The most commonly used equipment is inoculation needles, transfer loops, inoculation, Bunsen burner, autoclave (or pressure cooker) incubators, hot air oven centrifuge, spectrophotometer magnetic stirrer electric shaker and rotary shaker heating plate, heating mantle distillation plant, UV-lamp carbon dioxide cylinder, water-bath and a single-pan balance that has weights (for general use) chemical balance, fine analytical balance pH meters, Quebec colony counter, Laminar air flow, camera lucida electrophoresis and a high-quality microscope and many more. To perform photomicrography, a photomicrographic camera mounted in a microscope equipped with every accessory is essential in the microbiology lab.
The other requirements: A large-sized container to store disposable items is essential in a laboratory for microbiology. Cotton rolls and forceps, scissors, blades, sealing films, cellotape, aluminum foil, parafilm, enamel tray, different kinds of containers including glass marker pen, brush, rubber, pencil measuring scale, Vernier’s Calliper, etc. are employed in laboratories for microbiology. A few of the tools and equipment are described in this document.

List of Instruments used in Microbiology Lab
- Inoculation needle and inoculation loop
- Bunsen burner (Spirit lamp)
- Waterbath
- Autoclave
- Laminar Air Flow
- Incubator
- Hot air oven
- Quebec colony counter
- The pH meter
- Balance
- Spectrophotometer (Colorimeter)
- Centrifuges
- Microscope
- Incinerator
- Deep Freezer (Laboratory Refrigerators and Freezers)
- Homogenizer
- Hot plate
- Magnetic Stirrer
- Vortex Mixture/ Vortexer
- Water Distiller
1. Inoculation needle and inoculation loop
- In microbiology, inoculation loops and needles are laboratory instruments used to transfer microscopic samples of bacteria to culture medium for development and examination.
- Usually used for streaking plates, an inoculation loop is a bent wire instrument with a tiny loop at the end that scoops up liquid or semi-liquid microbial samples.
- Usually composed of heat-resistant materials such platinum, tungsten, or nichrome, inoculation loops are sterilized by running them under a flame until they are red hot.
- Perfect for choosing single colonies or doing stab cultures, inoculation needles feature a straight wire tip and are used for moving solid or dense microbiological samples.
- Since they must be sterilized before and after use to avoid cross-contamination between cultures, these instruments are absolutely necessary for aseptic methods in microbiology.
- Convenience and a less need for recurrent sterilizing are provided by disposable versions of inoculation loops and needles.1
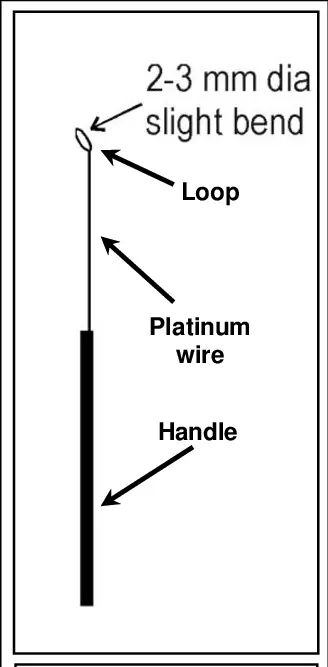
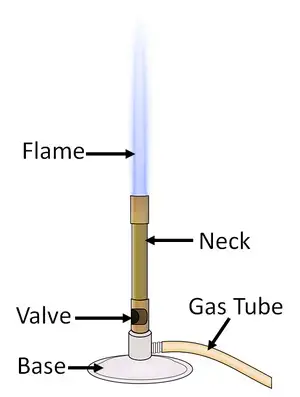
2. Bunsen burner (Spirit lamp)
Working Principle
Basic concept – it works on the principle of combustion of a fuel to produce a steady flame, the fuel may be alcohol in a spirit lamp or gas in bunsen burner, heat is generated by burning this fuel in presence of sufficient oxygen.
Fuel source – in spirit lamp ethanol or methanol is used as fuel, in bunsen burner it’s usually coal gas or LPG, the fuel is stored in a container and supplied to the burning tip.
Air mixing – air is mixed with the fuel before ignition, this is done through small holes or air inlets, this mixing helps in complete combustion, producing a hotter and cleaner flame.
Ignition – the wick in spirit lamp is soaked in fuel, in bunsen burner the gas is released through barrel and lit with match or spark, flame appears instantly.
Flame control – bunsen burner has an adjustable collar to control air intake, more air gives blue non-luminous flame which is hotter, less air gives yellow luminous flame with soot.
Heat transfer – the flame transfers heat to object placed above, in labs it’s used for heating chemicals, sterilizing tools, boiling liquids, the steady flame helps maintain constant temperature.
Safety aspect – flame must be monitored, excess air intake can cause flame blowout, fuel handling needs care to avoid spills and fires.
Key principle – combustion efficiency depends on proper fuel-air ratio, this is why adjusting air holes or wick length directly changes flame quality and heat output.
Uses
- Heating substances – it’s used to heat liquids or solids in test tubes, flasks or beakers during experiments, providing steady and controllable flame.
- Sterilizing equipment – metal tools like inoculating loops, needles, spatulas are passed through flame to kill microbes before and after use.
- Boiling liquids – water or chemical solutions are boiled for preparation or sterilization purposes.
- Evaporating solvents – solvents in solutions can be removed by gentle heating until only solid residue remains.
- Flame tests – chemicals are introduced into the flame to identify metal ions by characteristic flame color.
- Melting solids – low melting point solids are melted for analysis or preparation.
- Igniting reactions – some reactions need initial heat source for activation, bunsen or spirit flame is used to start them.
- Maintaining sterile field – in microbiology labs, bunsen flame creates updraft that helps prevent airborne contamination near work area.2
3. Water bath
Working Principle
Basic concept – it works on principle of transferring heat through water, the container with sample is placed inside the heated water so temperature stays uniform and stable.
Heating source – water is heated usually by an electric coil or sometimes gas burner under the bath, heat is passed into water by conduction which then spreads in all directions.
Temperature control – a thermostat or regulator is used, it turns heating on or off to keep water at a fixed set temperature, this avoid overheating and keeps results accurate.
Uniform heat distribution – convection currents are formed in water due to heating, these currents move the water continuously so all parts stay same temperature.
Indirect heating – sample is not in direct contact with flame or heating element, it prevents damage, burning or chemical breakdown due to too high temperature.
Thermal stability – because water has high specific heat capacity, it hold temperature for long time, small fluctuations in heating don’t affect sample much.
Safety aspect – no open flame is involved, risk of fire is reduced, also avoids sudden temperature spikes that could be dangerous.
Key principle – steady, even heating is achieved by conduction from heater to water and convection currents in water, making it ideal for lab procedures needing constant temperature.
Uses
- Heating samples gently – used when direct flame could damage chemical compounds, water provides controlled and gradual heat.
- Incubating cultures – microbiology labs use it to maintain required temperature for bacterial or fungal growth.
- Melting substances – fats, waxes, agar or gelatin are melted slowly to avoid burning or decomposition.
- Dissolving solids – helps dissolve substances in liquids by maintaining warm temperature for longer time.
- Enzyme reactions – used to keep enzyme solutions at optimum temperature for activity without denaturation.
- Thawing frozen samples – frozen biological or chemical samples are thawed slowly to preserve integrity.
- Maintaining constant temperature – ideal for experiments needing stable heat for long periods, such as chemical rate studies.
- Evaporating solvents slowly – allows solvent removal without overheating sensitive compounds.3
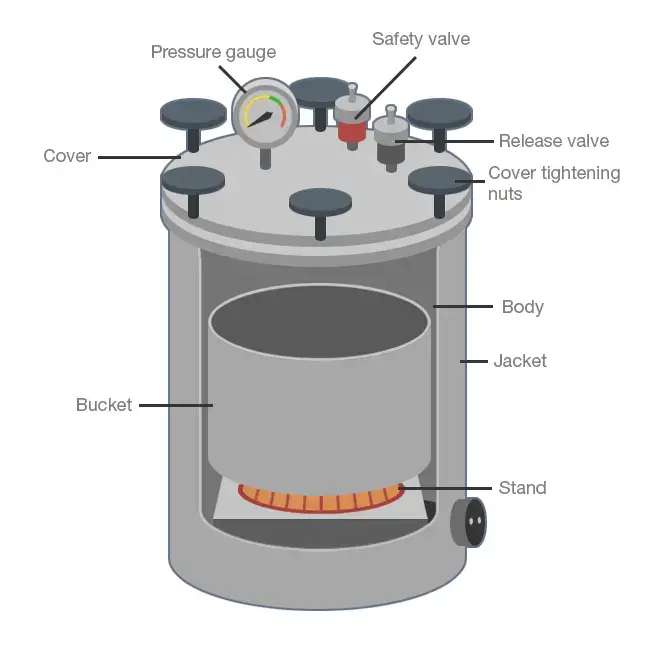
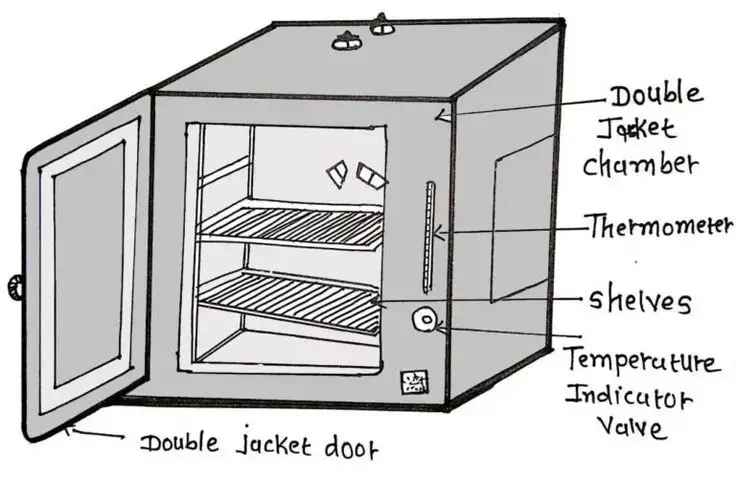
4. Autoclave
Working Principle
Basic concept – it works on moist heat sterilization where steam under high pressure kills microorganisms by denaturing proteins and breaking cell structures, making them non viable.
Steam generation – water inside chamber or a separate boiler is heated until it forms saturated steam, steam fills the inner space and pushes out the air.
Pressure build-up – because steam is trapped inside, pressure increases, normally around 15 psi above atmospheric level, this rise lets water boil at much higher temperature.
Temperature rise – the raised pressure makes steam temperature reach about 121°C or even more in some models, this hot moist heat is more effective than dry heat.
Heat penetration – steam condenses on surfaces of instruments or media, it releases large amount of latent heat instantly, this rapid heat transfer ensures all parts are sterilized evenly.
Holding time – materials are kept at required temperature for set period, usually 15 to 20 minutes, longer for large loads or highly resistant spores.
Cooling and depressurizing – after heating cycle, pressure is released slowly to avoid damage, then materials are allowed to cool down before opening lid.
Key principle – effectiveness depends on combination of high temp, correct steam saturation, right pressure and enough exposure time, any one missing reduces sterilization success.
Uses
- Sterilizing laboratory tools – glassware, pipettes, petri plates, metal forceps are sterilized so no microbes or spores remain, making them safe for reuse.
- Preparing culture media – nutrient agar, broth, selective media are autoclaved before use, this prevents unwanted contamination in experiments.
- Disposing biohazard waste – infectious lab waste, used culture dishes, contaminated gloves are treated inside autoclave before they’re thrown away.
- Sterilizing surgical instruments – scalpels, scissors, clamps, and other surgical tools are sterilized in hospitals to stop patient infections.
- Sterilizing dressings and fabrics – hospital gowns, bandages, drapes are made free from pathogens using steam under pressure.
- Sterilizing lab liquids – solutions like buffers, water for injections are processed in autoclave to remove any microbial life.
- Reusing containers safely – test tubes, flasks, bottles are sterilized so they can be used again without contamination risk.
- Research applications – essential in microbiology and molecular biology to keep cell cultures sterile, ensures experiments aren’t ruined by unwanted organisms.4
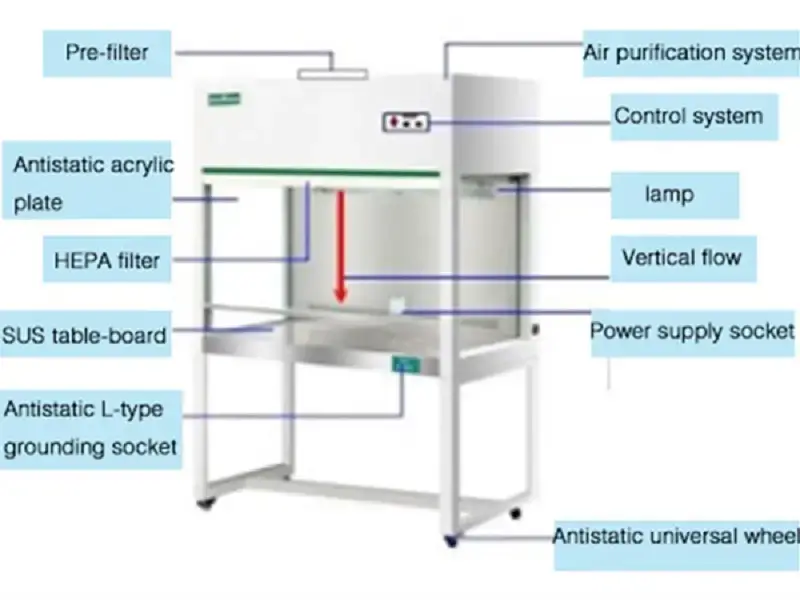
5. Laminar Air Flow
Working Principle
Basic concept – it’s based on forcing HEPA filtered air into the workspace as parallel, unidirectional streams that prevent turbulent mixing, contaminants are carried away, contamination is reduced.
Air intake – room air is drawn in by a blower, coarse particles are removed by a pre filter, air is preconditioned then routed to the main filtration stage.
Filtration process – air is passed through a HEPA filter which captures particles down to 0.3 µm at ~99.97% efficiency, capture occurs by impaction, interception and diffusion, particles and many microbes are trapped.
Laminar flow formation – clean air is expelled through a perforated plenum or honeycomb face to produce parallel low turbulence streams at controlled velocity, velocity is controlled to avoid eddies.
Air direction – directional flow is established as either horizontal or vertical, the choice then determines how the clean air sweeps over the work surface and how contaminants are displaced.
- Horizontal type – air moves from back to front, it’s good for product protection, operator may not be protected so it’s avoided for hazardous work.
- Vertical type – air moves from top to bottom, it gives a downward sweep and often better product protection in many lab setups.
Protection mechanism – a sterile field is created, clean air curtain isolates the critical zone, particles are displaced rather than mixed into the sample, product protection is achieved.
Continuous operation – blower runs continuously to maintain face velocity, pressure drop and velocity sensors are monitored, alarms warn when flow degrades and filters must be changed.
Key principle – uniform, unidirectional, low turbulence airflow combined with intact HEPA filtration and correct face velocity is what ensures a particle-free working zone, it’s dependent on filter integrity, proper installation and maintenance.
Uses
- Aseptic preparation of samples – used in microbiology and molecular biology labs to handle cultures without risk of airborne contamination.
- Media preparation – nutrient agar plates, broth tubes and other culture media are poured inside to keep them sterile.
- Plant tissue culture – protects plant explants during transfer and subculturing, preventing fungal or bacterial infection.
- Pharmaceutical compounding – sterile drugs, injections, and IV fluids are prepared in hospitals or industry inside laminar cabinets.
- Handling cell cultures – mammalian or bacterial cell lines are maintained without contamination from dust or microorganisms.
- Sterile packaging – small instruments, medical devices or research materials are packed in clean environment before sealing.
- Diagnostic testing – clinical samples like blood, swabs or other fluids are processed in sterile conditions for accurate results.
- Research experiments – any lab procedure needing particle free environment such as genetic engineering, cloning, or PCR setup is performed here.5
6. Incubator
Working Principle
Basic concept – it maintains a controlled environment of temperature, humidty, and sometimes CO₂ to support growth of microorganisms or cells, incubator working principle is based on keeping physical and chemical variables steady so experiments are reproducible.
Heating mechanism – electric heaters or water jackets supply heat, heat is transferred to chamber air and shelves by conduction and convection, temp is raised to setpoint then held, it’s done to provide uniform warmth.
Temperature regulation – temperature is sensed by probes (thermocouple or RTD), a thermostat or microprocessor controler modulates power, heating is switched on or off or PWM controlled to keep set temp within tight limits.
Humidity control – water trays or built in humidifiers add vapour, humidty is monitored so media don’t dry out, relative humidity is maintained within required range to preserve sample integrity.
Air circulation – fans or passive convection distribute warmed air, uniform conditions are produced across shelves, gradients are minimized so all cultures are exposed to same conditions.
CO₂ regulation – in CO₂ incubators, CO₂ is supplied from cylinder or generator, sensors (NDIR or others) measure concentration, CO₂ is controlled to maintain pH of bicarbonate buffered media.
- Typical setpoint – CO₂ is often kept at 5% for mammalian cell culture, ph is therefore stabilized for proper cell metabolism.
Sterility features – HEPA filtered air, UV lamps or periodic decontam cycles may be used, surfaces are cleaned and aseptic technique is applied, risk of contamination is reduced but not eliminated.
Insulation and safety – insulated walls and tight door seals reduce heat loss, alarms and over temp cutoffs are provided, samples are protected and operator safety is supported.
Key principle – precise control of temp, humidty and CO₂, plus uniform air distribution, produce a stable microenvironment, biological growth rates then become predictable and experiments can be repeated.
Uses
- Culturing microorganisms – used in microbiology labs for growing bacteria, fungi, yeast, temp is kept constant so microbial growth happens at optimum rate without outside interference.
- Cell culture work – supports mammalian or plant cell growth, CO₂ control is often added to keep pH stable in culture medium.
- Egg hatching – in poultry farms eggs are kept inside at right temp and humidty until chicks hatch, without need of parent bird.
- Biochemical testing – enzyme assays, fermentation studies or metabolic activity tests are carried out at fixed temperature for accurate results.
- Genetic research – provides stable environment for genetically modified organisms during gene expression or transformation experiments.
- Pharmaceutical stability tests – used to check drug stability, shelf life, microbial limit testing under controlled conditions.
- Food and dairy testing – milk, cheese, meat samples are incubated to detect microbial contamination levels.
- Environmental simulation – simulates climate conditions for studying microbial ecology, adaptation or stress response in controlled environment.6
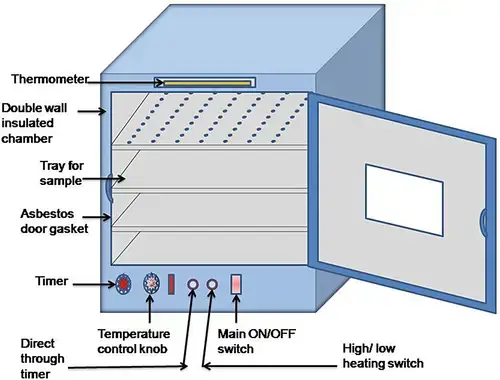
7. Hot air oven
Working Principle
Basic concept – it works on the dry heat sterilization method, hot air is circulated in a closed chamber to kill microorganisms by oxidative damage, dehydration and protein denaturation, since no moisture is involved heat transfer is slower but very effective for some materials.
Heating mechanism – electric heating elements convert electrical energy into heat, the chamber walls get hot, then heat is passed to the air inside which in turn warms the load by conduction and convection.
Air circulation – fans or natural convection move heated air around shelves, uniform distribution is needed because uneven temp zones can leave some microbes alive, movement speed is controlled to avoid temperature drop.
Temperature control – thermostat or digital controller senses chamber temperature through probes, power to heaters is switched on or off automatically, fluctuations are minimized but can still happen if door is opened mid-cycle.
Sterilization action – at about 160°C for 2 hrs or 170°C for 1 hr, the dry heat penetrates slowly but deeply into materials, it oxidizes cellular components, dehydrates cells, and causes irreversible damage to microbial enzymes and structural proteins.
Exposure time – depends on load size, wrapping, and material type, heavier or insulated loads need longer exposure to ensure all points reach sterilizing temp.
Cooling phase – after cycle ends, door must stay closed until temperature drops enough to handle items safely, sudden opening can cause burns or glassware breakage from thermal shock.
Key principle – efficiency depends on correct combination of temperature, holding time, and even air flow, all must be maintained or sterilization will fail.
Uses
- Sterilizing glassware – test tubes, petri dishes, pipettes, flasks are sterilized without moisture so they remain dry after process.
- Sterilizing metal instruments – forceps, scalpels, scissors, and other tools that can withstand high dry heat are processed here.
- Sterilizing powders – dry heat is used for powders like starch, talc, and some drugs that would clump or degrade with steam.
- Sterilizing oils and fats – substances like paraffin, glycerin, mineral oil are sterilized without risk of hydrolysis that happens in moist heat.
- Sterilizing glass syringes – reusable syringes or glass ampoules are treated to ensure they’re microbe free.
- Preparing lab materials – empty glass containers are sterilized before filling with sterile media or chemicals.
- Sterilizing sharp instruments – maintains sharpness better than steam sterilization which can cause corrosion or dulling.
- Industrial use – used in pharmaceutical, cosmetic and food industries where moisture-free sterilization is required.7
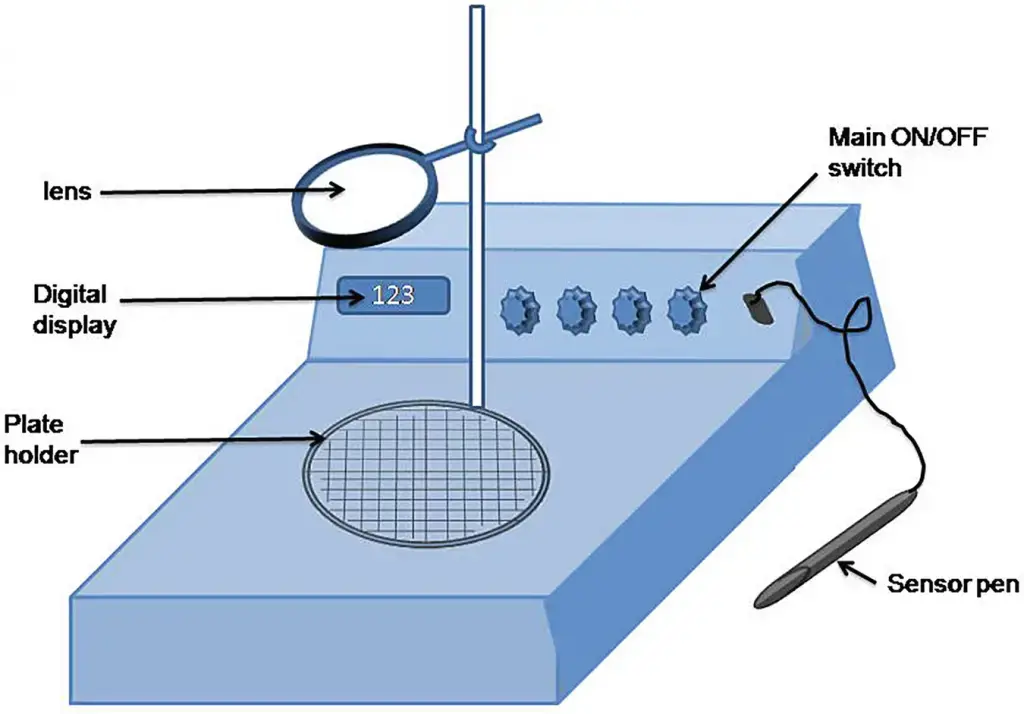
8. Quebec colony counter
Working Principle
- Definition – Quebec colony counter is a lab device used for bacterial or fungal colony enumeration, it’s widely used in microbiology cause it make counting faster, more acurate and less tiring for eyes.
- Basic concept – It works by using strong illumination from below the plate, with a clear magnifying lens on top, so colonies on agar appear bigger and more defined, this help operator to see each colony even when it’s small or very close to others.
- Illumination system – Light from LED or fluorescent lamp is passed through translucent plate holder, the bright background make colony edges sharper, so there’s less chances of missing them, even in dense cultures.
- Magnification lens – A fixed magnifier, often around 1.5x to 3x, is placed just above the plate, it reduce eye strain and allow counting for long time without fatigue, also making colony boundaries more visible.
- Grid reference – Under the plate there’s a marked grid pattern, it divides the agar into smaller zones, this let user count section by section then sum up, which lower human error in total calculation.
- Manual counting – Operator press a digital or mechanical counter button each time colony is seen, the device keep the running total displayed, so it’s easy to stop and resume without loosing track.
- Ergonomic design – Plates are kept stable during counting, so they won’t move or slip, this avoid double counting or skipping colonies by mistake.
- Accuracy principle – The combination of illumination, magnification, and grid system ensures consistent results, even if different people count the same plate, which is vital for microbiology quality control.
Uses
- Microbial enumeration: This is an important part of microbiology research, water testing, food safety analysis, and pharmaceutical quality control. It counts the number of bacterial or fungal colonies on agar plates.
- Testing the quality of water is done in public health labs to find out how many microbes are in drinking water, waste water, or swimming pool samples. This makes sure the water meets safety criteria.
- Food and drink testing is used in the dairy, meat, bakery, and beverage industries to check for contamination and make sure that hygiene is maintained during production and storage.
- Pharmaceutical analysis is used for evaluating pharmaceuticals, raw materials, and medical devices for sterility and microbiological limits before they are released to the public.
- Clinical diagnostics: In hospitals or diagnostic labs, they count pathogens in samples from patients, such as urine, sputum, or wound swabs, to help plan treatment.
- Environmental monitoring is used to check for microbes in soil, air, and surfaces in controlled conditions like cleanrooms or biosafety labs.
- Used at colleges and training institutes to teach microbiological skills and help students get better at counting colonies.
- Uses in research – Helps in microbiological research in genetics, antibiotic testing, and microbial ecology by giving precise colony counts for data analysis.8
9. The pH meter
Working Principle
A pH meter works on the principle of electrochemical potential difference between two electrodes — a glass electrode sensitive to hydrogen ion activity and a reference electrode with a constant potential.
The pH meter’s electronic circuit measures the potential difference between these electrodes and converts it into pH using the Nernst equation:
When the glass electrode is immersed in a solution, hydrogen ions interact with its special glass membrane, generating a potential (voltage) proportional to the hydrogen ion concentration.
The reference electrode provides a stable potential for comparison.

Here, E is the measured potential, and [H+] is the hydrogen ion concentration.
- In simple terms: The meter senses the small voltage change caused by hydrogen ion activity in the sample, and this voltage is directly related to pH.
Uses
- Checking the quality of water by measuring the pH in drinking water, effluent, swimming pools, and aquariums.
- Food and drink industry – Making sure that dairy, wine, beer, juices, and sauces have the right pH level.
- Soil and Agriculture Analysis- Checking the pH of the soil to make sure crops grow well and that fertilizer is used correctly.
- Pharmaceutical Industry – Keeping the pH level in drug formulations, buffer solutions, and quality control.
- Environmental Monitoring—checking the impacts of acid rain, the acidity of rivers and lakes, and industrial waste.
- Chemical and petrochemical industry: controlling the pH of chemical reactions and process streams.
- In the lab, we measure pH in experiments, culture medium, and chemical solutions.
- Cosmetics Industry – Changing the pH of skin-care and hair-care products to make them safer and more effective.9
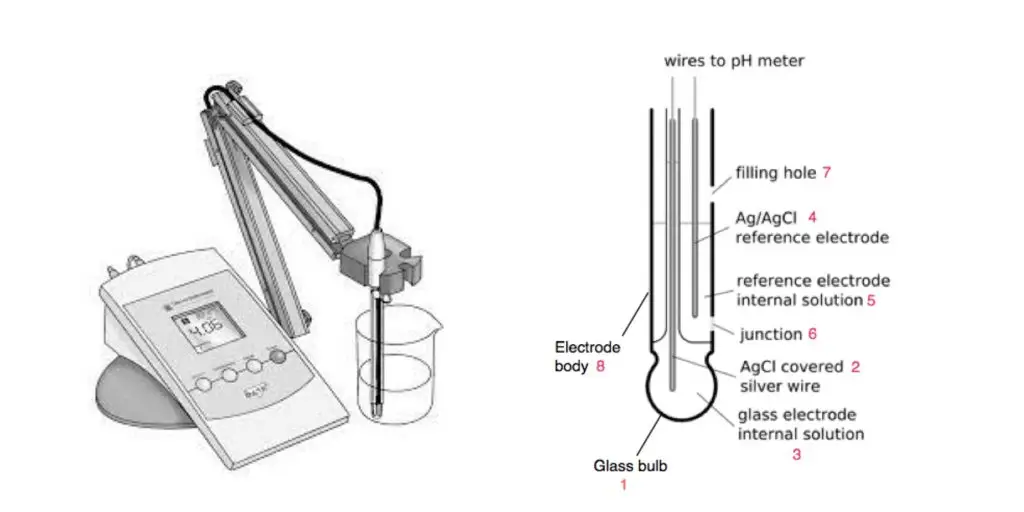
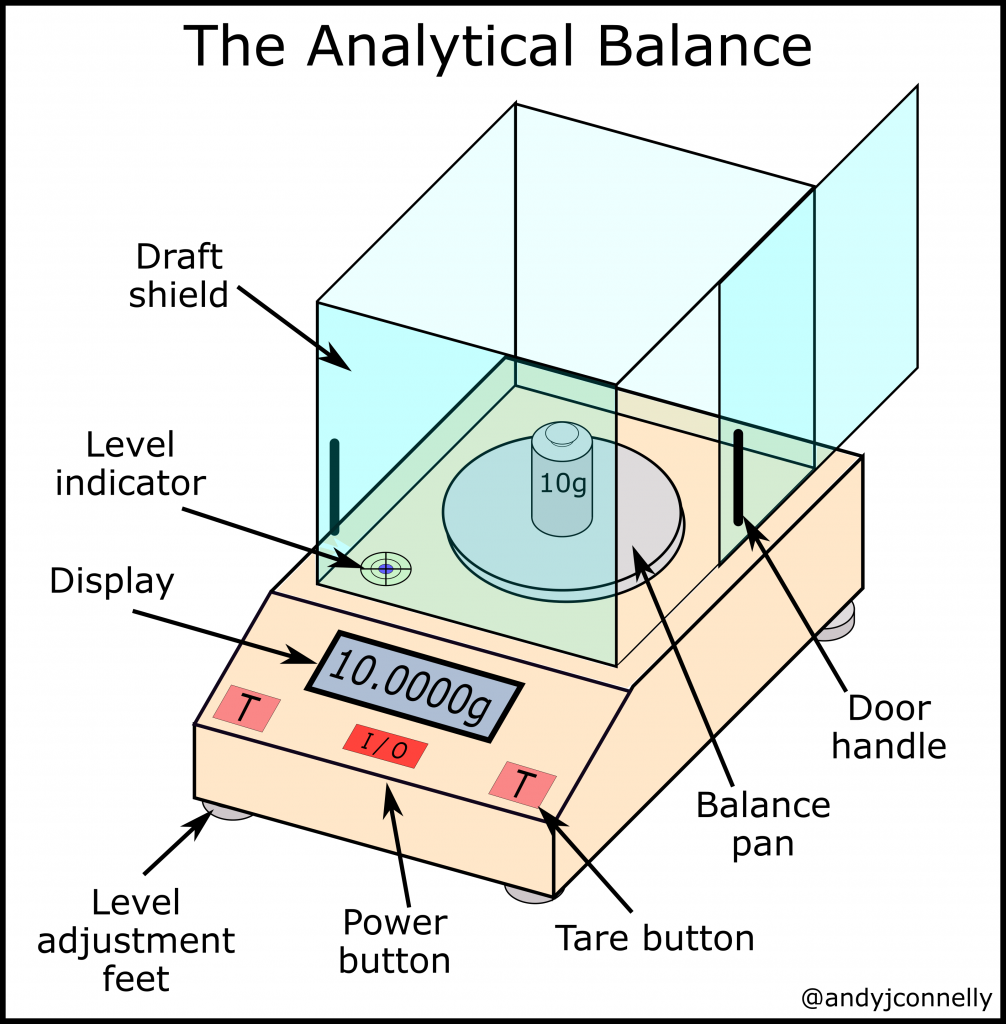
10. Balance
Working Principle
A balance works on the principle of equilibrium of moments — when the torques (rotational forces) on both sides of a pivot are equal, the beam remains horizontal, indicating equal masses.
- Traditional mechanical balance:
- Consists of a beam pivoted at its center with two pans at equal distances from the pivot.
- An unknown mass is placed on one pan and standard weights on the other.
- When the beam is in equilibrium (level), the masses are equal because weight is proportional to mass under constant gravity.
- Electronic balance:
- Uses a load cell that converts the force due to the object’s weight into an electrical signal.
- This signal is amplified and processed to display the mass digitally.
In short: Mechanical balances measure mass by comparing it to known masses, while electronic balances measure the force of gravity and convert it into a mass reading.
Uses
- Scientific Laboratories: Getting the exact amounts of chemicals, reagents, and biological samples.
- Pharmaceutical Industry: Weighing active chemicals and other substances to make drugs.
- Jewelry business: Very precise measurements of precious metals and diamonds.
- Food Industry: checking the quality of food, measuring ingredients, and controlling portions.
- Schools—Teaching how to measure mass in biology, chemistry, and physics labs.
- Industrial Uses: Measuring the raw materials used in making something.
- Environmental Studies: Weighing samples of soil, plants, and water for research.
- Forensic science is the study of things like fibers, powders, and residues that are left behind.
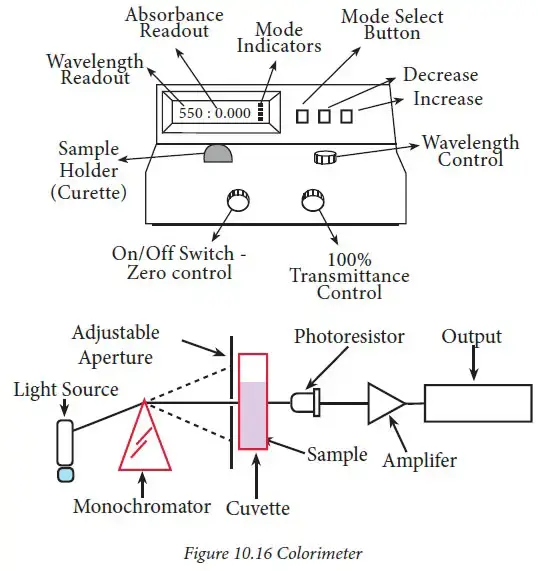
11. Spectrophotometer (Colorimeter)
Working Principle
A spectrophotometer or colorimeter works on the principle of Beer–Lambert’s law, which states that:
The absorbance of light by a solution is directly proportional to the concentration of the absorbing substance and the path length of the light through the solution.
Key idea:
- Light of a specific wavelength is passed through the sample solution.
- Molecules in the solution absorb some light, and the rest passes through to the detector.
- The amount of absorbed light (absorbance) is measured.
- Using Beer–Lambert’s law:
A=ε⋅c⋅l
Where:
- A = Absorbance (no units)
- ε = Molar absorptivity (L·mol⁻¹·cm⁻¹)
- c = Concentration of the solution (mol/L)
- l = Path length of the cuvette (cm)
In simple terms:
The instrument measures how much light is absorbed by the sample, and from this, the concentration of the substance can be determined.
Uses
- Biochemistry and Clinical Labs: Checking blood sugar, hemoglobin, cholesterol, and enzyme activity.
- Environmental Monitoring—checking the quality of water for things like nitrates, phosphates, and heavy metals.
- Pharmaceutical Industry: figuring out how much of a medicine is in a sample and making sure that the formulations are of good quality.
- Food and Beverage Industry: Checking the color, additives, and nutrient levels in products.
- Chemical Research – Looking at how fast reactions happen and how products are made.
- Microbiology: Using turbidity (optical density) to guess how fast microbes are growing.
- Forensic Science: Looking at pigments, colors, and poisonous compounds in evidence.
- Agriculture—figuring out how much nutrients are in plant extracts and soil.10
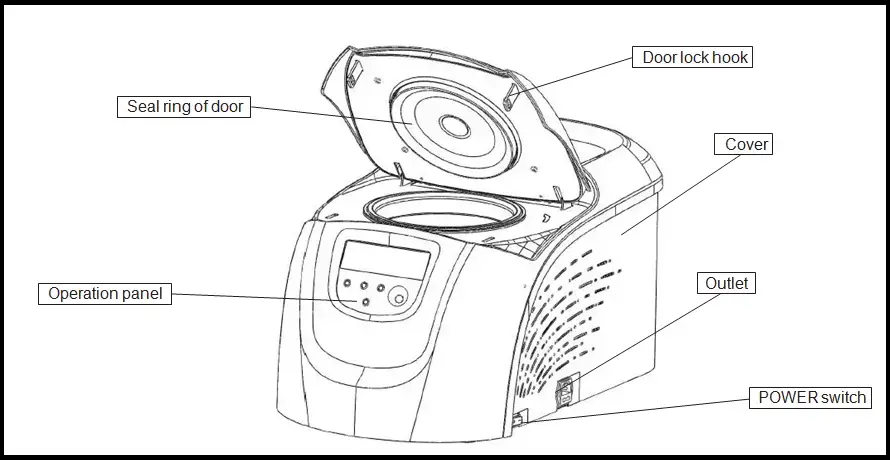
12. Centrifuges
Working Principle
- The Beer-Lambert law says that absorbance is directly proportional to concentration and path length. This means that when light of a certain wavelength passes through a solution, some of it is absorbed by the solution and the rest is transmitted. The amount of light absorbed is proportional to the concentration of the solute.
- Light Source: A light source emits light of a specific wavelength. For the visible range, a tungsten lamp is used, and for the UV range, a deuterium lamp is used. The light then goes through a monochromator or filter to get to the right wavelength.
- Sample Interaction: The monochromatic light goes through the cuvette that holds the sample solution. The solute molecules absorb certain wavelengths based on their chemical structure.
- The transmitted light hits the photodetector, which turns the light intensity into an electrical signal. Then, the absorbance or transmittance is measured.
- Output / Calculation: The instrument uses the formula A = log10(I₀/I) to figure out the absorbance (A). I₀ is the intensity of the light that hits the instrument, and I is the intensity of the light that passes through it. Then, the concentration of the solute can be found using a calibration curve or directly from the Beer-Lambert equation.
- Colorimeter Specifics: This is a simpler version that employs colored filters instead of a monochromator. It only measures absorbance at one wavelength, generally in the visible range. It is helpful for routine analysis of colored solutions.
- Spectrophotometer Advantage: It is more accurate, can scan more wavelengths, is better for quantitative analysis in UV and visible ranges, and is more sensitive than a colorimeter.
Uses
- Quantitative Estimation of Substances – it is used to measure concentration of biomolecules like proteins, nucleic acids, enzymes, vitamins, pigments, then allows precise determination of solution strength.
- Enzyme Assays – enzyme activity can be measured by following changes in absorbance of substrate or product over time, useful in biochemical and clinical labs.
- Clinical Diagnostics – it is used for measuring blood glucose, hemoglobin, bilirubin, cholesterol, then helps in disease diagnosis and monitoring.
- Water and Environmental Analysis – detects pollutants, heavy metals, nitrates, phosphates in water samples, then ensures environmental safety and compliance.
- Pharmaceutical Industry – used to check drug purity, assay of active ingredients, and concentration of formulations, then quality control can be maintained.
- Food Industry – measures color intensity, pigment concentration, and additives in food, then ensures consistency and safety of food products.
- Research and Development – used in molecular biology, chemistry, microbiology for studying reaction kinetics, DNA/RNA quantification, and chemical analysis.
- Color Matching and Quality Control – ensures consistency in dyes, paints, textiles, then standardizes color in industrial production.11
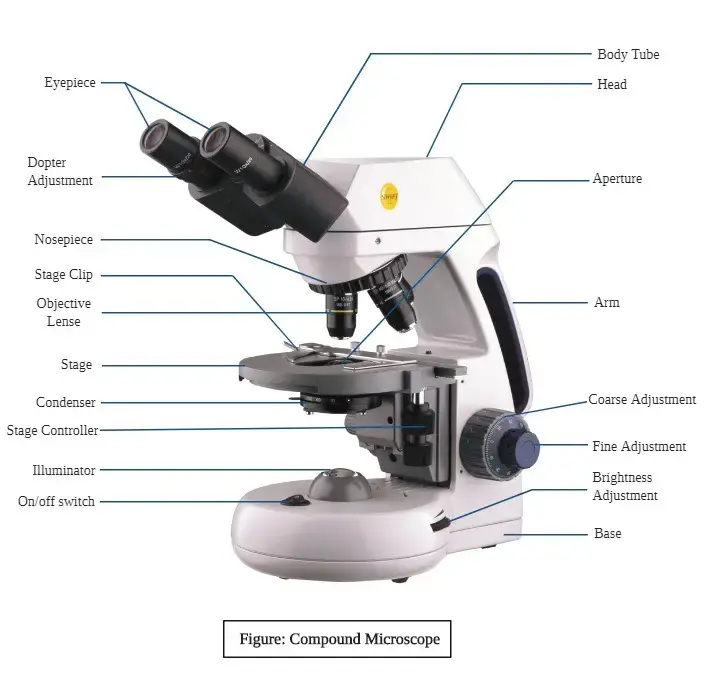
13. Microscope
Working Principle
- Working Principle of Microscope – it is based on the principle of magnification, where small objects are made visible by bending and focusing light or electrons through lenses, then the image appears larger than actual size.
- Light Microscope – uses visible light that passes through or reflects from the specimen, then lenses bend the light to form a magnified image, the total magnification is product of objective and ocular lens magnification.
- Electron Microscope – uses beam of electrons instead of light, electrons have shorter wavelength, then allows much higher resolution and magnification, images formed on fluorescent screen or detector.
- Optical Path – light from source passes through condenser to illuminate specimen, then objective lens collects light from specimen to form real image, eyepiece lens magnifies this image to form virtual image visible to eye.
- Contrast Enhancement – staining, phase contrast, or fluorescence can be used to enhance visibility of structures, then fine details of cells or tissues can be observed.
- Resolution Principle – ability to distinguish two closely spaced points depends on wavelength of illumination and numerical aperture of lens, then higher resolution gives clearer, detailed images.
- Focusing Mechanism – coarse and fine focus adjust lenses to bring specimen into sharp view, then precise observation of microscopic details is possible.
Uses
- Observation of Microorganisms – it is used to study bacteria, fungi, protozoa, algae, then identification and classification of microbes can be done.
- Cell Structure Study – allows examination of cell organelles, nucleus, cytoplasm, then understanding of cellular morphology and functions is possible.
- Medical Diagnostics – used in pathology labs to detect blood disorders, tissue abnormalities, parasites, then helps in disease diagnosis and treatment.
- Research and Education – widely used in biology, microbiology, histology for teaching and scientific investigations, then students and researchers can observe microscopic details.
- Material Science – examines metals, crystals, polymers, then microstructure, defects, and composition analysis is possible.
- Forensic Analysis – used to study hair, fibers, bodily fluids, then evidence analysis in criminal investigations is supported.
- Quality Control – inspects industrial products, semiconductors, pharmaceuticals, then ensures product consistency and defect detection.
- Environmental Studies – observes plankton, soil microorganisms, water contaminants, then helps in ecological monitoring and pollution assessment.12
14. Incinerator
Working Principle
Working Principle of Incinerator – it is based on the principle of thermal oxidation, where waste materials are burned at high temperature to convert them into ash, gases, and heat, then volume and toxicity of waste are greatly reduced.
Primary Combustion Chamber – waste is fed into chamber where it is exposed to high temperature flames, then organic matter is oxidized into carbon dioxide, water vapor, and heat energy.
Secondary Combustion Chamber – gases and particulates from primary chamber enter here, then incomplete combustion products are burned completely at higher temperature to reduce pollutants and odor.
Air Supply – controlled supply of primary and secondary air ensures complete combustion, then temperature and oxygen levels are maintained for efficiency.
Heat Recovery / Energy Utilization – heat generated can be used to produce steam, electricity, or hot water, then energy from waste is recovered.
Emission Control – flue gases pass through filters, scrubbers, or electrostatic precipitators, then particulate matter, acidic gases, and toxic compounds are minimized before release into atmosphere.
Ash Collection – remaining incombustible residue is collected as ash, then can be safely disposed or used in construction materials depending on composition.
Uses
- Medical Waste Disposal – it is used to safely burn infectious hospital waste like syringes, bandages, and pathological waste, then prevents spread of diseases.
- Hazardous Waste Management – disposes of chemical, pharmaceutical, and toxic industrial wastes, then reduces environmental contamination.
- Municipal Solid Waste Reduction – reduces volume of household and municipal waste, then saves landfill space and lowers waste management costs.
- Animal Carcass Disposal – used in farms and veterinary hospitals to dispose of dead animals safely, then prevents disease outbreak and odor problems.
- Laboratory Waste Treatment – burns biological and chemical lab waste, then ensures safe handling and reduces risk of contamination.
- Energy Recovery – heat generated can be used to produce steam or electricity, then contributes to energy generation from waste.
- Environmental Protection – minimizes release of untreated waste, then reduces soil, water, and air pollution.
- Industrial Waste Management – disposes of manufacturing residues and by-products, then maintains cleanliness and compliance with safety regulations.13
15. Deep Freezer (Laboratory Refrigerators and Freezers)
Working Principle
- Working Principle of Deep Freezer – it is based on vapor compression refrigeration, where heat is removed from the storage chamber to lower temperature far below ambient, then preserves biological samples, chemicals, and reagents.
- Refrigerant Cycle – a refrigerant circulates through compressor, condenser, expansion valve, and evaporator, then absorbs heat from inside freezer and releases it outside.
- Compressor Function – compresses low-pressure refrigerant vapor into high-pressure, high-temperature gas, then forces it through condenser coils.
- Condenser Function – high-pressure gas releases heat to surrounding environment and condenses into liquid, then heat is removed from system.
- Expansion Valve Function – liquid refrigerant passes through valve and expands into low-pressure cold vapor, then temperature drops drastically before entering evaporator.
- Evaporator Function – cold refrigerant absorbs heat from freezer interior, then interior temperature decreases and stored items are preserved.
- Temperature Control – thermostat monitors and regulates compressor operation, then maintains constant ultra-low temperature required for lab samples.
- Insulation – thick walls with high-quality insulation prevent heat ingress, then energy efficiency and stable low temperature are ensured.
Uses
- Storage of Biological Samples – it is used to preserve blood, plasma, serum, tissues, then prevents degradation and maintains sample integrity for research or clinical use.
- Preservation of Microorganisms – bacteria, viruses, fungi, and other microbial cultures can be stored at ultra-low temperatures, then ensures long-term viability for experiments.
- Chemical and Reagent Storage – stores temperature-sensitive chemicals, enzymes, antibodies, and reagents, then maintains their stability and activity.
- Pharmaceutical Storage – vaccines, insulin, and other drugs requiring low temperature can be kept safely, then ensures efficacy and shelf life.
- Molecular Biology Applications – DNA, RNA, and protein samples are preserved, then prevents enzymatic degradation and supports experimental reproducibility.
- Clinical Laboratories – preserves diagnostic kits and patient samples, then supports accurate testing and analysis.
- Research and Development – maintains experimental samples under controlled conditions, then allows long-term studies and reproducible results.
- Food and Nutrient Storage – in some labs, preserves specialized diets, culture media, and supplements, then prevents spoilage and contamination.

16. Homogenizer
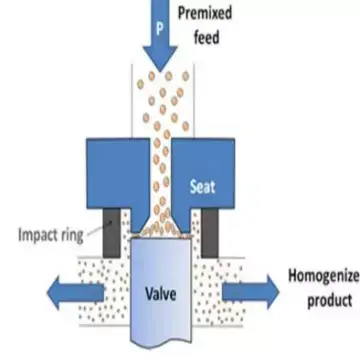
Working Principle
Working Principle of Homogenizer – it is based on mechanical disruption and size reduction, where particles or droplets in a liquid are broken down and uniformly dispersed, then creates stable and homogeneous mixtures.
Mechanical Shearing – liquid is forced through narrow gaps or orifices at high pressure, then intense shear forces break larger particles or droplets into smaller ones.
Impact and Cavitation – particles collide with surfaces or each other, and cavitation bubbles collapse, then energy from these effects further reduces particle size.
Mixing and Emulsification – solids, liquids, or immiscible liquids are dispersed evenly in the medium, then prevents settling or phase separation.
Pressure Application – high-pressure homogenizers use pressures up to 2000 bar, then ensures extremely fine particle size and uniform consistency.
Continuous Flow – liquid passes continuously through the homogenizing valve or chamber, then large batches can be processed efficiently.
Temperature Control – often cooling is applied during homogenization, then prevents heat-sensitive materials from denaturation or degradation.
Uses
- Food Industry – it is used to create uniform mixtures like milk, dairy products, sauces, and beverages, then improves texture, taste, and shelf life.
- Pharmaceutical Industry – disperses active ingredients in suspensions, emulsions, and creams, then ensures uniform dosage and product consistency.
- Biotechnology and Research – breaks cells to release intracellular contents like proteins, enzymes, and DNA, then supports extraction and analysis.
- Cosmetic Industry – produces lotions, creams, and gels with smooth, uniform texture, then enhances product quality and stability.
- Chemical Industry – used for making paints, inks, and adhesives with consistent particle size, then prevents settling and improves performance.
- Microbiology – homogenizes tissue or microbial samples, then aids in sample preparation for culturing or analysis.
- Environmental Analysis – prepares soil, sludge, or water samples for testing, then ensures representative sampling and accurate results.
- Nanotechnology and Emulsions – produces nanoemulsions and fine dispersions, then improves solubility, bioavailability, and stability of compounds.14
17. Hot plate
Working Principle
- Working Principle of Hot Plate – it is based on electrical resistance heating, where electric current passes through a resistive element, then converts electrical energy into heat energy, which is transferred to the surface and sample.
- Resistive Heating Element – a coil or plate of high-resistance material heats up when current flows, then generates uniform heat across the surface.
- Heat Transfer – heat is conducted from the plate to the container or sample in direct contact, then allows controlled heating of liquids or solids.
- Temperature Control – thermostat or digital controller regulates current to maintain desired temperature, then prevents overheating or underheating.
- Magnetic Stirring (Optional) – some hot plates include built-in magnetic stirrer, then rotates a magnetic bar inside the container for uniform mixing during heating.
- Safety Features – thermal cut-off or overheat protection prevents damage or accidents, then ensures safe laboratory operation.
- Applications – suitable for heating solutions, melting solids, chemical reactions, and sample preparation in labs, then provides steady and controllable heat source.
Uses
Heating Solutions – it is used to heat liquids in beakers, flasks, and test tubes, then allows chemical reactions or sample preparation.
Melting Solids – melts wax, paraffin, or low-melting-point compounds, then aids in experimental procedures or sample processing.
Evaporation – helps evaporate solvents from solutions, then concentrates samples for further analysis.
Chemical Reactions – provides controlled heat for organic or inorganic reactions, then ensures reproducible reaction conditions.
Laboratory Experiments – used in educational labs for demonstrations and practicals, then supports teaching of heating and reaction principles.
Sample Preparation – heats media, buffers, or reagents before use, then ensures proper experimental conditions.
Magnetic Stirring – combined with magnetic stirrer, it allows uniform mixing during heating, then prevents localized overheating or sedimentation.
Industrial and Research Applications – used in small-scale synthesis, formulation, and testing, then provides reliable heating for lab-scale operations.15
18. Magnetic Stirrer
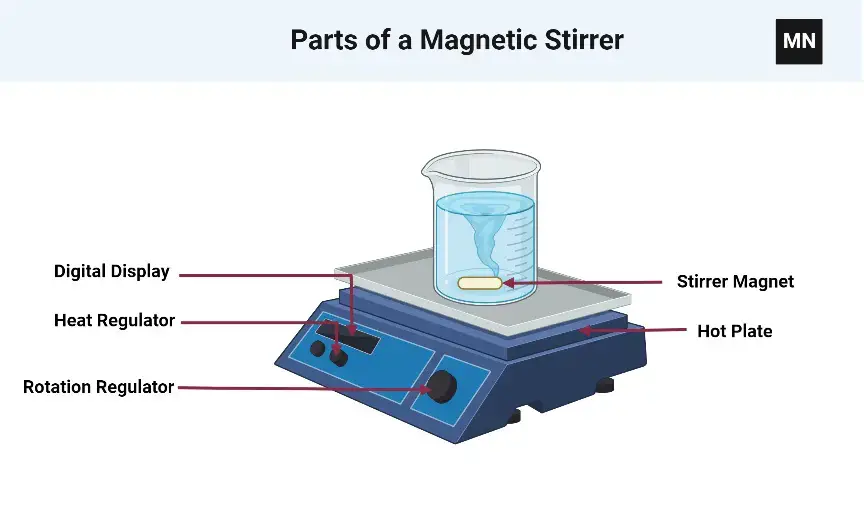
Working Principle
Working Principle of Magnetic Stirrer – it is based on magnetic induction, where a rotating magnetic field beneath the platform causes a magnetic stir bar immersed in the liquid to spin, then mixes the solution uniformly without direct contact.
Magnetic Field Generation – electric current passes through coils or rotates a permanent magnet under the plate, then creates a rotating magnetic field.
Stir Bar Rotation – a small coated magnetic bar placed inside the container aligns with the magnetic field, then rotates synchronously to stir the liquid.
Speed Control – variable speed controller adjusts rotation rate of magnetic field, then allows gentle to vigorous mixing depending on requirement.
Heat Integration (Optional) – some magnetic stirrers include heating element beneath platform, then allows simultaneous stirring and heating of the solution.
Non-Contact Mixing – stir bar does not touch motor directly, then reduces contamination risk and allows sterile or closed system mixing.
Versatility – works with beakers, flasks, or other containers, then suitable for chemical reactions, solution preparation, and sample homogenization.
Uses
- Mixing Solutions – it is used to uniformly mix liquids, buffers, and reagents, then ensures homogenous solutions for experiments.
- Chemical Reactions – provides continuous stirring during reactions, then improves reaction kinetics and consistency.
- Dissolving Solids – helps dissolve powders, salts, or other solutes in liquids, then speeds up preparation of solutions.
- Sample Homogenization – ensures even distribution of components in suspensions, emulsions, or colloids, then prevents settling or phase separation.
- Heating and Stirring – combined models allow simultaneous heating and stirring, then supports reactions requiring controlled temperature and mixing.
- Laboratory Demonstrations – used in educational labs for showing mixing, reactions, and solution preparation, then facilitates practical learning.
- Biological Applications – gently stirs cell cultures, microbial suspensions, or enzyme solutions, then maintains uniform conditions without contamination.
- Environmental and Analytical Studies – mixes soil, water, or chemical samples for testing, then ensures representative sampling and accurate analysis.16
19. Vortex Mixture/ Vortexer
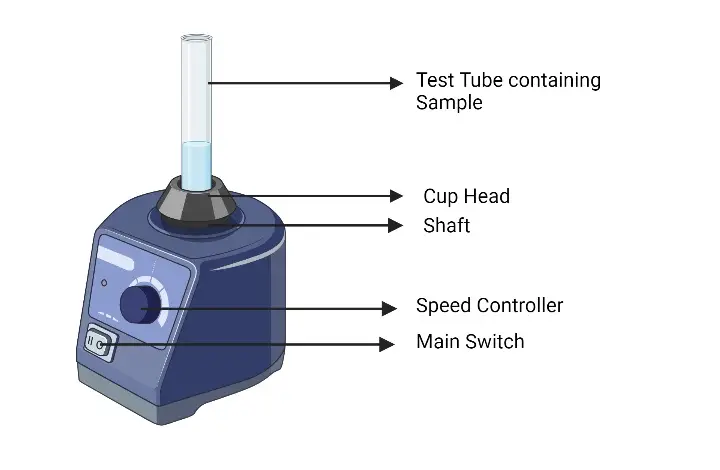
Working Principle
Working Principle of Vortex Mixer / Vortexer – it is based on rapid circular motion, where a motor-driven platform oscillates in a circular or orbital motion, then creates a vortex in the liquid to mix samples quickly and efficiently.
Oscillating Platform – the platform or cup holder moves in small circular or orbital paths, then imparts kinetic energy to the container placed on it.
Vortex Formation – when a test tube or small container touches the moving platform, liquid inside swirls and forms a vortex, then ensures thorough mixing of contents.
Speed Control – variable speed dial adjusts rotation intensity, then allows gentle or vigorous mixing depending on sample type.
Touch or Continuous Mode – can operate in touch mode (mixing only when pressed) or continuous mode (mixing continuously), then provides flexibility for different experimental needs.
Non-Destructive Mixing – no direct mechanical stirring inside the liquid, then minimizes risk of contamination or sample damage.
Versatility – works with test tubes, microcentrifuge tubes, small flasks, then suitable for biological, chemical, and clinical sample preparation.
Uses
Mixing Small Volumes – it is used to quickly mix small amounts of liquids in test tubes, microcentrifuge tubes, or vials, then ensures uniform sample preparation.
Dissolving Solids – helps dissolve powders, salts, or reagents into liquids, then speeds up solution preparation.
Sample Homogenization – ensures even distribution of components in suspensions, emulsions, or cell suspensions, then prevents settling or phase separation.
Biological Applications – mixes bacterial cultures, enzymes, or DNA/RNA samples gently, then maintains sample integrity for experiments.
Chemical Reactions – provides rapid mixing for small-scale reactions, then improves reaction consistency and efficiency.
Laboratory Assays – used in immunoassays, ELISA, and other diagnostic tests, then ensures proper reagent mixing and reliable results.
Preparation for Centrifugation – mixes samples before centrifugation, then prevents stratification and ensures accurate separation.
Clinical and Diagnostic Labs – mixes blood, serum, or other clinical specimens, then supports accurate testing and analysis.17
20. Water Distiller
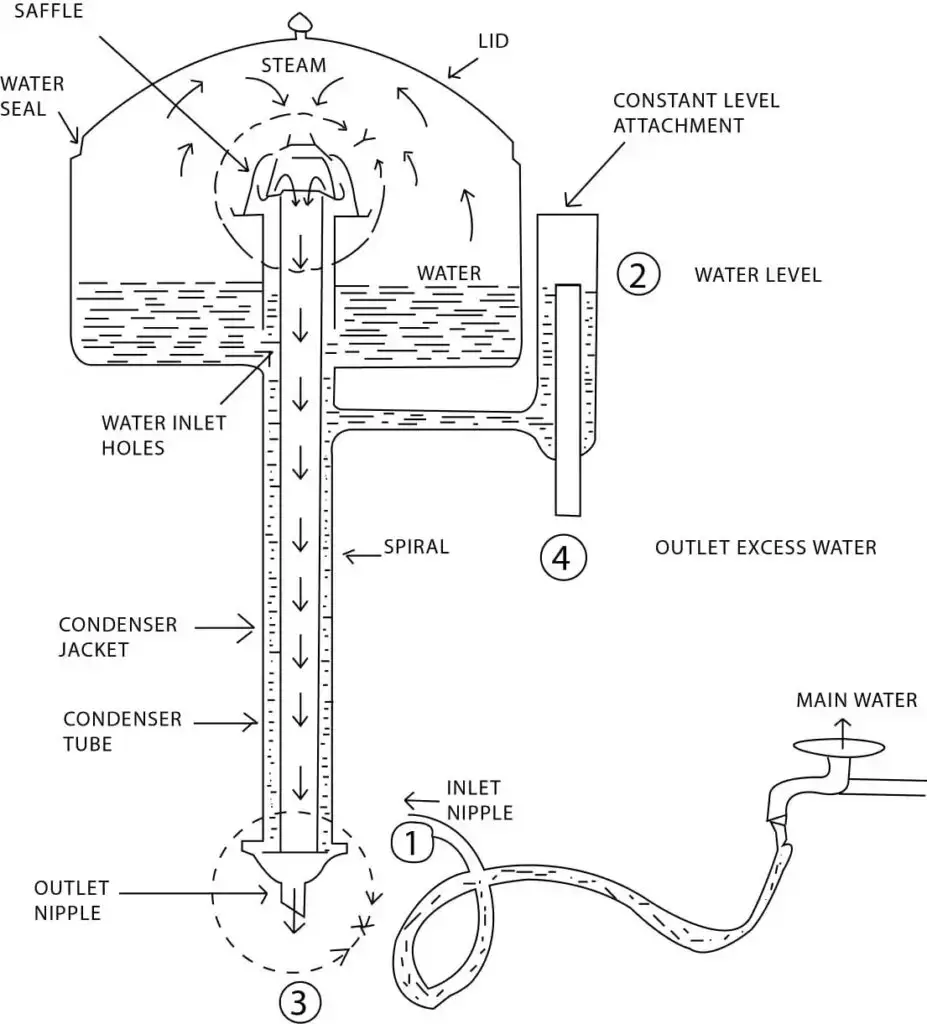
Working Principle
Working Principle of Water Distiller – it is based on the principle of evaporation and condensation, where impure water is heated to produce vapor, then condensed back into liquid form to obtain purified water.
Boiling / Evaporation – water is heated in a boiling chamber until it vaporizes, then dissolved salts, minerals, and most impurities are left behind.
Vapor Rise – water vapor rises from the boiling chamber, then carries no solid impurities or contaminants.
Condensation – vapor passes through a cooling coil or condensing chamber, then loses heat and changes back into liquid form.
Collection – condensed pure water is collected in a separate container, then ready for use as distilled water.
Heating Source – electric or gas heater provides controlled heat, then ensures continuous and efficient distillation process.
Optional Filtration – some distillers include pre-filters to remove large particles, then reduces load on boiling and improves efficiency.
Purity Assurance – separates water from salts, heavy metals, microorganisms, and many organic compounds, then produces safe and contaminant-free distilled water.
Uses
- Laboratory Use – it is used to produce high-purity water for experiments, reagent preparation, and analytical procedures, then prevents contamination and ensures accurate results.
- Medical and Pharmaceutical Applications – provides sterile water for injections, cleaning instruments, and preparing solutions, then ensures patient safety and product quality.
- Industrial Processes – used in boilers, cooling systems, and manufacturing that require mineral-free water, then prevents scaling and equipment damage.
- Drinking Water – produces purified drinking water by removing salts, heavy metals, and microorganisms, then ensures safe consumption.
- Cosmetic and Personal Care – supplies purified water for creams, lotions, and other formulations, then maintains product quality and stability.
- Electronics and Laboratory Equipment – provides deionized or distilled water for cleaning sensitive equipment, then prevents mineral deposits and corrosion.
- Research Applications – used in molecular biology, chemistry, and microbiology for preparing buffers, media, and solutions, then ensures reproducibility and accuracy.
- Food and Beverage Industry – produces clean water for beverages, cooking, and processing, then improves taste and safety of products.18