advanced, and regeneration and transformation have been achieved in many species.
- In red algae, protoplasts have been isolated and used for genetic transformation and fusion in a few species.
- In brown algae, protoplast isolation and regeneration protocols are less standardized, and challenges remain in achieving stable regeneration.222324
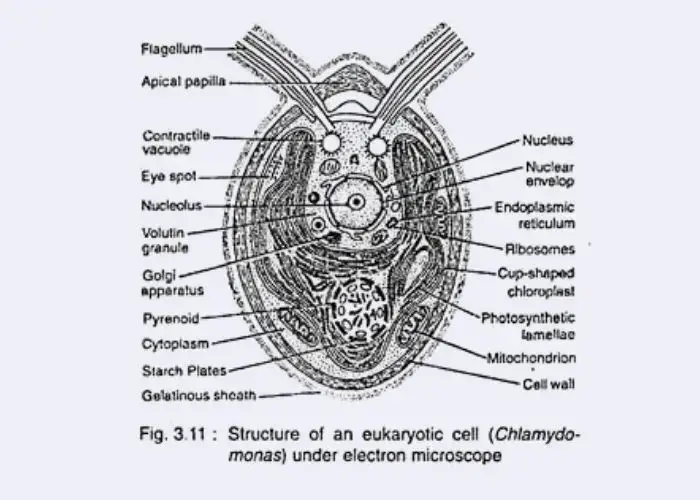
I. Chloroplast Variations in Algae
- The chloroplast in algae is recognized as the most prominent organelle, since photosynthetic pigments are housed in it and energy capture is performed.
- It is always surrounded by a double-membrane envelope, and the internal structure is highly organized into distinct regions.
- Three principal structural regions were identified, and they are considered universal:
- Envelope – composed of two membranes, with an intermembrane space enclosed.
- Stroma – a fluid matrix, in which soluble enzymes, proteins for metabolism, machinery for protein synthesis and starch storage granules are contained.
- Internal lamellar system – made of thylakoids, arranged into discs or vesicles, which may be stacked to form grana; electron carriers and pigment systems are embedded here.
- Each thylakoid encloses a distinct interthylakoid space, and together the entire system forms a continuous cavity separated from stroma by the thylakoid membrane.
- The pigments and carriers built into the thylakoid membrane are responsible for the light-dependent phase of photosynthesis, and energy transduction occurs therein.
- In Cyanophyceae, true chloroplasts are absent, and instead thylakoids remain free in the cytoplasm; phycobilisomes appear as vesicular granules on their surface.
- Forms of Chloroplasts in Algae
- Cup-shaped (Chlamydomonas, Volvox)
- Discoid (Chara, Vaucheria, centric diatoms)
- Parietal (Chaetophorales, Phaeophyceae, Rhodophyceae, Chrysophyceae, pinnate diatoms)
- Girdle-shaped / C-shaped (Ulothrix)
- Spiral (Spirogyra)
- Reticulate (Oedogonium, Hydrodictyon, Cladophora)
- Stellate (Zygnema)
- Ribbed (Volvocales)
- The diversity of form is striking, and the chloroplast nearly fills the cell in certain species, while only a single small chloroplast is present in others.
- Pigmentation
- Chlorophylls were reported in five types: chlorophyll a (in all algae), b (in Chlorophyceae), c (in Phaeophyceae, Cryptophyceae, Bacillariophyceae, Chrysophyceae), d (in some red algae), and e (in certain Xanthophyceae).
- Carotenoids are present as carotenes and xanthophylls. Five carotenes were identified: α-carotene (Chlorophyceae, Cryptophyceae, Rhodophyceae), β-carotene (in nearly all groups except Cryptophyceae), c-carotene (Chlorophyceae), e-carotene (Bacillariophyceae, Cryptophyceae, Phaeophyceae, Cyanophyceae), flavacene (restricted to Cyanophyceae).
- Xanthophylls occur in many variations: lutein, violaxanthin, neoxanthin in Chlorophyceae and Phaeophyceae; fucoxanthin as the characteristic pigment in Phaeophyceae and Bacillariophyceae; myxoxanthophyll, myxoxanthin, oscilloxanthin confined to Cyanophyceae.
- Phycobilins are found in Rhodophyceae and Cyanophyceae only, and they are soluble biliproteins. Phycoerythrin (red) and phycocyanin (blue) function as accessory pigments for light capture and transfer.
- Pyrenoids
- Pyrenoids are present as proteinaceous inclusions in chloroplasts, regarded as highly characteristic of algal plastids.
- Their main function is the synthesis and storage of starch, although in Bacillariophyceae lipids are accumulated instead.
- Number of pyrenoids is variable: only one is observed in Chlamydomonas, but more than one in Oedogonium.
- From these features it can be concluded that algal chloroplasts are highly variable, both in form and in pigment composition, but a fundamental plan of double-membrane structure, stroma and thylakoid system is maintained.
- The presence of multiple accessory pigments and structural modifications allow algae to occupy diverse habitats, because light absorption can be optimized for different depths, intensities, and spectral qualities.
- It must be noted that evolutionary continuity is reflected in similarities of chloroplast structure across the plant kingdom, although striking variation is retained among algal groups.21
II. Mitochondria in Algae
- The mitochondrion is present in all eukaryotic algal cells except Cyanophyceae (blue-green algae) which lack the organelle.
- It is enveloped by a double membrane, and an intermembrane space lies between the outer and inner membranes.
- The inner membrane is more extensively folded than outer membrane, and invaginations called cristae are formed, usually by narrow necks.
- The cristae shapes vary in different algae; they may be tubular, sheet-like, or finger-like.
- The mitochondrial matrix occupies the interior, and a granular, protein-rich milieu with soluble enzymes, mitochondrial DNA, ribosomes, and metabolites is contained therein.
- Mitochondria are semiautonomous, because a circular DNA and mitochondrial ribosomes permit synthesis of some proteins independently of the nucleus.
- More than one mitochondrion per algal cell is the usual condition, however in certain chlorophyte taxa (e.g. Micromonas) a single mitochondrion was reported.
- In Cyanophyceae, respiration is conducted on invaginations of the cytoplasmic membrane (mesosomes), since mitochondria are absent.
- Genome architecture in algal mitochondria is variable; in red algae lineage Stylonematophyceae, mitochondrial genomes are fragmented into minicircles encoding one or two genes per circle.
- Translation code in mitochondrial genomes may deviate: in Chlorophyceae, UAG can code for an amino acid (leucine) instead of serving as stop codon.
- The number and arrangement of mitochondria are dynamic: under some stress or metabolic demand mitochondria relocate or change morphology to adjust bioenergetic capacity.
- Mitochondrial function is integrated with chloroplast and cellular metabolism: electron transport, ATP production, redox balance, and retrograde signaling are coordinated.
- The ultrastructure, genome content, and regulatory control of mitochondria are adapted to specific algal lineages, reflecting ecological specialization and evolutionary history.181920
III. Endoplasmic Reticulum (ER)
- The endoplasmic reticulum (ER) in algal cells is a membranous network of interconnected tubules and cisternae that spans much of cytoplasm, and its membranes are continuous with the nuclear envelope.
- In many algae two functional forms are distinguished: the rough ER (RER), bearing ribosomes on its cytoplasmic face, and the smooth ER (SER), lacking ribosomes and involved in lipid / membrane metabolism.
- In rough ER, the cisternae are flattened sacs, and ribosomes attached ensure nascent polypeptides are translocated into the lumen or membrane; this system supports protein synthesis targeted for secretion or organelles.
- In smooth ER, tubular elements and membranes are more abundant, and functions such as lipid synthesis, detoxification, and membrane formation are conducted.
- In certain algae, a chloroplast‐associated ER (chloroplast ER, cER) is observed, which is continuous or closely associated with both the ER and chloroplast envelope membranes; this specialized ER participates in synthesis / transport of lipids or proteins for plastids.
- The ER membranes traverse the cytoplasm and may be closely associated with Golgi apparatus, nuclear envelope, and plastid membranes, enabling vesicular trafficking and membrane continuity across organelles.
- In brown algae, portions of cytoplasmic ER are sometimes continuous with Golgi stacks or nuclear envelope regions, forming structural channels for secretory precursors or carbohydrate transport.
- The lumen (internal space) of ER cisternae is used for folding of proteins, posttranslational modifications (glycosylation), and as a buffer compartment for ions / small molecules.
- Variation exists in ER abundance, tubule vs sheet prevalence, and connectivity in response to cell type, metabolic demand, developmental stage, or environmental condition.
IV. Dictyosomes or Golgi Apparatus
- Golgi Apparatus is present in algae as a stack of membrane-bound cisternae (dictyosomes), and secretory trafficking is mediated through it.
- In many green algae, multiple Golgi bodies are scattered in cytoplasm and function in secretion, wall polysaccharide synthesis, and vesicle sorting.
- In scale-forming algae (e.g. Prymnesiophytes), Golgi architecture differs from higher plants: “prosecretory vesicles” derived from trans-Golgi network (TGN) are used for scale formation, and cisternal progression is not strictly followed.
- Golgi-derived vesicles are deployed in red algae (Polysiphonia) for cell wall formation, and during reproductive development Golgi morphology and vesicle types shift.
- In marine chrysophycean algae (e.g. Pleurochrysis), cellulosic wall fragments / scales are produced by the Golgi, indicating that wall synthesis is a Golgi function in algae.
- In brown algae, the Golgi is closely associated with the nuclear envelope, and vesicles for secretion are derived from Golgi and possibly from nuclear envelope membranes.
- Near mitosis, Golgi bodies in algal (green) cells divide, but unlike animal Golgi they do not completely fragment; division is coordinated with cell cycle.
- The distribution pattern of Golgi in algae is variable, and cisternae number, stack size, and activity are modulated during growth or secretion demand.
- In secretion of scales (in algae), the TGN is active in packaging of scale precursors and routing them via vesicles to cell surface.
- Structural features such as tubular connections between cisternae, cis/trans polarity, and dilations of cisternae have been documented in algal Golgi, contributing to morphological diversity.
- The role of Golgi in algae includes: polysaccharide modifications, membrane / protein trafficking, scale / cell wall component secretion, vesicle sorting.
- Because algae show great diversity, Golgi organization is adapted: some algae have compact Golgi stacks, others more dispersed bodies.
- Secretory load in algae (wall formation, scales, extracellular polysaccharide secretion) places demand on Golgi function, thus Golgi is prominent in many algal cells.
- Vesicles budding off Golgi are destined to plasma membrane, extracellular matrix, or wall assembly.
- The Golgi in algae is semiautonomous in the sense that its dynamics are responsive to cellular metabolic state and developmental stage.
- Consider that comparative Golgi studies in algae help understand evolution of secretory pathways in plant lineage.12131415
V. Eye-Spot or Stigma
- Eye-spot / Stigma in algae is a photoreceptive organelle, and light direction / intensity is sensed by it.
- Within algae, the stigma is composed of carotenoid pigment granules or lipid globules, which form a pigmented shield and act as a shading / screening device.
- The pigment granules are orange-red in color, and they lie in a fixed plane relative to flagellar bases in many motile algae.
- Adjacent to the pigmented layer, photoreceptor proteins are located in membranes, which receive light signals that are modulated by the shading from pigment granules.
- The term “eyespot” is sometimes misleading, because the stigma does not itself contain the photoreceptor in all cases, but only provides directional shading; the photoreceptor may be in membrane regions next to it.
- Structural Types / Variations
- In Chlamydomonas and Volvox, a cup-shaped stigma is observed, with pigment granules arranged in layers.
- In algae with parietal chloroplasts, eyepots are often situated against chloroplast periphery, underlying the chloroplast envelope membranes.
- Stigmata can act also by reflecting or focusing light (like a concave mirror) to direct photons toward photoreceptor membranes, enhancing sensitivity.
- In Euglenophyta, the eyespot lacks the reflective properties seen in green algae, and is more purely shading / screening device.
- Functions / Physiological Roles
- Light direction detection is mediated through differential shading by the stigma, and the flagellar beating pattern is modulated accordingly to achieve phototaxis (toward or away from light).
- The stigma helps the cell in finding favorable light intensity for photosynthesis, by orienting movement (positive or negative phototaxis).
- In certain algae, absorption spectra of the eyespot reveal two maxima (A-band in blue, B-band in green), and anisotropy in B-band suggests structured pigment arrangement (e.g. in Euglena).
- The pigmentation (carotenoids) is essential, because the absorption / shading of unwanted light directions is achieved by these pigment granules.
- The stigmatic shading ensures that only light from preferred direction reaches photoreceptors, and interference by background illumination is reduced.
- From evolutionary perspective, the presence of stigma in many flagellate algae indicates that primitive photoreceptive systems evolved early in algal lineages, and later more complex eyes developed in some dinoflagellates / ocelloids.
- It must be understood that stigma architecture (pigment stack geometry, spacing, membrane proximity, photoreceptor coupling) varies across taxa, and functional performance is adapted to habitat light conditions.
- The coupling between stigma and photoreceptor membranes is critical, because light must be either screened or passed in a directional sense, and precise spatial alignment is required.
- Signal transduction from photoreceptor to flagellar motor apparatus is triggered by light capture, and flagellar beating is modulated accordingly, resulting in directional movement.
- Consider that in studies, the stigma is seen under microscope as an orange or red spot, often just beneath chloroplast membranes, and is stained by carotenoid-binding stains or osmium tetroxide (forming black precipitates).
- Care is needed in assuming that all algae have stigma; many nonmotile algae or nonflagellate forms lack it, or have reduced / vestigial photoreceptive structures.
- The shading functionality of stigma is essential in noisy light environments (e.g. fluctuating light), because it permits differential light detection rather than absolute light sensing.
- In some dinoflagellates, more elaborate eyespots or ocelloid structures are present, which include lens / retinal-like components and more complex pigmentation / membrane systems.17

VI. Vacuoles in Algae
Vacuoles are present in almost all algal cells, except Cyanophyceae, and they are bounded by a membrane known as the tonoplast.
In motile algal forms, three main types of vacuoles are identified, and each type shows distinct structure / function.
- Simple vacuole – very small, contractile; contraction and expansion occur periodically; they expel metabolic wastes; water content is regulated by discharging excess water at short intervals (secretion / osmoregulation).
- Complex vacuole – characteristic of Dinophyceae and Euglenophyceae; composed of a cytopharynx (tube-like), a large reservoir plus several vacuoles of varying sizes; osmoregulatory function is primary, and sometimes storage of reserve food (e.g. laminarin, chrysolaminarin) is also performed.
- Gas vacuole – found in Cyanophyceae (though Cyanophyceae lack true vacuoles in other respects); stacks of transparent cylindrical cavities occur; walls are permeable to gases; buoyancy is conferred to planktonic forms and protection from bright light is provided.
The tonoplast is the delimiting membrane of each vacuole, and selective permeability is mediated through it, allowing movement of solutes / water.
In contractile (simple) vacuoles, osmoregulation is maintained by cyclic filling and expulsion, and excess water is actively pumped out (thus cell lysis is prevented).
Complex vacuoles integrate multiple subcompartments (reservoir + smaller vacuoles) so that a coordinated osmotic balance is sustained.
Reserve storage in vacuoles is sometimes enabled (in complex vacuoles) where polysaccharides like laminarin or chrysolaminarin are deposited until needed.
Gas vacuoles, though structurally simpler, aid vertical positioning in water column, and modulate light exposure, because gas volume / buoyancy influences cell depth.
The relationship of vacuole type to cell motility is significant: motile algae often show contractile or complex vacuoles to cope with variable osmotic conditions in aquatic media.
Vacuolar dynamics (contraction, expansion, reservoir filling) are energy-dependent and regulated, and coordination with cytoskeletal / membrane systems is required.
In motile forms, multiple small vacuoles or vesicles may supply water to central contractile vacuole during diastole (filling phase).
Osmoregulation via vacuoles is particularly critical in freshwater algae, because water influx due to osmotic gradient is continuous.
In Cyanophyceae (blue-green algae), classical vacuoles are largely absent, and instead gas vacuoles or membrane invaginations (mesosomes) may take over some functions of buoyancy or intracellular compartmentation.
The efficiency of vacuolar function affects ion homeostasis, turgor regulation, waste removal, and survival under varying salinity or osmotic stress.
From evolutionary / ecological perspective, vacuolar types are adapted to habitat: freshwater motile algae favor contractile vacuoles, marine / planktonic forms favor gas vacuoles or reduced vacuolar complexity.
Structural variation in tonoplast proteins, pumps, channels is expected across algal taxa, enabling variation in solute specificity, responsiveness, and regulation.
Rapid vacuolar adjustments are often required when algae are shifted between media of different osmotic strength, and delay in response may result in cell stress or rupture.
In microscopy / ultrastructure studies vacuoles are visualized by their clear lumen, tonoplast boundary, and associated substructures (contractile vacuole complexes, cytopharynx, spongiome).
Considering application, knowledge of algal vacuole types is important in physiological experiments, stress physiology, and in manipulation of osmotically sensitive cells.
The presence / absence, size, dynamics of vacuoles may be used as taxonomic / ecological markers in algae classification and habitat inference.16
VII. Flagella and Motility Structures in Algae
Flagella and other motility structures are considered vital in many algae, especially those in which movement is needed (gametes, zoospores, motile cells).
In most motile algae, flagella (sometimes termed eukaryotic cilia) are constructed with a classical “9 + 2” microtubule axoneme inside a membrane sheath, surrounded by a basal body (centriole‐derived) at base.
The dynein arms on the microtubule doublets use ATP hydrolysis to generate sliding forces between microtubules, bending the flagellum and producing motility.
In many algae, flagella are equipped with mastigonemes (tubular hairs) which reverse thrust or assist in propulsion, particularly in stramenopile / heterokont algae.
The number, length, and arrangement of flagella vary: some algae bear two equal flagella, others bear unequal or numerous flagella.
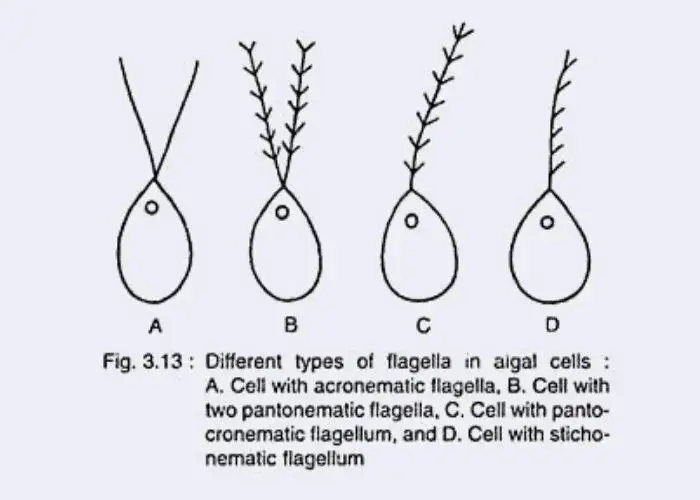
Characteristics / Details
- Basal apparatus and coupling – Basal bodies are connected by fibers and structural linkages, ensuring synchronization of multiple flagella; motility is constrained by these couplings.
- Synchronization / mechanical coupling – In Chlamydomonas, the two flagella are mechanically synchronized: when they lose synchronous beating, the cell rocks, which restores coordination.
- Waveforms / beat patterns – Flagella may beat with symmetric or asymmetric waveforms, or even novel patterns (lasso-like in some algae) to generate locomotion.
- Motility types – Swimming in water (planktonic cells), gliding over surfaces (in some species via intraflagellar transport), or even swarming in colonial forms is mediated.
- Developmental / functional shifts – In some species (e.g. volvocine algae), flagellar distribution changes during gamete formation; these changes affect swimming speed and modes (e.g. sperm packets vs colonial flagellated forms).
Examples / Functional Implications
- In Volvox, thousands of surface somatic cells carry flagella; their coordinated action produces colony swimming and interactions (waltzing, attraction) at surfaces.
- In colonial algae like Gonium, peripheral cells beat flagella to rotate and translate the colony; internal cells may assist stability or orientation.
- In summary, flagella and associated motility structures in algae have been evolved with variations in design, coupling, beat patterns, and distribution; these variations have functional, ecological, and evolutionary significance in algal life cycles.891011

Specialized Morphological Structures in Algae
- A holdfast is produced at the base of many macroalgae
- It is used to anchor the alga to substrate (rock, shell, other)
- In some cases flexibility is given by basal cushions or rhizoids
- Often damage is resisted by thickening of cells adjacent to it
- A stipe is formed in many large seaweeds
- Elevation of the photosynthetic parts is allowed by it
- It is supported internally by strengthened cell walls or thicker filaments
- In some species, secondary growth is observed in stipe tissues
- A blade or lamina (sometimes called frond) is developed for photosynthesis
- The blade is flattened to increase light capture area
- Gas exchange and nutrient absorption are facilitated by it
- In some algae, veins or internal support filaments are embedded
- Pneumatocysts (gas bladders) are contained in some thalli
- Buoyancy of the alga is thereby aided, lifting blades toward surface
- Oxygen or other gases are stored; their regulation is controlled
- Damage to pneumatocysts can cause deflation and reduced buoyancy
- Segmented thallus / articulation is seen in certain green algae
- The thallus is divided into discrete segments or joints
- Flexibility and resistance to breaking by waves is provided
- Example: Halimeda has calcified segments separated by nodes
- A conceptacle is formed in some brown and red algae
- Reproductive organs (gametangia, sporangia) are enclosed inside it
- It opens to the outside via an ostiole (pore)
- Types include asexual, male, female, and cystocarpic conceptacles
- Sterile analogues are cryptostomata (produce hairs) and caecostomata (ostiole blocked)
- A carpogonium (with associated trichogyne) is specialized in red algae reproduction
- It is the female gametangium containing the reproductive nucleus
- A trichogyne may be present which receives sperm before fertilization
- Carpospores are derived after fertilization
- A pyrenoid is a subcellular specialization in chloroplasts
- Carbon dioxide fixation is concentrated around it, enhancing efficiency in aquatic environment
- RuBisCO is packed into the pyrenoid matrix
- It is traversed by thylakoid membranes (in many algae)
- A starch sheath often surrounds the pyrenoid
- A phycoplast is formed during cytokinesis in many green algae (Chlorophyceae)
- It is a microtubule array oriented parallel to the plane of division
- The daughter nuclei are separated and the division plane is guided by it
- It contrasts with phragmoplast mechanism in higher plants
- Differentiated heterocysts are produced by some cyanobacteria (though strictly not algae in strict eukaryote sense)
- Nitrogen fixation is allowed in heterocysts under anaerobic microenvironment
- Thick cell wall is formed to limit oxygen diffusion
- Thick-walled spores (e.g. akinetes, hypnospores) are produced in some algae / cyanobacteria
- Survival under unfavorable conditions (drought, freezing, nutrient lack) is allowed
- Dormancy is maintained until favorable conditions return
- Inconsistent cell-wall / extracellular matrix modifications are observed
- Cell walls of green algae vary: cellulose, pectin, hydroxyproline-rich glycoproteins etc
- Sulfated polysaccharides, extensins, arabinogalactan proteins are also found
- Colonial / multicellular arrangements with specialization
- Colonial forms with extracellular matrix connecting cells are produced
- Some cells in colonies are somatic, others reproductive (division of labor)
- E.g. in volvocine algae, some flagellar cells perform motility, others reproduction
Key Specialized Structures in Algae
| Structure | Type / location | Function(s) | Example(s) / Notes |
|---|---|---|---|
| Holdfast | A basal anchoring organ | Attaches the alga to the substrate (rocks, other surfaces) | Widely seen in large brown algae (kelps, Fucales) |
| Stipe | A stalk- or stem-like extension | Elevates the photosynthetic parts (fronds) | In brown algae (e.g. Fucus, Laminaria) |
| Blade / Lamina / Frond | Flattened (leaf-like) photosynthetic portions | Increase surface area for light capture and gas exchange | In many seaweeds (brown, red, green multicellular algae) |
| Pneumatocysts (Gas Bladders / Air Bubbles) | Gas-filled vesicles in the thallus | Provide buoyancy to lift the alga toward light / surface | Seen in many brown seaweeds (e.g. some Sargassum) |
| Segmented Thallus / Articulation | Thallus divided into segments | Flexibility, reduce breakage, growth control | E.g. in Halimeda (a green alga) |
| Conceptacle | Specialized cavity or chamber in the thallus | Houses reproductive organs (gametangia, sporangia) | Found in brown algae (Fucales) and in red coralline algae. |
| Cryptostomata / Caecostomata | Sterile cavities / blocked pores | May produce hairs or act as protective structure | In some Fucus species: cryptostomata produce hair tufts, caecostomata have blocked ostiole. |
| Carpogonium (and trichogyne) | Female gametangium in red algae | Receives male gametes; site of fertilization | In red algae (Rhodophyta) |
| Pyrenoid | Organelle inside chloroplast | Concentrates CO₂ around RuBisCO, enhancing carbon fixation in aquatic environments | Present in many green and red algae. |
| Phycoplast | Microtubule arrangement during cytokinesis | Guides division plane in some green algae | Seen in Chlorophyceae during cell division (as opposed to phragmoplast in land plants) |
- Text Highlighting: Select any text in the post content to highlight it
- Text Annotation: Select text and add comments with annotations
- Comment Management: Edit or delete your own comments
- Highlight Management: Remove your own highlights
How to use: Simply select any text in the post content above, and you'll see annotation options. Login here or create an account to get started.