UV Spectroscopy is a scientific method that’s like a detective tool for scientists. It helps them understand what’s happening in a molecule when it interacts with light, specifically ultraviolet light. This type of light is invisible to our eyes but plays a crucial role in the world of molecules.
Imagine a molecule as a tiny, busy city. The buildings in this city are the electrons, and they have different levels, just like floors in a building. Usually, these electrons are on the ground floor (ground state). But when UV light, which is kind of like an energy-packed beam, hits the molecule, it’s like an elevator that takes some electrons from the ground floor to higher floors (higher energy states). This process is called excitation.
Not all molecules get excited in the same way. Some have special kinds of electrons, known as π-electrons or nonbonding electrons (n-electrons), that are really good at catching and absorbing this energy from UV light. When they do, they move up to even higher floors, called anti-bonding molecular orbitals. It’s a bit like these electrons moving to a penthouse suite!
The interesting part is, some electrons are easier to excite than others. If they get excited easily, they can absorb light that has a longer wavelength. Wavelength is just a fancy word for the color of the light. In UV Spectroscopy, we see four main types of these electron moves or transitions: π–π, n–π, σ–σ, and n–σ. These are just different ways electrons can jump to higher energy levels. The order of these from needing the most energy to the least is: σ–σ* > n–σ* > π–π* > n–π*.
Now, the coolest part of UV Spectroscopy is that each molecule has its own unique way of absorbing UV light, which creates a special pattern or spectrum. This is like a fingerprint for the molecule. By looking at this fingerprint, scientists can tell what kind of molecule it is, just like detectives figuring out who was at the crime scene by looking at fingerprints.
So, UV Spectroscopy is a super useful tool for scientists to understand more about molecules, how they’re structured, and what makes them unique, all by looking at how they interact with light!
What is UV Spectroscopy?
UV Spectroscopy, short for Ultraviolet Spectroscopy, is an analytical technique fundamentally used for measuring the absorption of ultraviolet light by a substance. This method plays a crucial role in understanding the electronic structure of molecules, particularly in the fields of chemistry and biology.
The principle behind UV Spectroscopy is based on the fact that every molecule absorbs or transmits ultraviolet light over a certain range of wavelength. This absorption is primarily due to electronic transitions within the molecules, specifically transitions of electrons from lower energy levels to higher energy levels. The wavelength at which a substance absorbs UV light is indicative of various properties of that substance, such as chemical bonding, molecular structure, and the presence of specific functional groups.
In a typical UV Spectroscopy setup, a beam of UV light is passed through a sample, and the intensity of the light passing through the sample is measured. By comparing the intensity of the light before and after passing through the sample, one can determine the amount of light absorbed by the sample at various wavelengths. This information is then used to derive qualitative and quantitative data about the substance under study.
UV Spectroscopy is widely used for the analysis of organic compounds, especially in identifying and quantifying substances in a mixture. It is a non-destructive technique and requires only a small amount of sample. Common applications include the purity analysis of pharmaceuticals, the detection of impurities in drinking water, and the study of biomolecules like proteins and nucleic acids.
Principle of UV Spectroscopy
UV Spectroscopy is a fascinating scientific technique that revolves around how light, specifically ultraviolet light, interacts with various substances. It’s like shining a special kind of light on a molecule to see how it reacts:
- Interaction of Light and Matter: When UV light (part of light that’s invisible to the naked eye) hits a molecule, it can add energy to the molecule’s atoms or electrons. This is similar to how a person feels more energetic on a sunny day.
- Excitation of Electrons: This added energy causes the electrons in the molecule to get excited. Imagine the electrons are sitting calmly in a chair (the ground state), and then they jump up to a higher step or level (a higher energy state) because they’ve absorbed energy from the light.
- Types of Electrons Involved: The molecules that react to UV light often have specific types of electrons, called π-electrons or nonbonding electrons. These are special because they can absorb UV light and move to a higher energy state. It’s like these electrons are more sensitive to the ‘UV dance’.
- Electron Transitions: There are various ways electrons can make these jumps or transitions when they absorb UV light. They can move from one type of orbital (a region where the electron usually hangs out) to another. The way they move depends on how much energy they absorb from the light. The easier it is for an electron to get excited, the longer the wavelength of UV light it can absorb.
- Spectrum and Identification: Each time a molecule absorbs UV light, it creates a unique pattern or spectrum, like a molecular fingerprint. This pattern helps scientists identify what the substance is, as each compound will have a different reaction to the UV light.
In summary, the principle of UV Spectroscopy is about understanding how molecules respond to UV light, particularly how their electrons get excited and move to higher energy levels. This response is unique for different substances, making UV Spectroscopy a powerful tool for identifying and studying chemical compounds.
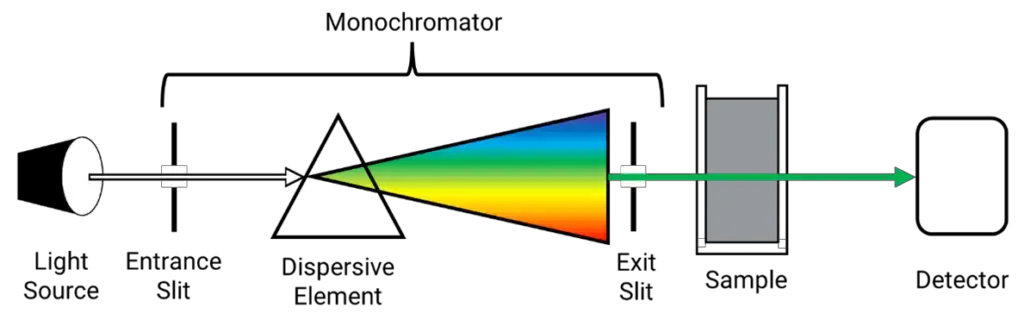
Instrumentation (Parts of UV Spectroscopy)
UV Spectroscopy is a method used in chemistry and biology to identify the chemical makeup of a substance by analyzing how it interacts with ultraviolet light. This technique relies on various parts or instruments, each playing a crucial role in the process. Let’s break down these components and their functions:
- Light Source:
- Tungsten Filament Lamps: These are known for their emission of red radiations, particularly around 375 nm. They are essential for covering certain parts of the UV spectrum.
- Hydrogen-Deuterium Lamps: These lamps are significant for their ability to cover the UV spectrum below 375 nm. They complement the tungsten lamps by filling in the lower wavelength range.
- Monochromator:
- The monochromator is a critical component that uses prisms and slits to disperse the light emitted from the source.
- It’s often part of double beam spectrophotometers, which are common in UV spectroscopy.
- The prisms rotate to separate different wavelengths of light. The slits then select specific wavelengths, allowing only monochromatic light (light of a single wavelength) to pass through. This light is then split into two separate beams.
- Sample and Reference Cells:
- These cells hold the sample solution and a reference solution.
- They are typically made from silica or quartz because glass absorbs UV light, making it unsuitable.
- One beam passes through the sample solution, and the other through the reference solution, allowing for comparative analysis.
- Detector:
- The detector usually comprises two photocells.
- These cells receive the beams from the sample and reference cells.
- The intensity of the radiation from the reference cell is typically stronger than from the sample cell, leading to the creation of alternating electrical currents in the photocells.
- Amplifier:
- The alternating current from the photocells is then sent to an amplifier.
- The amplifier, often connected to a servometer, boosts the signal strength.
- This amplification is vital as the original signals are usually too weak to be recorded directly.
- Recording Devices:
- These devices, often connected to the amplifier, include pen recorders and computers.
- They are crucial for storing data and generating the spectrum of the analyzed compound.
Additional Information
- Wavelength Selection: Modern spectrophotometers may use diffraction gratings instead of prisms for more precise wavelength selection.
- Software Integration: Advanced software is often integrated for data analysis, allowing for more sophisticated interpretation and visualization of the spectral data.
- Temperature Control: Some systems include temperature control units for samples, as temperature can affect absorption.
- Deuterium Lamps: These lamps, in addition to Hydrogen-Deuterium lamps, are sometimes used for a broader UV range coverage.
Applications of UV Spectroscopy
- Detection of Impurities:
- UV Spectroscopy excels in identifying impurities in organic molecules. The presence of additional peaks in the UV spectrum, compared to a standard, indicates impurities.
- By measuring absorbance at specific wavelengths, it can detect and quantify these impurities, ensuring the purity of a substance.
- Structure Elucidation of Organic Compounds:
- This technique is crucial in determining the structure of organic molecules. It helps in identifying unsaturation levels, the presence of heteroatoms, and other structural features.
- The absorption spectra provide clues about molecular structures, making it invaluable in organic chemistry.
- Quantitative and Qualitative Analysis:
- UV Spectroscopy is used for both quantitative and qualitative analysis of compounds that absorb UV radiation.
- It characterizes compounds based on their UV absorption, aiding in identification by comparing their spectra with known substances.
- Functional Group Analysis:
- The technique is adept at detecting the presence or absence of specific functional groups in a compound. The lack of an absorption band at certain wavelengths can indicate the absence of a particular group.
- Studying Reaction Kinetics:
- UV Spectroscopy is used to monitor the progress of chemical reactions. By observing changes in absorbance during a reaction, it provides insights into reaction kinetics.
- Pharmaceutical Applications:
- Many drugs, in raw or formulated states, are analyzed using UV Spectroscopy. The absorbance of drug solutions at specific wavelengths helps in drug assay and quality control.
- Molecular Weight Determination:
- By preparing suitable derivatives of compounds, UV Spectroscopy can be used to estimate their molecular weights, an essential aspect in molecular biology and chemistry.
- Integration with HPLC:
- A UV spectrophotometer can be coupled with High-Performance Liquid Chromatography (HPLC) as a detector, enhancing the capabilities of both techniques.
- Environmental Monitoring:
- UV Spectroscopy is used to detect and measure pollutants in water and air. It’s effective in monitoring the presence of organic and inorganic compounds in environmental samples.
- Food Industry:
- In the food industry, it helps in the quality control of food products, detecting additives, preservatives, and contaminants.
- Biochemical Applications:
- It plays a vital role in biochemical research, including the study of nucleic acids and proteins. It helps in understanding protein-ligand interactions and DNA/RNA analysis.
- Cosmetic Industry:
- Used for analyzing the components in cosmetics, ensuring the safety and efficacy of these products.
Advantages of UV Spectroscopy
- Sensitivity: UV Spectroscopy is highly sensitive, capable of detecting even minute amounts of substances. This sensitivity is particularly beneficial in fields like pharmaceuticals, where precise measurements are crucial.
- Non-destructive Analysis: The technique does not destroy the sample being analyzed. This is particularly valuable when dealing with rare or expensive substances, or when further analysis is required.
- Rapid and Simple: UV Spectroscopic analysis is generally fast and straightforward, allowing for quick data collection. This makes it suitable for high-throughput environments and situations where rapid decision-making is essential.
- Quantitative Analysis: UV Spectroscopy is excellent for quantitative analysis, providing accurate and reproducible results. It can determine the concentration of substances in a mixture, which is critical in many industrial and research applications.
- Qualitative Analysis: It’s also useful for qualitative analysis, helping identify substances based on their spectral patterns. This is particularly useful in chemical identification and purity assessment.
- Versatility: The technique can analyze a wide range of samples, including liquids, solids, and gases. This versatility makes it applicable in numerous fields, from environmental science to biochemistry.
- Cost-Effective: Compared to other spectroscopic methods, UV Spectroscopy is relatively cost-effective in terms of both equipment and operation. This makes it accessible for many laboratories and research institutions.
- Small Sample Size Requirement: UV Spectroscopy can be performed with a relatively small amount of sample, which is beneficial when samples are scarce or costly.
- Compatibility with Other Techniques: It can be easily combined with other analytical methods, such as High-Performance Liquid Chromatography (HPLC), to enhance analysis and provide more comprehensive data.
- Automation and Integration with Software: Modern UV Spectrophotometers can be automated and integrated with software for data analysis, improving efficiency and reducing the chances of human error.
- Structural Information: It provides valuable information about the molecular structure, functional groups, and chemical bonding in a substance.
- Real-Time Monitoring: UV Spectroscopy can be used for real-time monitoring of chemical reactions, processes in production, and quality control in various industries.
Limitations of UV Spectroscopy
- Limited to UV Absorbing Compounds: UV Spectroscopy is only applicable to substances that can absorb ultraviolet light. Compounds without UV-absorbing groups (like non-conjugated systems) cannot be analyzed effectively using this method.
- Sample Purity: The technique requires relatively pure samples for accurate analysis. Impurities can interfere with the readings, leading to incorrect interpretations.
- Sensitivity to Environmental Conditions: UV measurements can be affected by environmental factors such as temperature and the presence of solvents. These factors can alter the absorption characteristics of the sample.
- Overlap of Spectral Bands: In complex mixtures, the absorption bands of different components can overlap, making it difficult to distinguish between them. This overlap can complicate the analysis and interpretation of results.
- Limited Information on Structure: While UV Spectroscopy can provide some structural information, it is limited compared to other spectroscopic methods like NMR or IR spectroscopy. It cannot give detailed information about molecular structure.
- Quantitative Limitations: For quantitative analysis, the technique requires calibration with standard solutions. If standards are not available or not accurate, the quantitative results may not be reliable.
- Photodegradation: Prolonged exposure to UV light can lead to photodegradation of some samples, potentially altering their chemical composition during analysis.
- Solvent Selection: The choice of solvent is critical in UV Spectroscopy. The solvent itself must not absorb UV light in the range of interest, which can limit the choice of solvents for some analyses.
- Instrument Limitations: The performance and accuracy of UV Spectroscopy are also dependent on the quality and calibration of the instrument used. Poor calibration or maintenance of the spectrophotometer can lead to errors in results.
- High Concentration Samples: UV Spectroscopy can be less effective for samples with very high concentrations, as they may absorb too much light, leading to inaccuracies in absorbance measurements.
- Fluorescence Interference: Some compounds may fluoresce upon UV irradiation, which can interfere with the absorbance measurements and skew the results.
FAQ
How does UV-Vis Spectroscopy work?
UV-Vis Spectroscopy works by measuring the absorbance or transmittance of UV or visible light by a sample. Light from a lamp passes through a monochromator to select a specific wavelength, which then passes through the sample. The detector measures how much light is absorbed by the sample.
What is UV-Vis Spectroscopy?
UV-Vis Spectroscopy is a technique used in analytical chemistry to measure the absorbance or transmittance of ultraviolet (UV) and visible light by a sample. This helps in identifying and quantifying various substances.
Why are quartz cuvettes used in UV Spectroscopy?
Quartz cuvettes are used because they are transparent to UV light, unlike glass, which absorbs UV light. This allows accurate measurement of light absorption by the sample.
Why is UV-Vis Spectroscopy used?
It’s used for its sensitivity, ability to provide rapid and accurate analysis, and because it’s a non-destructive method suitable for a wide range of samples.
What is absorbance in UV Spectroscopy?
Absorbance in UV Spectroscopy is a measure of how much UV light is absorbed by a sample. It’s calculated from the intensity of light before and after it passes through the sample.
Which detectors are used in Ultraviolet (UV)‑Visible Spectroscopy?
Photomultiplier tubes (PMTs) and semiconductor detectors like photodiodes and charge-coupled devices (CCDs) are commonly used.
What is UV Spectroscopy used for?
It’s used for analyzing the chemical structure of substances, identifying compounds, and quantifying the concentration of substances in a sample.
What does UV Spectroscopy measure?
Similar to UV-Vis, UV Spectroscopy specifically measures the absorption of ultraviolet light by a substance, providing information about the concentration and chemical properties of the substance.
What does UV-Vis Spectroscopy tell you?
UV-Vis Spectroscopy provides information about the concentration of a substance in a solution and can also give insights into the chemical bonding and molecular structure of the substance.
What does UV-Vis Spectroscopy measure?
It measures the intensity of light before and after passing through a sample. The difference in these intensities is used to calculate the absorbance, which is related to the concentration of the absorbing species in the sample.
What is UV-Vis Spectroscopy used for?
It’s used for both qualitative and quantitative analysis in various fields like chemistry, biology, and environmental science. It helps in identifying compounds and determining their concentration in solutions.
References
- Chemistry LibreTexts. (n.d.). 4.4: UV-Visible Spectroscopy. Retrieved from https://chem.libretexts.org.
- Wikipedia contributors. (n.d.). Ultraviolet–visible spectroscopy. In Wikipedia, The Free Encyclopedia. Retrieved from https:/en.wikipedia.org.
- Technology Networks. (n.d.). UV-Vis Spectroscopy: Principle, Strengths and Limitations and Applications. Retrieved from https://www.technologynetworks.com.
- Chemistry LibreTexts. (n.d.). 4.5: Ultraviolet and Visible Spectroscopy. Retrieved from https://chem.libretexts.org.
- Chemistry LibreTexts. (n.d.). 2.1: Introduction to UV Spectroscopy. Retrieved from https://chem.libretexts.org.
- Chemistry LibreTexts. (n.d.). 3.5: UV-Visible Spectrometer. Retrieved from https://chem.libretexts.org.