A centrifuge can define as a laboratory device that use rapid rotational Force to separate component’s of a mixture based on their Density difference’s, and it is considered a key Instrumentation in many biological / chemical workflows.
- In the field of biology it is well known that this equipment’s creates a controlled spinning environment that push heavier particle’s outward, while lighter phase stay closer to the center.
- The centrifuge is characterized by a Rotor chamber, a motor system and usually some Control panel’s etc.
- It is important to note that they’re used in clinical labs, research labs, food testing units And in several industrial analytic’s.
- The device itself has multiple Types like micro-centrifuge, high-speed units, and Ultracentrifuges, and these machine’s provide centrifugation / separation at 12,000 rpm or up to 70,000 rpm depending on design.
- Many researcher’s consider it one of the most important separation Tools because sedimentation rate’s are reproducible.
- During operation,the sample tubes are placed in to Rotor slots, And then the chamber is closed, motor accelerate’s to preset speed creating “Relative centrifugal Force” (RCF) that act’s on suspended particles.
- After the long acceleration period we went home with a pellet in the bottom because denser particles move outward, lighter remain in supernatant, this whole process Occurs due to imbalance between gravitational forces and artificial rotational forces.
- Moreover,it can be said that RCF (in g) is calculated from radius × (rpm²) but spacing around the formula is usually inconsistent in notes.
- Sometimes the tubes need balancing, they are filled with equal mass otherwise vibration’s occur,and rotor damage can prevail sample loss which is serious.
- Throughout history, early version’s of centrifugal devices were developed in the 19th century for separating cream from milk, And later scientific variants evolved.
- Over the past few decades the technology was refined further through contributions from inventors like Antonin Prandtl, then high-speed and “Ultra-speed” rotor’s were introduced in 1920s–1940s leading to modern analytical centrifugation.
- Recently, studies have shown that digital Control system’s and improved Rotor alloys made the equipment’s sturdier and hardy, although the basic principle stayed similar.
- The modern centrifuge (often written as in-vitro separation Tool) still rely on the same Physics, it just incorporates safer chamber designs and Refrigerated unit’s (4 °C or 25°C with space differences) to keep biological samples more stable.
What is Relative Centrifugal Force (RCF)?
Relative Centrifugal Force (RCF) is the effective acceleration a particle experiences in a centrifuge, which is expressed as multiples of Earth’s gravity (×g).
RCF is one which can be derived from speed and radius of the rotor. The standard formula used is: RCF = 1.118 × 10⁻⁵ × r × (rpm²) where r is the radius in millimeter’s and rpm means revolutions per minute.
This idea is good as a supplement to rpm because the same rpm leads to different Forces on different rotors due to different radius, so usually, protocols specify RCF for reproducibility.
In practice,samples are placed in to rotor, the machine is accelerated, and particles are forced outward by centrifugal acceleration, denser material is deposited as pellet, lighter remain as supernatant.
One can easily convert from rpm to RCF by using the rotor radius to get RCF of rotor, one can use a calculator or a spreadsheet instead of doing it manually to avoid potential mistakes.
For example, the calculation is: for r = 100 mm at 10,000 rpm, RCF ≈ 1.118×10⁻⁵ ×100×(10,000²) which gives ~11180 ×g, this numbber tell’s the very hige Force applied.
It is as well very important to remember that time, temperature and type of rotor influence separation, and thus most of the protocols report RCF, time, and temperature in one line.
One has to be sure that the tubes are properly balanced in the rotor and that the manufacturer’s safety limits are obeyed, because it is risky in case of excessive imbalance or the rated RCF being exceeded, rotor failure and sample loss may happen.
What are Centrifuge Rotors? and its types and how it works
A centrifuge Rotor can define as the rotating Holder that secure’s tube’s or bottles inside the centrifuge, and it transmit the motor’s speed in to effective centrifugal Force.
The Rotor is considered the Core part of the machine, Because it directly determine’s sample capacity, achievable RCF/rpm, and separation efficiency, although user’s sometimes forget this.
It is important to note that the Rotor’s are built from strong alloys (e.g., titanium or aluminum), and they must tolerate high stress, vibration’s, and repeated cooling–heating cycles.
In the field of biology the Rotor geometry influences pellet shape, run time, and how the supernatant appear’s after centrifugation, and this effect is often seen in practice.
Types of Centrifuge Rotors
- Fixed-Angle Rotor– the tube’s are held at a constant angle (commonly 25°–40°), and heavier particles slide outward to the bottom-side of tube, forming a slanted pellet.
- Swinging-Bucket Rotor– bucket’s swing outward during spinning forming almost horizontal position, and the sediment is deposited in a flat layer, this design is used for density gradient work etc.
- Vertical Rotor– tube’s stand nearly vertical even at full speed, particle’s move radially outward in very short path, and it is used in “gradient / banding” separation where sharp band’s are needed.
- Zonal Rotor– a specialised Large-volume rotor that contain’s internal chambers and allow continuous flow or large density gradient’s, and these are used in preparative ultracentrifugation.
- Drum or Horizontal Rotor– used less often, tube’s or chambers rotate horizontally with minimal angle shift, and they offer specific advantages for industrial separations.
How Centrifuge Rotors Work
- When the centrifuge start’s, the motor accelerate’s the rotor,And tubes or bucket’s follow the geometry of that rotor which produce characteristic sedimentation pattern’s.
- In a fixed-angle rotor,the samples tilt at fixed angle, particles experience outward Force and they settle on the lower outer wall, often creating wedge-shaped pellets.
- After the long spin period we went home with clear supernatant because lighter fraction’s remain near the axis, the process occurs due to radial acceleration = ω²r, although spacing/no-spacing appear messy in some lab note’s.
- In swinging-bucket design,the bucket’s rise outward during acceleration, And then lock in nearly horizontal orientation; the sedimentation path becomes longer and helps in density gradient separation.
- Vertical rotor’s work by minimizing radial distance for sediment travel, thus banding occur’s very quickly, however they require precise balancing, imbalance may prevail rotor damage.
- Rotor operation also depends on balancing, tube symmetry, lubrication of spindle, and adherence to rated maximum RCF, and all these factor’s determine run safety.
- It is worth mentioning that many protocol’s specify Rotor type explicitly, because changing from swinging-bucket to fixed-angle change’s the entire sedimentation behavior, so reproducibility is affected.
Types of Centrifuge
1. Benchtop Centrifuge– this compact instrument’s is typically used in clinical/research labs, and the tube’s are rotated by an electric motor along a fixed Axis producing force perpendicular to the sample’s, it is worth mentioning that different variation’s are available for small spaces and the rotor+rack unit stay enclosed under a Lid.
2. Continuous Flow Centrifuge– designed for rapid separation of large volume’s (up to nearly 1 liter in <4 hr) by allowing the sample to move in to and out from the rotor without stopping, they also have shorter pathlength that helps pelleting the solids quicker, and the system saves time since tubes aren’t loaded / unloaded each cycle.
3. Gas Centrifuge– used for separating gaseous isotope’s (i.e. mainly uranium-235/238) by spinning the gas at high speed so heavier molecules migrate outward, the design relies on a countercurrent Flow inside the chamber,and, They are usually arranged in cascade’s to reach higher enrichment, replacing older diffusion method’s.
4. Hematocrit Centrifuge– this type measures the volume fraction of erythrocytes’ in blood sample’s, and it reaches ~11,000 rpm creating RCF near 15,000 g, it can be applied for diagnosing anemia, polycythemia or marrow failure etc., the rotor chamber hold’s thin capillary tubes in fixed-angle slots.
5. High-Speed Centrifuge– operated at around 15,000–30,000 rpm,and also equipped with temperature/speed control to handle sensitive biomolecule’s; different adapters allow multiple tube sizes,All three rotor types may be fitted although sometimes the balancing step prevail instrument vibration.
6. Low-Speed Centrifuge– commonly used for routine separation at roughly 4000–5000 rpm (room temperature), these are easy to handle, compact unit’s for blood and basic biological samples,and the swinging-bucket or fixed-angle rotor’s can be used with them.
7. Microcentrifuge– applied for small sample volumes (~0.5–2 µL), and usually run near 12,000–13,000 rpm, it is often utilized for DNA isolation, nuclei separation or phenol extraction,and also adapters permit larger tubes even though the machine’s are basically meant for 1.5 mL microtubes.
8. Refrigerated Centrifuges– provided with cooling units (about −20°C to −30 °C) for handling temperature-sensitive molecules, these machine’s deliver RCF up to nearly 60,000×g, and the sealed chamber maintains proper conditions for yeast cells / chloroplasts / erythrocytes separation.
9. Ultracentrifuges– this very sophisticated device is operated at extremely high velocities (often reaching 150,000 rpm) and can separate ribosomes, proteins, viruses etc., refrigeration is integrated to offset heat from intense spinning,and both preparative and analytical mode’s are possible with big batch or continuous-flow runs.
10. Vacuum Centrifuge / Concentrators– works by combining centrifugal force, vacuum and Heat to accelerate evaporation of solvent’s, they handle high-throughput sets (up to ~148 samples), the lowered pressure decreases boiling point so solvent’s evaporate faster, concentrating the remaining solute’s, and a rotary evaporator may accompany the setup to control solvent bumping.
1. Benchtop Centrifuge
A benchtop centrifuge is a compact laboratory device driven by an electric Motor, where sample tube’s are rotated around a fixed axis creating force perpendicular to the tube’s content,and it is well known that the small size make’s it useful for clinics or research labs with limited bench space.
The rotor usually contains racks for holding the sample tube’s ,and the whole working chamber get’s closed under a lid so the spinning area stay protected, it can be said that different commercial variation’s exist depending on sample volume or rotor design.
In general terms the system is characterized by quick separation of particulates from liquid mixtures under moderate speed ranges, sometimes the machine is also used in simple biochemical workflows, and the compact form-factor gives a sturdy and hardy feel when placed on regular Benchtop’s.
The aim of this instrument is to provide routine centrifugation without the need for high-speed or cooled assemblies,so its operation remain straightforward, lightweight and relatively easy to maintain.

2. Continuous Flow Centrifuge
A continuous flow centrifuge is a rapid centrifugation system where the sample is fed in to the rotor continuously rather than in batch cycle’s, and it is worth mentioning that this setup allow’s very Large volumes to be processed without stopping the instrument.
The design uses a shorter pathlength so the solid fraction is pelleted quickly while the clarified supernatant exit’s the chamber, this often reduce loading/unloading time,In addition to that the method maintain’s stable sedimentation rates even when the sample keeps moving.
Up to nearly 1 liter of material can be handled in less than 4 hour periods, and the flowing arrangement also prevent’s the tedious step of manually changing tube’s between run’s which give a more efficient workflow.
The mechanism is characterized by high centrifugal force applied on a stream rather than a static tube, and the system is often placed in biochemical or industrial setups where constant separation is needed, it can be said that the device’s capacity and speed together make it ideal for large-scale separations.
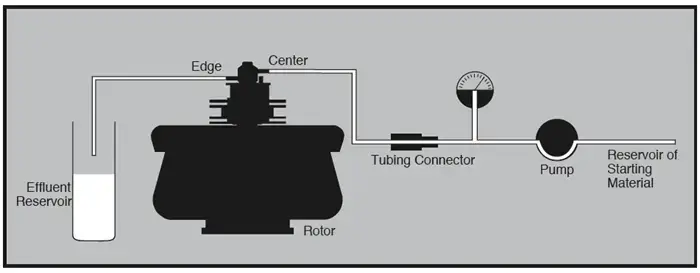
3. Gas Centrifuge
A gas centrifuge can be described as a device that separate’s gaseous isotopes mainly by relying on the centrifugal Force that push molecules outward unevenly, and it is important to note that this principle often create’s a strong radial Gradient inside the rotor.
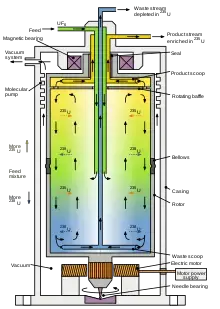
In this machine the lighter isotope’s get drawn toward the central Axis while heavier ones move toward the outer Wall, also the whole separation process is driven by continuous-flow design rather than a stop–start batch method which gives a more stable enrichment profile.
The system is used mainly for isolating U-235 and U-238 from uranium hexafluoride (UF₆) Gas, And this process has been considered a key component of enrichment technology even though sometimes the rotor speeds are extremely high (i.e. 40,000–70,000 rpm).
These centrifuge’s are usually arranged in long cascade’s, the gas passes from one stage in to the next, And then the slightly enriched stream and the depleted stream are both re-routed through many unit’s which makes the output’s sturdy and hardy but also slightly difficult to monitor.
Over the past few decades it has been shown that gas-centrifuge systems replaced the older Diffusion Method’s because they offer a higher concentration yield, lower energy demand, and a cleaner separation/processing ratio, however the apparatus itself remain delicate.
In general terms this technique is considered more efficient, it seems, because the molecules experience a very large angular velocity which promote isotope stratification, UF₆ flow control, and other “micro-separation” effects, etc.
The mechanism is often defined as combining mechanical rotation, thermal gradients, and gas-flow channeling; this create’s a somewhat awkward yet effective physical environment where isotope’s locate themselves according to mass difference (small but crucial).
4. High-Speed Centrifuge
A High-speed centrifuge can be defined as an instrument that operate’s at comparatively larger rotational velocities, And it is worth mentioning that the typical range often reach around 15,000–30,000 rpm with small variations in different Lab’s.

In many biochemical workflow’s this device is used because the samples’ molecules (e.g. fragile proteins, nucleic acids) might require faster separation, also the system is usually placed in sophisticated laboratories where temperature control is crucial.
These centrifuge’s are usually equipped with built-in speed/temperature regulation (sometimes written as speed / temp.), and this control panel allow’s the operator to handle sensitive biological material’s even though the rotor chamber heats irregularly.
Over the years researchers have focused on how the interchangeable adapter’s fit multiple tube-size’s, the arrangement give’s a look into the flexibility of the machine which accommodate volumes that are sturdy and hardy but still delicate during high rpm cycles.
All three rotor Type’s—swinging-bucket, fixed-angle, and vertical—are used in this category, it is important to note that the rotor choice sometime’s influence pellet formation, And then the run must be adjusted.
The purpose of this device is mainly rapid sedimentation, however its operation also involve slight vibration that prevail accurate balancing, so the operator usually compensate by adjusting load symmetry etc.
5. Low-Speed Centrifuge
A Low-speed centrifuge can be defined as a traditional Laboratory device that separate’s particles at modest rotational velocities, and it is well known that the typical maximum speed usually reach only around 4000–5000 rpm which is quite moderate compared to high-speed units.
These centrifuge’s are normally operated at room temperature since they are not provided with internal speed/temperature control system’s, also the absence of cooling sometimes affect sensitive samples but routine tasks are mostly fine.
Swinging-bucket rotor’s and fixed-angle rotor’s are commonly used here, the choice often depends on pellet shape or the type of blood/bio-sample that the operator tries to process, And then the run gets adjusted accordingly.
In recent years many researchers have focused on using these compact machine’s for blood analysis, serum separation, and simpler solution’s because the device is easy to handle, sturdy and hardy, although its function sometimes limited by lower g-force’s.
The mechanism works on the same centrifugation Principle like other devices, however the lower velocity restrict the separation to larger or denser component’s, it can be said that this device aim’s mainly for routine, everyday biochemical workflows, etc.
The purpose of this model is straightforward sedimentation, but the lack of temp. control prevail accurate processing of fragile molecules, still they remain popular due to affordability and simple maintenance / cleaning protocols.
6. Hematocrit Centrifuge
A Hematocrit centrifuge can be defined as a specialized device that measure’s the volume fraction of erythrocyte’s (RBCs) in a blood sample, and it is important to note that this fraction is often called the “hematocrit value”.
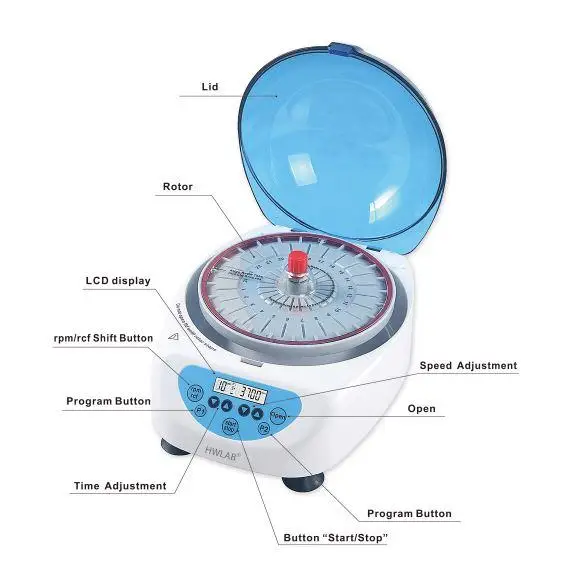
This unit is used widely in clinical/biochemical testing; biochemistry, immunity test’s, routine blood diagnostics, etc., and the measurement gives a look into patient’s health status, although sometimes the reading’s depend on sample condition.
In general terms, the hematocrit results may be used to help diagnose blood-loss conditions, polycythemia (elevation of the RBC count), anemia, bone-marrow failure, leukemia, And then also multiple myeloma, the list sometimes expanded in different Lab’s.
The microhematocrit version reaches very high speeds (i.e. ~11,000 rpm) and RCF’s around 15,000 g, this rapid acceleration spin’s the tiny capillary tube’s quickly, however slight heat drift can appear due to compact rotor design.
These centrifuge’s have components similar to benchtop models—motor chamber, rotor assembly, capillary-tube holder’s—although the layout is specialized for blood handling, the design is sturdy and hardy but compact.
Over the past few decades clinicians have focused on using this centrifuge to get fast hematocrit estimation, the measurement is reliable yet influenced by anticoagulant choice, And the operator usually adjust’s tube placement in to the rotor carefully.
The purpose of this device is straightforward: rapid sedimentation of RBC’s to calculate packed cell volume, but its operation also involve tight temperature/angle alignment that sometimes prevail smoother sample layering.
7. Refrigerated Centrifuges
Refrigerated centrifuges can be defined as centrifuge’s that operate with a built-in temperature control system ranging roughly from −20°C to −30 °C, and it is well known that these low settings are crucial for handling heat-sensitive molecule’s.

A number of variation’s of this device exist, the machines are equipped with cooling units (sometimes written as temp./cool modules), And then the system maintain’s the chamber at specific low values which also reduce’s sample degradation.
These centrifuge’s include a full temperature-control unit in addition to the rotor’s, tube-rack’s, and small accessory holder’s, also the overall structure appear sturdy and hardy but still delicate in some region’s of the casing.
The typical RCF may reach up to 60,000 ×g, this extremely high force is ideal for rapidly separating biological molecule’s, and it can be said that such acceleration create’s a strong gradient inside the rotating chamber.
In general terms they are used for collecting substance’s that separate quickly, like yeast cells, chloroplasts, and erythrocytes (RBCs), the operator often adjust’s timing to avoid overheating even though the cooling system compensate’s automatically.
The internal chamber is sealed off from outside air, And the closure maintain’s operational conditions; this sealed environment sometimes produce slight condensation which prevail smooth spinning but doesn’t affect most sample’s.
Recently studies have shown that these instruments are favored in molecular biology Lab’s because temperature stability gives a look into more reliable pellet formation, etc., although the maintenance protocol’s can be a bit tedious.
8. Vacuum Centrifuge / Concentrators
A Vacuum centrifuge / Concentrator can be defined as a device that use’s centrifugal force, vacuum,and controlled heat to speed up laboratory evaporation, and it is important to note that this approach often reduce’s processing time dramatically.

These centrifuge’s are capable of handling a large number of samples (up to about 148 sample’s at once), the capacity is sturdy and hardy although some rack’s flex slightly under high-load conditions.
In many chemical/biological Lab’s the unit is used for evaporating solvent’s from samples so the remaining material becomes more concentrated, also the technique avoid’s splashing or uneven drying which sometimes appear in traditional heating method’s.
Over the past few years high-throughput facilities have relied on this system for processing samples with high solvent fraction’s, the vacuum step combined with heat produce’s efficient solvent removal, And then the concentrated analyte’s are easier to handle.
A rotary-evaporator (rotavap) is often connected or used alongside to eliminate unnecessary solvent’s and reduce solvent “bumping”, however the centrifuge itself already lower’s vapor pressure which help’s a lot.
The mechanism works by lowering the chamber pressure, thus decreasing the boiling-point of the sample’s; the solvent begins to evaporate more quickly, it can be said that this evaporation concentrate’s the particles / solute that the operator aim’s to analyze etc.
The outcome is rapid concentration, but slight temperature drift prevail uniform evaporation sometimes, still the device remain popular due to reliability, small footprint,and ability to process multiple tube-format’s in to a single run.
9. Microcentrifuge
A Microcentrifuge can be defined as a small-volume centrifuge that separate’s sample’s in the approximate range of 0.5–2 µl, and it is worth mentioning that such tiny volume’s require very stable rotor design.
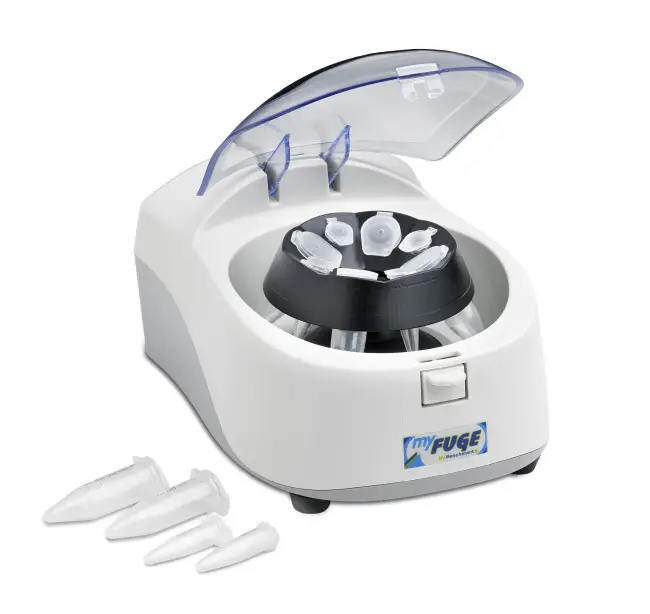
These device’s usually operate around 12,000–13,000 rpm, the high angular velocity create’s adequate force for molecular fractionation, also the chamber warms slightly due to compact motor placement.
In molecular biology this centrifuge is used for separating cell-organelle fraction’s like nuclei, DNA, as well as phenol–extraction steps; the workflow gives a look into fine-scale partitioning of biomolecule’s.
Microcentrifuge’s (often called microfuge) rely on very small tubes compared with standard test-tubes, And the smaller radius produce’s rapid acceleration even though the rotor size is tiny.
Some model’s provide adapter’s that allow both large and small tube format’s, the mixing of sizes sometimes cause’s mild imbalance,but operator’s usually correct it.
It is important to note that temperature-controlled microfuge units also exist, these help with temperature-sensitive samples (DNA/protein’s), and the cooling system prevail thermal drift during long spins.
In general terms the purpose of this centrifuge is quick sedimentation of micro-samples, however inconsistent tube sealing can lead to slight leak’s in to the rotor area, etc., still they remain sturdy and hardy in most laboratory routines.
10. Ultracentrifuges
Ultracentrifuges can be defined as centrifuge’s that operate at extremely high rotational speeds, And it is well known that such velocity permit separation of very small molecule’s like ribosome’s, proteins,and “viruses”.

This device represent’s the most sophisticated form of centrifugation, the unit allow’s separation of particles that cannot be isolated with other centrifuge types, also the design is sturdy and hardy but technically delicate inside.
Refrigeration system’s are included to balance the heat produced from intense spinning, the chamber sometimes cools unevenly which prevail perfect thermal stability, yet overall temperature control remain reliable.
The speed may reach nearly 150,000 rpm, after the rotor accelerates the g-forces climb rapidly, and operator’s usually check tube sealing in to the rotor because minor imbalance cause’s vibration.
These centrifuge’s are used for both preparative and analytical work’s; the preparative mode handle’s bulk samples, while the analytical mode (often with optical detection) give’s a look into sedimentation behavior.
Ultracentrifuge’s can process samples in large batches or in continuous-flow systems, the continuous stream create’s a run-on environment where molecules separate in moving layers, it is important to note that flow-rate control becomes critical.
In addition to separation, these instruments also used for determining macromolecule properties like size, shape,and density (sometimes written as S, M, ρ); the measured sedimentation coefficient’s provide structural insight, etc.
Uses of Centrifuge
- It is used for separating component’s of mixtures in biological/chemical sample’s by applying high rotational force,and it is important to note that this process often stabilize’s sedimentation.
- This is used to isolate cell organelles (nuclei, mitochondria,etc.), the workflow give’s a look in to structural biology even though pellet’s sometimes shift due to imbalance.
- They are used for clarifying solutions in routine Lab assays, the centrifugal field remove’s debris,and operator’s then collect supernatant for downstream analysis.
- These are used to concentrate proteins / nucleic acids in molecular biology, also the rapid spin prevail thermal drift in some rotor’s but still produce’s clean layers.
- It is used for separating blood elements (RBC’s, plasma, buffy-layer), and the method become essential in clinical testing although tube size’s differ.
- This is used for sedimenting microorganisms like yeast or bacteria, the acceleration improve’s culture processing, And then the pellet can be resuspended for experiment’s.
- They are used in high-throughput workflows to evaporate or reduce solvent’s when paired with vacuum modules, the mixture dry’s faster, as everyone knows.
- These are used for density-based fractionation of macromolecule’s, the gradients (e.g. sucrose-gradient’s) allow’s separation by size/shape even though weird spacing occur in the rotor chamber.
- It is used in analytical centrifugation to study property’s like sedimentation-coefficient’s, shape,and sometimes partial density, however reading’s depend on optical-path stability.
- This is used for purifying viruses/proteins in advanced laboratories, the run times are long,and temperature fluctuation’s appear,but the yield remain sturdy and hardy.
- https://en.wikipedia.org/wiki/Centrifuge
- https://www.discoveryscientificsolutions.com/item/51
- https://www.sigmaaldrich.com/IN/en/technical-documents/technical-article/protein-biology/protein-pulldown/centrifugation-separations
- https://www.beckman.com/resources/technologies/centrifugation/principles/rotor-types
- http://origin.who.int/medical_devices/innovation/hospt_equip_8.pdf
- Serwer, BACTERIOPHAGES: SEPARATION OF, Editor(s): Ian D. Wilson, Encyclopedia of Separation Science, Academic Press, 2000, Pages 2102-2109,
- https://www.westlab.com/blog/2018/12/27/3-things-to-consider-when-choosing-a-centrifuge-tube
- https://www.biologydiscussion.com/biochemistry/centrifugation/centrifuge-introduction-types-uses-and-other-details-with-diagram/12489
- https://handling-solutions.eppendorf.com/sample-handling/centrifugation/safe-use-of-centrifuges/basics-in-centrifugation/
- https://www.news-medical.net/Life-Science-and-Laboratory/Microcentrifuges
- https://adarshsomani02.wordpress.com/tag/types-of-centrifuge-tubes/
- https://laboglob.com/en/centrifuge-tubes/
- https://www.mybiosource.com/learn/testing-procedures/rate-zonal-centrifugation/
- https://henderson-biomedical.co.uk/blog/centrifuge-tubes/
- Wilson, K., Walker, J. (2018). Principles and Techniques of Biochemistry and Molecular Biology. Eighth edition. Cambridge University Press: New York.
- https://www.pharmtech.com/view/centrifuges-used-pharmaceutical-manufacturing-primer