Ziehl–Neelsen staining is the differential staining technique that is used for demonstrating the acid-fast bacteria. It is the method developed first by Ziehl and later modified by Neelsen, and it is the reason why this staining procedure is referred to as Ziehl–Neelsen stain. It is the hot method of Acid-Fast staining because heat is applied during staining, and the use of acid-alcohol as the decolorizing agent gives it the name Acid-Fast stain. It is mainly used for microorganisms which are not easily stained by the basic stains and the organisms that possess a thick waxy cell wall made up of mycolic acid. These cell wall components do not allow penetration of simple stains, therefore harsher treatment is required to penetrate the dye.
In this staining method, the smear is first flooded with carbol-fuchsin, and heat is applied so that the stain penetrates the hydrophobic cell wall. After cooling, the dye remains trapped within the lipid rich wall and this property is referred to as acid-fastness. When the smear is treated with acid-alcohol, the non-acid-fast cells are decolorized immediately but the acid-fast bacteria retain the primary red stain. A counterstain such as methylene blue is then applied, so the background material and non-acid-fast organisms appear blue while the acid-fast bacilli appear red. Mycobacterium spp., Nocardia, Actinomycetes, Cryptosporidium, and some fungi show such acid-fast characteristics because of the presence of mycolic acid in their cell wall.
Ziehl–Neelsen staining is used mostly for rapid microscopic detection of Mycobacterium tuberculosis and other acid-fast organisms, especially in clinical samples. It is a simple method that helps in differentiating acid-fast and non-acid-fast organisms for identification and examination.
Objective of Ziehl-Neelsen Staining (ZN Staining)
The main objective of this stinging method is to differentiate between the acid-fast and non-acid fast bacteria. It also used to stain Mycobacterium species.
Principle of the Ziehl-Neelsen Staining (ZN Staining principle)
The principle of Ziehl–Neelsen staining is based on the presence of a thick waxy cell wall in acid-fast bacteria. It is the process where the organisms like Mycobacterium spp. that contain high lipid content made up of mycolic acid (β-hydroxycarboxylic acids) are stained by using carbol-fuchsin with heat. The cell wall is hydrophobic and impermeable, therefore simple stains do not penetrate the wall and harsh treatment is required to allow the entry of the dye. It is the heat and phenol that helps in softening the lipoidal complex, allowing the primary stain to enter the cell.
In this staining method the smear is first flooded with carbol-fuchsin, and the heat is applied until steaming. This helps in driving the stain deep into the cell wall and forms a strong dye-lipid complex. After cooling, the waxy layer becomes rigid again and traps the stain inside the cell. The smear is then treated with acid-alcohol, which acts as the decolorizing agent. The acid-fast bacteria retain the red color because the dye complex is not removed easily, but the non-acid-fast bacteria lose the primary stain as they do not have the waxy lipid layer.
A counterstain such as methylene blue is added at the end. The non-acid-fast organisms take up the blue color, while the acid-fast bacilli remain bright red due to the retained carbol-fuchsin. This is referred to as the hot method of Acid-Fast staining and it is used for identifying organisms like Mycobacterium tuberculosis and Mycobacterium leprae for microscopic examination.
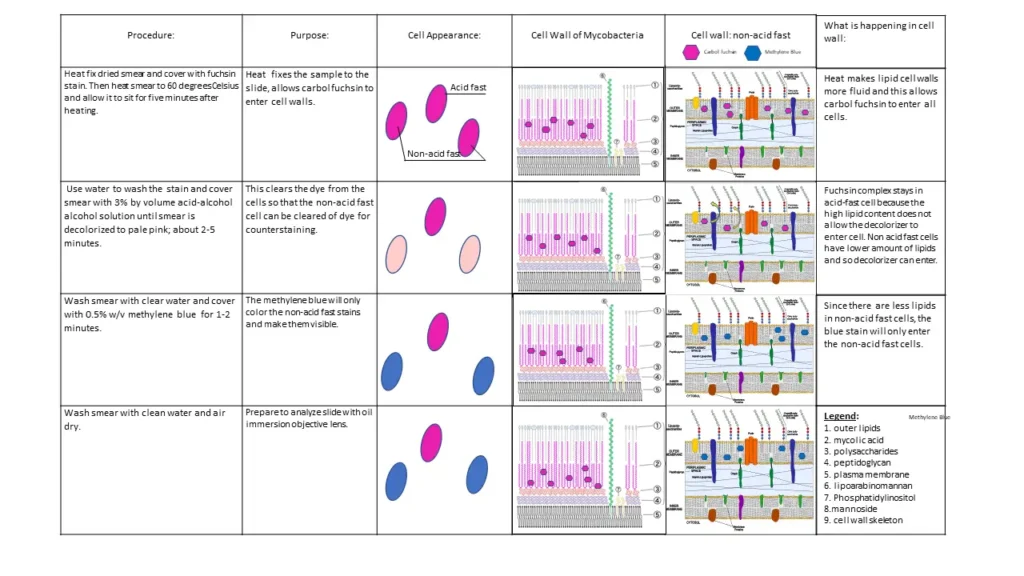
Requirement for Ziehl-Neelsen Staining
- Carbol-fuchsin (Primary stain).
- Acid-alcohol or 20% sulphuric acid (Decolorizer).
- Methylene blue dye or malachite green (Counterstain).
- Distilled water for preparing and rinsing the smear.
- Basic fuchsin (1 g) used for making the primary stain.
- Ethyl alcohol (100% ethanol, 10 ml) for dissolving the dye.
- Phenol crystals (5 ml) required for preparing the carbol-fuchsin solution.
- Acid-alcohol reagent prepared by mixing 95 ml ethyl alcohol + 2 ml distilled water + 3 ml concentrated hydrochloric acid.
- 0.25% methylene blue prepared by mixing methylene blue (0.25 g) + distilled water (99 ml) + acetic acid (1 ml).
- Clean grease-free microscopic slides for smear preparation.
- Heating source such as a spirit lamp or Bunsen burner for steaming the primary stain.
- Distilled water for gentle rinsing between each step of the staining.
- Oil immersion microscope for examining acid-fast bacilli under 1000× magnification.
Ziehl Neelsen Stain Steps/Procedure
Ziehl–Neelsen staining is the hot method of acid-fast staining. It is used to detect acid-fast bacilli (AFB) which retain the primary stain even after strong decolorization. It is mainly done for specimens like sputum and FNAC materials.
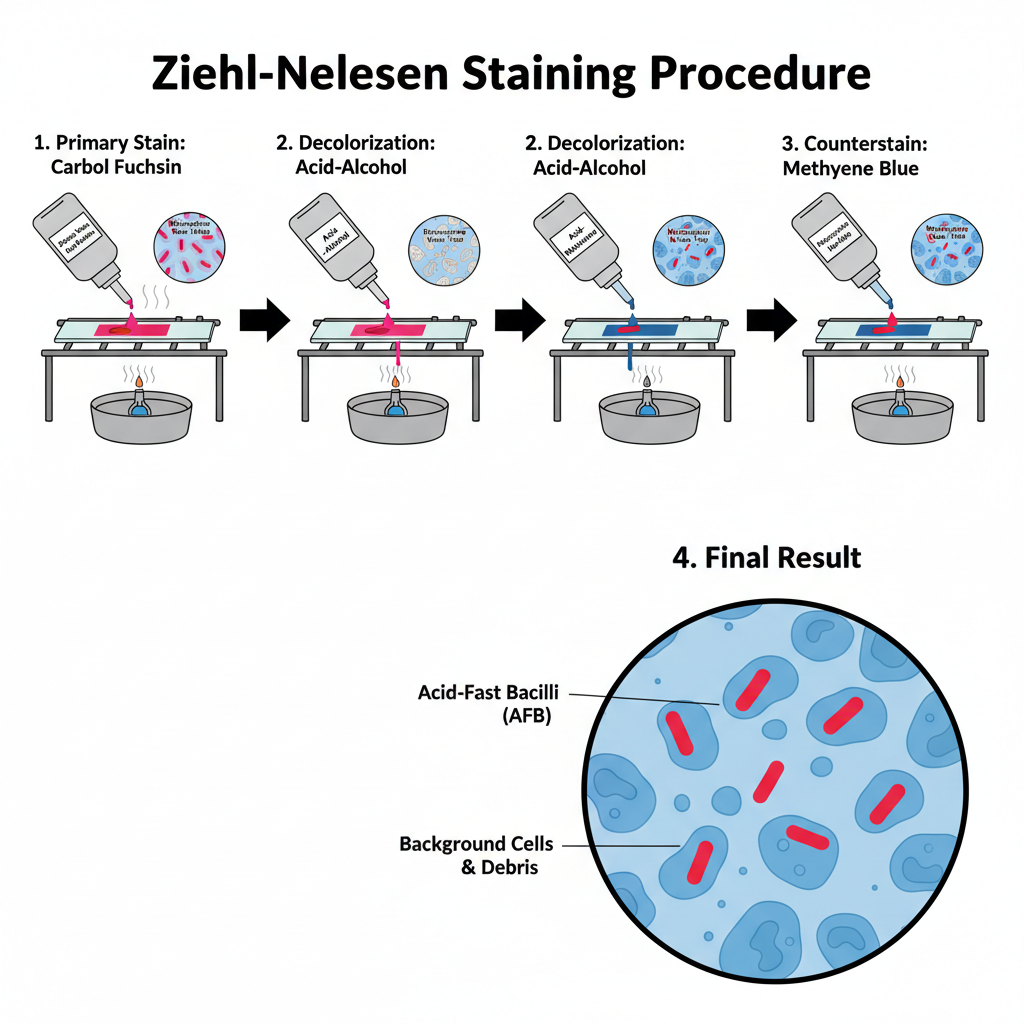
Step 1- Smear Preparation and Fixation
- Preparation of smear– It is prepared on a clean glass slide. A mucopurulent or blood-stained portion of the sample is taken for making the smear.
- Spreading– The material is spread thinly over an area of about 1.5–2 cm × 2–3 cm. A good smear is thin and it can be seen through.
- Air drying– The smear is allowed to air dry completely. This is done without keeping the slide directly in sunlight or flame.
- Heat fixation– The organisms are killed and the smear is fixed to the slide. It is done by passing the backside of the slide over flame 2–3 times or by keeping the slide on slide warmer (65°C–75°C) or by heating at 80°C for 15 minutes.
- Cooling– The slide is cooled before staining.
Step 2- Primary Staining (Carbol Fuchsin)
- Filtering of stain– Carbol fuchsin stain is filtered before use.
- Flooding of smear– The fixed smear is kept on a staining rack and completely covered with carbol fuchsin (0.3%).
- Heating– The underside of the slide is gently heated till steam rises. It should not be boiled or dried.
- Steaming– The steaming is maintained for 5 minutes. Some procedures use 10 minutes but the stain must remain moist.
- Rinsing– The slide is washed gently with clean water until no colour appears in the runoff.
Step 3- Decolorization (Acid–Alcohol)
- Decolorizer addition– The smear is flooded with 3% acid-alcohol.
- Reaction time– It is kept for 2–3 minutes. This step is repeated until the smear becomes faint pink and the washing becomes colourless.
- Rinsing– The slide is again washed with water.
Step 4- Counterstaining and Examination
- Counterstain– The smear is covered with methylene blue stain (0.3%).
- Time– It is kept for about 1 minute or upto 20–30 seconds.
- Final wash– The slide is washed with water until clear.
- Drying– The stained slide is dried in air. It should not be wiped.
- Microscopy– The smear is examined under oil immersion objective (100×) giving total magnification of 1000×.
- Results– Acid-fast bacilli appears bright red or pink rods against blue background. At least 300 fields are examined before a negative report is given.
| Reagent | Acid Fast | Non-Acid Fast |
| Carbol Fuchsin with heat | Red (Hot Pink) | Red (Hot Pink) |
| Acid Alcohol | Red | Colorless |
| Methylene Blue/Malachite Green | Red | Blue/Green |
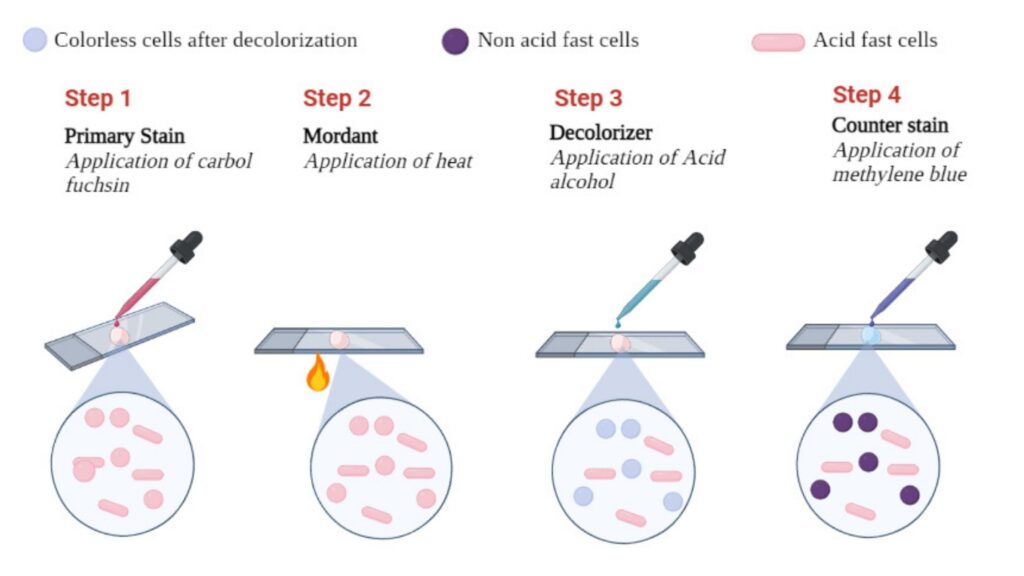
Result of Ziehl-Neelsen Staining or acid-fast staining
Microscopic Appearance
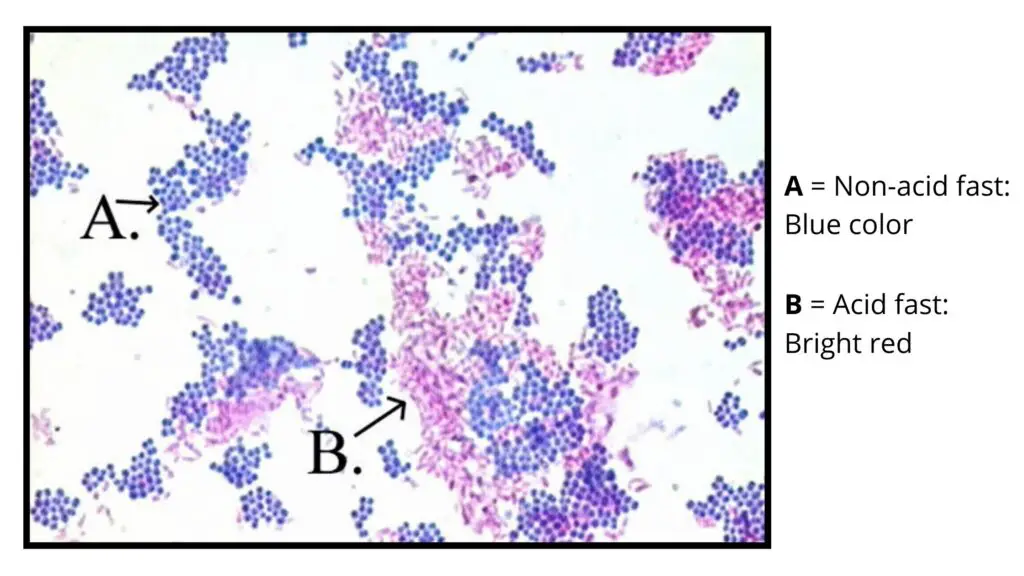
In Ziehl–Neelsen staining, the result is based on the retention of the primary stain. Acid-fast organisms keep the carbol fuchsin dye even after decolorization. Non-acid-fast organisms take the counterstain.
Acid-fast bacilli (AFB)
These appear as bright red or pink rods. They may be straight or slightly curved. Sometimes they show deeply stained portions which are referred to as beads. These organisms include Mycobacterium tuberculosis and other members of this group.
Non–acid-fast bacteria
These organisms lose the primary stain during decolorization and take the methylene blue stain. They appear blue in the background. If malachite green is used, the background is green.
The smear is examined under oil immersion (100× objective) where the background shows blue structures while AFB remain red.
Basis of Differentiation
The result depends on the presence of mycolic acid in the cell wall.
- Primary staining
The heated carbol fuchsin enters all cells. At this time all cells become red. - Decolorization
Acid-fast cells resist acid-alcohol due to high mycolic acid content. They remain red. Non–acid-fast cells become colorless. - Counterstaining
The counterstain colours the decolorized cells blue. Thus a contrast is produced.
Reporting of Results
The reporting is done by observing several microscopic fields under oil immersion. It is expressed in a semi-quantitative way depending on the number of AFB seen.
Some of the common grades are–
- Negative – No AFB seen in 300 fields.
- Doubtful – 1–2 AFB in 300 fields.
- 1+ – 1–9 AFB in 100 fields.
- 2+ – 1–9 AFB in 10 fields.
- 3+ – 1–9 AFB in every field.
- 4+ – More than 9 AFB in every field.
These grades indicate the bacterial load present in the sample.
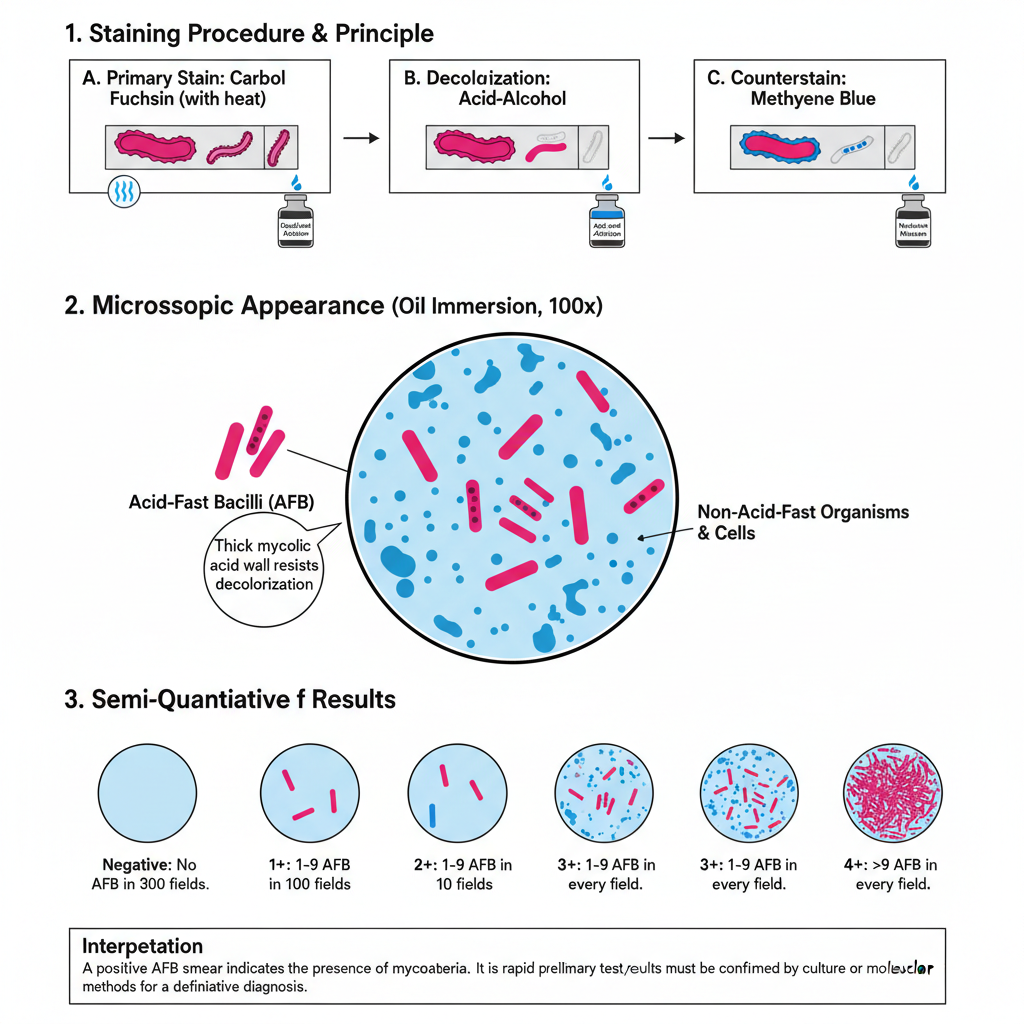
Interpretation
Acid-fast bacilli indicates the presence of mycobacteria. But the test cannot differentiate between Mycobacterium tuberculosis and other non-tuberculous mycobacteria. It is the rapid and preliminary method only.
Loss of acid-fastness sometimes occurs in dormant bacilli. These organisms may remain viable even when they do not appear red which may give low counts in smear.
Thus, any positive AFB result must be confirmed by culture or other diagnostic tests.
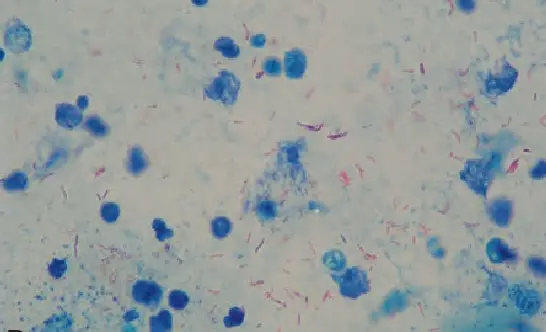
Modified Ziehl Neelsen Staining Technique
The modified Ziehl–Neelsen staining technique refers to the different changes made to the conventional hot ZN method in order to increase safety, sensitivity, and applicability for specific organisms. It is the process where the basic principle of acid-fast staining is kept same, but certain steps or reagents are altered so that the staining becomes simpler or more suitable for particular samples. One of the important modification is the Kinyoun cold staining method. It is a technique where heating of the slide is not done. The primary stain in this method contains higher concentration of basic fuchsin and phenol, so the dye penetrates the cell wall without heat. It is safer because no phenol fumes are produced and there is no risk of flame or slide breakage. Many recent studies show that Kinyoun technique can show higher sensitivity for acid-fast bacilli detection, especially in FNAC materials, making it suitable for laboratories with limited facilities.
Another type of modification is the concentrated ZN staining. In this method, the sputum sample is first digested and decontaminated and then centrifuged to concentrate the bacilli before preparing the smear. It is the procedure used when the sample contains low number of organisms. The concentration step increases the chance of finding AFB, and it improves the sensitivity of smear microscopy. It is more helpful in paucibacillary cases where the direct ZN smear often gives negative result. There is also a specific modified ZN method for Mycobacterium leprae. The cell wall of M. leprae is more easily decolorized, so a weaker decolorizing agent like 5% sulfuric acid is used. This helps in retaining the red colour of carbol fuchsin in these organisms.
There are other improvements like fluorescent staining using Auramine O or Auramine–Rhodamine which are not exactly ZN stains but are considered improved alternatives. These use fluorescence microscopy and are more sensitive and allow rapid examination at lower magnification. Because of this higher sensitivity, WHO recommends LED fluorescent microscopy in high-burden areas. Thus, the term modified Ziehl–Neelsen staining includes these different approaches which improve the detection of acid-fast organisms while keeping the basic principle same.
Examples of Acid-fast
- Mycobacterium tuberculosis which is the main causative agent of pulmonary tuberculosis and shows bright red acid-fast bacilli in sputum smear.
- Mycobacterium leprae which causes leprosy and is demonstrated by the modified Ziehl–Neelsen method in slit skin smear.
- Members of Mycobacterium genus in general because these organisms contain mycolic acids in their cell wall and remain acid-fast.
- Non-tuberculous mycobacteria (NTM) like Mycobacterium avium complex, M. bovis, M. phlei, M. abscessus, M. intracellulare, and M. fortuitum.
- Nocardia species which also show partial acid-fastness with weaker decolorizing agents like 1% sulfuric acid.
- Some Corynebacterium species and a few bacterial endospores that may appear acid-fast under modified conditions.
- Cryptosporidium oocysts which are detected due to their acid-fast property in stool smears.
- Cystoisospora (Isospora) oocysts which also stain acid-fast.
- Cyclospora oocysts that retain the stain and can be visualized in intestinal samples.
- Histoplasma species which may show acid-fast characteristics in certain preparations.
- Eggs of Schistosoma species where the ZN stain can highlight structural features useful for diagnosis.
- Paragonimus eggs which are detected in sputum smears in endemic regions and can show acid-fast staining.
Applications of Ziehl-Neelsen Staining
- It is used for the identification of acid-fast bacilli (AFB) mainly belonging to Mycobacterium species and also some Nocardia species.
- It is the differential staining method that clearly distinguishes acid-fast organisms which appear red from the non–acid-fast organisms appearing blue.
- It is applied for the rapid diagnosis of pulmonary tuberculosis because the smear gives early indication of Mycobacterium tuberculosis infection.
- It is used in extrapulmonary tuberculosis diagnosis by staining materials like FNAC of lymph nodes, gastric washing, and bronchoalveolar lavage.
- It helps to detect non-tuberculous mycobacteria (NTM) including organisms of Mycobacterium avium complex.
- It is used for assessing infectiousness as the smear grading correlates with the bacterial load, helping in deciding isolation and public health measures.
- It is used to monitor the treatment progress of tuberculosis patients by comparing smear findings during therapy.
- A modified ZN method is used in diagnosing leprosy by detecting Mycobacterium leprae in slit skin smears and also helps in classifying the disease based on bacillary load.
- It is applied in detecting coccidian parasites like Cryptosporidium, Cystoisospora, and Cyclospora whose oocysts show acid-fast property.
- It can be used to demonstrate some fungal and parasitic structures, like Histoplasma and the characteristic features in eggs of Schistosoma species in stool and urine samples.
Limitations of Ziehl-Neelsen Staining
- It has limited sensitivity and requires a high number of bacilli (about 5,000–10,000 AFB/ml) to give a positive smear, so many paucibacillary cases remain negative.
- It gives low diagnostic yield in extrapulmonary tuberculosis because these samples usually contain fewer organisms than sputum.
- It cannot differentiate between viable and dead organisms, so smear may remain positive even when culture becomes negative during treatment.
- It cannot identify the species of mycobacteria because all mycobacteria are acid-fast, and NTM also appear red, so further tests are always needed.
- The hot method requires heating of carbol fuchsin which produces phenol fumes, fire hazards, and may break slides, making the technique unsafe and cumbersome.
- The primary stain fades quickly in direct sunlight or high temperature and humidity, which may convert positive smears into negative on storage.
- Loss of acid-fastness occurs in dormant bacilli (Koch’s paradox), so these organisms may not stain and the bacterial load appears lower than actual.
- It needs observation under oil immersion which gives a very small field and requires examination of many fields, taking more time and causing eye strain.
- Technical errors easily affect the result such as thick smear, excessive heating, old reagents, stain precipitates, or reused slides leading to false positive or false negative findings.
- Personnel with color blindness may face difficulty because acid-fast bacilli appear red on a blue background.
- Association of Public Health Laboratories (APHL). (2007). External Quality Assessment for AFB Smear Microscopy.
- Centers for Disease Control and Prevention. (2025). Provider Performed Microscopy Procedures: A Focus on Quality Practices. U.S. Department of Health and Human Services.
- Centers for Disease Control and Prevention, Division of Laboratory Systems. (2024). Provider-Performed Microscopy Procedures | Laboratory Quality. U.S. Department of Health and Human Services.
- Chopra, S., Mahajan, S., Singh, Y., & Chopra, M. (2022). Comparative evaluation of Ziehl- Neelsen staining and kinyoun’s staining in the diagnosis of clinically suspected cases of tuberculosis. IP International Journal of Medical Microbiology and Tropical Diseases, 8(2), 149–153. https://doi.org/10.18231/j.ijmmtd.2022.031
- Gebre-Selassie, S. (2003). Evaluation of the Concentration Sputum Smear Technique for the Laboratory Diagnosis of Pulmonary Tuberculosis. Tropical Doctor, 33(3), 160–163.
- Golia, S., Hittinahalli, V., Nirmala, A. R., & Kamath B., A. S. (2013). A comparative study of auramine staining using led fluorescent microscopy with Ziehl Neelsen staining in the diagnosis of pulmonary tuberculosis. J Evol Med Dent Sci, 2(20), 3450–3456. https://doi.org/14260/jemds/714
- Hussey, M. A., & Zayaitz, A. (2008). Acid-Fast Stain Protocols. American Society for Microbiology.
- Law, Y. N., Jian, H., Lo, N. W.-S., & Wu, X. (2018). Low cost automated whole smear microscopy screening system for detection of acid fast bacilli. PLoS One, 13(1), e0190988. https://doi.org/10.1371/journal.pone.0190988
- Médecins Sans Frontières. (2022). Appendix 4. Sputum smear microscopy. MSF Medical Guidelines.
- Mohan, N., Sunil, M., Akhil, G., Priya, S. K., Vijayakumar, V., Vineetha, M., Celine, M. I., & Ajithkumar, K. (2023). Modified Ziehl–Neelsen staining for Mycobacterium leprae. J Skin Sex Transm Dis, 5(2), 120–122. https://doi.org/10.25259/JSSTD_34_2022
- Ratsavong, K., Quet, F., Nzabintwali, F., Diendéré, J., Sebert, J., Strobel, M., & Buisson, Y. (2016). Usefulness and limits of Ziehl-Neelsen staining to detect paragonimiasis in highly endemic tuberculosis areas. Parasitology International, 6(1), 1–7. https://doi.org/10.1016/j.parepi.2016.12.001
- Saeed, M., Rasheed, F., Iram, S., Hussain, S., Ahmad, A., Riaz, S., & Ahmad, M. (2018). False Negativity Of Ziehl-neelsen Smear Microscopy: Is The Scale-up The Worth It In Developing Countries? Journal of the College of Physicians and Surgeons–Pakistan: JCPSP, 28(3), 201–205. https://doi.org/10.29271/jcpsp.2018.03.201
- Sharma, M., Broor, S., Maheshwari, M., Sharma, M., & Sudan, D. S. (2023). Auramine-O Staining vs Ziehl Neelsen Staining: Advantages and Disadvantages. The Indian Journal of Chest Diseases and Allied Sciences, 65(1), 39–43. https://doi.org/10.5005/jp-journals-11007-0062
- Vilchèze, C., & Kremer, L. (2017). Acid-Fast Positive and Acid-Fast Negative Mycobacterium tuberculosis: The Koch Paradox. Microbiol Spectr, 5(2). https://doi.org/10.1128/microbiolspec.tbtb2-0003-2015
- WebPath: Internet Pathology Laboratory. (n.d.). Acid-Fast Bacteria – Ziehl-Neelsen Stain (AFB).
Wikipedia. (n.d.). Ziehl–Neelsen stain. Retrieved from https://en.wikipedia.org/w/index.php?title=Ziehl%E2%80%93Neelsen_stain&oldid=1322930842 - Yumul, J., Ghosh, J. K., Hwang, S., Green, N., & Kerndt, P. (2015). Acid-fast Bacilli (AFB) Smear Grade Standardization in Los Angeles County, California [Poster presentation]. 2015 National Tuberculosis Controllers Association Conference, Atlanta, GA.