The Davson–Danielli model (or paucimolecular model) was proposed in 1935 by Hugh Davson and James Danielli as a model of the plasma membrane of a cell. The model describes a trilaminar and lipoprotinous phospholipid bilayer sandwiched between two layers of globular proteins. Gorter and Grendel had postulated the phospholipid bilayer in 1925; however, the flanking proteinaceous layers in the Davson–Danielli model were original and were designed to explain Danielli’s results on the surface tension of lipid bilayers (It is now known that the phospholipid head groups are sufficient to explain the measured surface tension).
The hypothesis was supported by electron microscopy, which revealed three distinct layers within a cell membrane, including a central white core and two bordering black layers.
The micrographs were interpreted as depicting a phospholipid bilayer sandwiched between two protein layers, as proteins typically appear dark and phospholipids white. The model gave an explanation for why some molecules can pass through the cell membrane and others cannot, while simultaneously accounting for the thinness of cell membranes.
Despite being academically recognised, the Davson–Danielli model contained assumptions, such as that all membranes had the same structure, thickness, and lipid-protein ratio, which contradicted the observation that membranes might have specific functions. In addition, the Davson–Danielli model could not account for several observable events, including the active transport of large numbers of molecules across the plasma membrane. A further flaw of the Davson–Danielli model was that many membrane proteins were known to be amphipathic and predominantly hydrophobic; hence, the existence of membrane proteins outside of the cell membranes in direct contact remained a mystery.
The Davson–Danielli model was accepted by the scientific community until 1972, when Seymour Jonathan Singer and Garry L. Nicolson introduced the fluid mosaic model.
The fluid mosaic model extended the Davson–Danielli model by include transmembrane proteins and eliminating the previously proposed bordering protein layers that were not supported by experimental evidence. The experimental evidence that disproved the Davson–Danielli model consisted of membrane freeze-fracturing, which revealed irregular rough surfaces in the membrane, representing trans-membrane integral proteins, and fluorescent antibody tagging of membrane proteins, which demonstrated their fluidity within the membrane.
What is Sandwich (Davson–Danielli) model of cell membrane?
The Sandwich (Davson-Danielli) model was presented in the 1930s by scientists James Danielli and Hugh Davson as a model for the structure of cell membranes.
According to this hypothesis, the cell membrane was believed to consist of two layers of phospholipid molecules sandwiching a layer of protein molecules. The hydrophobic (“water-afraid”) tails of the phospholipid molecules faced one another, but the hydrophilic (“water-loving”) heads faced the watery environment inside and outside the cell.
It was previously believed that the protein layer in the centre of the membrane provided structural support and acted as channels or holes for the transit of substances across the membrane.
Although the Sandwich model was generally accepted for many years, it was ultimately determined to be overly simplistic and inaccurate. Recent research has revealed that the cell membrane is a significantly more intricate structure, with a dynamic and fluid composition that enables a range of tasks including cell signalling, transport of chemicals, and cell recognition.
Key Features of the Davson–Danielli Model
According to the Davson-Danielli model, proteins are sandwiched between two layers of phospholipid molecules in the cell membrane. Some of the most notable aspects of this model are:
- Phospholipid bilayer: The cell membrane consists of a phospholipid bilayer, which is a double layer of phospholipid molecules. The phospholipids’ hydrophilic heads are exposed to the water surrounding them, but their hydrophobic tails are tucked in close to one another.
- Protein layer: The Davson-Danielli model includes a protein layer between its phospholipid bilayers. It is believed that this protein layer serves as both a structural support for the membrane and a set of channels or pores through which substances can pass.
- Fluid mosaic structure: The concept additionally postulated that proteins in the membrane are organised in a mosaic-like fashion, with each protein serving a unique purpose. It was previously believed that proteins and phospholipids may move about inside the membrane because of its fluid nature.
- Selective permeability: The model claimed that the membrane is selectively permeable, enabling some molecules to enter and leave the cell while blocking the passage of others.
Overall, the Davson-Danielli model helped shed light on the structure of the cell membrane in the past, but it has subsequently been altered in light of new information on the membrane’s intricacy and dynamic nature.
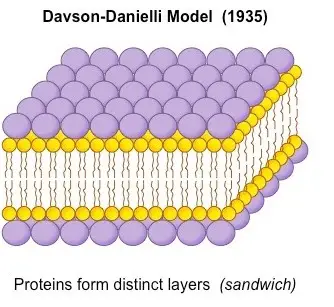
Support of Davson–Danielli model
Many experimental observations and data were available at the time the Davson-Danielli model was proposed, and these provided support for the hypothesis.
- Electron microscopy: It was determined that there were two layers of phospholipids in the model based on early electron microscopy studies of cell membranes, which revealed two dark lines.
- Biochemical studies: Biochemical research demonstrated the presence of lipids and proteins in the cell membrane, supporting the model’s hypothesised bilayer shape and protein layer.
- Functionality: The model explained how chemicals are transported across a cell membrane via protein channels and how the membrane might be selectively permeable.
- Consistency with other models: When compared to competing hypotheses of the time, such as the lipid bilayer model and the unit membrane model, the Davson-Danielli model was shown to be consistent with all of them.
In spite of the model’s initial success and widespread adoption, it was ultimately disproven as inadequate and inaccurate. Recent advances in technology and experimental methods have revealed a more intricate and dynamic structure of the cell membrane, as seen through freeze-fracture electron microscopy and biochemical research.
Problems in the Davson–Danielli Model
Although the Davson-Danielli model was widely accepted for a long time, various flaws and inconsistencies were subsequently discovered.
- Membrane fluidity: Fluidity of the membrane was overlooked in the model, despite its importance to membrane function. In reality, proteins in the membrane can move laterally and change their shape, allowing for dynamic modulation of membrane permeability and signalling, whereas the model assumed a rigid structure with fixed protein channels.
- Membrane asymmetry: The model did not account for the fact that the inner and outer surfaces of the cell membrane contain distinct lipids and proteins. Several membrane-based processes, including cell signalling and membrane trafficking, rely on the asymmetry.
- Transmembrane proteins: Unfortunately, the existence of proteins that traverse the entire membrane thickness, known as transmembrane proteins, was not factored into the model. The Dawkins–Danielli model’s protein layer is in conflict with the hydrophobic portions of these proteins, which interact with the lipid bilayer.
- Lack of evidence: Not enough evidence: The model relied on a small amount of anecdotal information and failed to account for advances in technology and experimental methods that have since shown a more nuanced structure to the cell membrane.
Even though the Davson-Danielli model contributed to our earliest knowledge of the cell membrane, it has since been upgraded to take into account both new experimental evidence and the increasingly complex and dynamic structure of the membrane.
Falsification Evidence for the Davson–Danielli Model
Several pieces of data eventually combined to disprove the Davson-Danielli concept.
- Freeze-fracture electron microscopy: In freeze-fracture electron microscopy, cells are frozen and then broken apart to show the underlying membrane structure. It turned out that the membrane had particles inside of it (later identified as integral membrane proteins) that the Davson-Danielli model had overlooked.
- Membrane protein mobility: Researchers found that membrane proteins were not immobile, as predicted by the Davson-Danielli model. They did this by employing fluorescence recovery after photobleaching (FRAP) and single-particle tracking. Instead, proteins would be free to transverse the membrane and interact with its many compartments.
- Membrane thickness: The thickness of the membrane was assumed to be constant in the model, but experimental evidence from X-ray diffraction and other methods shown that it actually changed with lipid and protein content.
- Membrane fusion: The model could not adequately account for membrane fusion, a process essential for several cellular activities including cell division and vesicular transport. Subsequent studies revealed the essential role that specialised proteins like SNARE proteins play in membrane fusion.
- Insolubility and dimensions of membrane proteins: Membrane proteins are not soluble in water because their hydrophobic surfaces are too large, a fact that was not incorporated into the model. It was also discovered that membrane proteins had different sizes, making it difficult for them to arrange themselves in a neat, continuous layer on the membrane’s surface.
- Mobility of membrane proteins: Proteins in membranes are not fixed in place as the Davson-Danielli model would have you believe; fluorescent antibody labelling of membranes has shown this. Experiments showing the addition of membrane proteins from two separate cells that had been tagged with different coloured markers provided evidence for this. The markers became distributed over the membrane of the fused cell, showing that the membrane proteins were able to move.
- Transmembrane proteins: Rough and uneven membrane surfaces detected by freeze-fracture electron microscopy were interpreted as transmembrane proteins. This contradicts the Dawson-Danielli hypothesis, which postulates that proteins can only exist on the membrane’s exterior.
Ultimately, additional experimental data and observations invalidated the Davson-Danielli model, leading to the development of new models like the fluid mosaic model, which more accurately describes the actual structure of the cell membrane.
Facts about Sandwich (Davson–Danielli) model of cell membrane
- Hugh Davson and James Danielli put forth the concept in 1935.
- The hypothesis postulated that the cell membrane was composed of a protein layer and a lipid bilayer, much like the structure of a sandwich.
- It was believed that the protein layers formed a continuous and homogeneous barrier between the cell’s inside and exterior.
- Phospholipids made up both layers of the lipid bilayer, with the hydrophilic ends of the lipids facing outside of the membrane and the hydrophobic ends remaining inside.
- In this conception, the membrane is assumed to be a stiff and immobile structure, with predetermined protein channels allowing for selective chemical transit across the membrane.
- After decades of success, the paradigm was disproved by experiments that showed the cell membrane to be more dynamic and complicated than previously thought.
- The information gained by the Sandwich model helped scientists better understand the cell membrane and led to the creation of other models like the fluid mosaic model.
FAQ
What is the Sandwich (Davson-Danielli) model of cell membrane?
The Sandwich (Davson-Danielli) model of cell membrane is an early model proposed in 1935 to describe the structure of the cell membrane. It suggests that the cell membrane is made up of two layers of phospholipids, with proteins sandwiched in between.
What is the structure of the cell membrane according to the Sandwich model?
According to the Sandwich model, the cell membrane is made up of two layers of phospholipids, with proteins embedded in between. The phospholipid layers are arranged with their hydrophobic tails facing inward and their hydrophilic heads facing outward, towards the extracellular and intracellular environments.
How do the proteins in the Sandwich model contribute to the cell membrane structure?
In the Sandwich model, proteins are embedded in the phospholipid bilayer, with their hydrophilic regions exposed to the extracellular and intracellular environments. These proteins provide structural support to the membrane, as well as serving as channels, pumps, and receptors for molecules to enter and exit the cell.
What is the role of the phospholipids in the Sandwich model?
The phospholipids in the Sandwich model form the basic structure of the cell membrane, providing a barrier between the inside and outside of the cell. Their hydrophobic tails repel water, while their hydrophilic heads interact with water, allowing for the formation of the bilayer.
How does the Sandwich model explain the fluidity of the cell membrane?
The Sandwich model suggests that the cell membrane is fluid, with the phospholipids and proteins able to move freely within the membrane. This fluidity is due to the properties of the phospholipids, which are able to move laterally within the membrane.
Is the Sandwich model still accepted today?
No, the Sandwich model is no longer accepted as an accurate representation of the cell membrane structure. While it was an important early model, subsequent research has shown that the membrane is much more complex than originally thought, with a variety of different lipids and proteins playing important roles.
What are some limitations of the Sandwich model?
One limitation of the Sandwich model is that it does not account for the presence of other types of lipids in the membrane, such as cholesterol. Additionally, the model suggests that all membrane proteins are evenly distributed throughout the bilayer, which is not necessarily the case.
How does the modern fluid mosaic model differ from the Sandwich model?
The modern fluid mosaic model of cell membrane structure describes the membrane as a fluid mosaic of lipids, proteins, and other molecules. Unlike the Sandwich model, it allows for the presence of a variety of different types of lipids and proteins, and suggests that these components are not evenly distributed throughout the membrane.
Why was the Sandwich model important despite its limitations?
The Sandwich model was an important early model of cell membrane structure, and helped to lay the groundwork for subsequent research in this field. While it has since been supplanted by more accurate models, it was an important first step in understanding the structure and function of the cell membrane.
Who were Davson and Danielli, and why is their model named after them?
Hugh Davson and James Danielli were two British scientists who proposed the Sandwich model of cell membrane structure in 1935. Their model was an important early contribution to the field of cell biology, and has since been superseded by more accurate models. The model is named after them as a tribute to their work in this field.
References
- Danielli, J. F., & Davson, H. (1935). A contribution to the theory of permeability of thin films. Journal of Cellular and Comparative Physiology, 5(4), 495–508. doi:10.1002/jcp.1030050409
- https://www.biologydiscussion.com/cell-biology/the-danielli-davson-membrane-model-with-diagram/3789
- https://people.wou.edu/~guralnl/gural/102Chapter%2004%20-%20Plasma%20Membrane.pdf
- http://www.esalq.usp.br/lepse/imgs/conteudo_thumb/Cell-membrane-structure.pdf
- http://www.wou.edu/~guralnl/gural/102Chapter%2004%20-%20Plasma%20Membrane.pdf
- https://nptel.ac.in/courses/102103012/module1/lec3/2.html
- http://www.biologydiscussion.com/cell-biology/the-danielli-davson-membrane-model-with-diagram/3789
- https://www.researchgate.net/figure/Evolution-of-the-membrane-models-during-the-XX-th-Century-A-the-Davson-Danielli-model_fig1_31730771
- http://ib.bioninja.com.au/standard-level/topic-1-cell-biology/13-membrane-structure/membrane-models.html
- https://www.revolvy.com/page/Davson%E2%80%93Danielli-model
- https://sebiology.weebly.com/blog/plasma-membrane-the-davson-danielli-model-vs-the-singer-nicolson-model
- http://www.oxfordreference.com/view/10.1093/oi/authority.20110810104725251
- http://yourdescribeinfo.blogspot.com/2018/05/davson-danielli-model.html
- http://www.library.armstrong.edu/eres/docs/eres/BIOL11071_NIXON/Membranes%20&%20Transport%20[Compatibility%20Mode].pdf
- http://www.library.armstrong.edu/eres/docs/eres/BIOL11071_NIXON/Membranes%20&%20Transport%20%5bCompatibility%20Mode%5d.pdf
- https://yourdescribeinfo.blogspot.com/2018/05/davson-danielli-model.html
- http://psystems.disco.unimib.it/download/BiancoPhDThesis.pdf
- https://www.oxfordreference.com/display/10.1093/oi/authority.20110810104725251
- https://biology.stackexchange.com/questions/20264/why-was-the-davson-danielli-model-rejected
- Lodish, H. F., Berk, A., Kaiser, C., Krieger, M., Scott, M. P., Bretscher, A., Ploegh, H. L., Matsudaira, P. T. (2008). Molecular cell biology. New York: W.H. Freeman.
- Smith, C. M., Marks, A. D., Lieberman, M. A., Marks, D. B., & Marks, D. B. (2005). Marks’ basic medical biochemistry: A clinical approach. Philadelphia: Lippincott Williams & Wilkins.
- Text Highlighting: Select any text in the post content to highlight it
- Text Annotation: Select text and add comments with annotations
- Comment Management: Edit or delete your own comments
- Highlight Management: Remove your own highlights
How to use: Simply select any text in the post content above, and you'll see annotation options. Login here or create an account to get started.