Salt Tolerance Test is a biochemical test which is used to determine the ability of an organism to survive and grow in the presence of high concentration of salt. It is generally performed using a medium containing 6.5% sodium chloride (NaCl). It is based on the principle that high salt concentration creates osmotic stress which affects membrane permeability and cellular activities of salt-intolerant organisms, thereby inhibiting their growth, while salt-tolerant organisms are able to grow under such conditions.
In this test, the medium commonly used is Brain Heart Infusion (BHI) broth or Tryptic Soy Broth (TSB) supplemented with sodium chloride. Dextrose and a pH indicator such as bromcresol purple is also present in the medium. If the organism is salt tolerant, growth is observed in the form of turbidity in the broth. In some cases, acid production from sugar fermentation may cause a colour change of the medium from purple to yellow, but the presence of turbidity alone is considered as a positive result.
Salt Tolerance Test is mainly used for the differentiation of Enterococcus species from other Group D streptococci. Enterococcus species are able to grow in 6.5% NaCl, whereas non-enterococcal Group D streptococci usually fail to grow in such conditions. This test is also useful in the identification of Aerococcus species and in differentiating certain rapidly growing Mycobacterium species.
Objective of Salt Tolerance Test
- It is used to determine the salt resistance of an organism in 6.5% NaCl medium.
- It is used to differentiate Enterococcus species from other Group D streptococci that cannot grow in high salt.
- It is used to identify salt-tolerant Aerococcus species and separate them from similar salt-intolerant genera.
- It is used to distinguish rapidly growing mycobacteria, especially Mycobacterium abscessus and M. chelonae.
- It is used to characterize bacterial groups like Actinobacteria and Vibrio based on their halotolerance.
- It is used to select and isolate Staphylococcus species on media containing high salt concentration.
Principle of Salt Tolerance Test
The principle of the Salt Tolerance Test is based on the ability of high salt concentration to restrict the growth of salt-intolerant organisms, while salt-tolerant organisms continue to survive in such conditions. It is the process in which increased sodium chloride (NaCl) concentration makes a hypertonic environment that affects the permeability of the bacterial cell membrane. In this condition, salt-sensitive cells lose water causing plasmolysis, and their growth is inhibited. These are the organisms that cannot adjust their internal ion concentration with the external medium.
Salt-tolerant organisms like Enterococcus species and Staphylococcus species can grow because they have cellular mechanisms for maintaining osmotic balance. It is the process where compatible solutes are accumulated inside the cell or the cytoplasmic ions are adjusted so that cell turgor is maintained even in high salt concentration.
In this test, a medium containing 6.5% NaCl is generally used, and a fermentable carbohydrate like dextrose is added together with a pH indicator. The reaction is as follows– when the organism grows in this medium, acid is produced from carbohydrate fermentation, which lowers the pH. This is indicated by the colour change of the indicator (for example, purple to yellow). Visible turbidity is also considered as a positive result because growth itself indicates salt tolerance.
Requirements for Salt Tolerance Test
- A suitable broth medium is required like Brain Heart Infusion broth or Tryptic Soy Broth, and the medium must contain 6.5% NaCl.
- A fermentable carbohydrate (dextrose) and a pH indicator such as bromcresol purple is needed.
- Chemicals required are sodium chloride, bromcresol purple, and dextrose.
- Test tubes, sterile loops, incubator, autoclave, weighing balance, and PPE are required for setting the test.
- A fresh pure culture (18–24 hours) is required as the inoculum and a light inoculum is used.
- Control strains like Enterococcus faecalis (positive) and Streptococcus gallolyticus or S. pyogenes (negative) are required.
- Incubation is done at 35°C approximately and the tubes are examined at 24 hours, and if required, further incubation is continued.
Composition of Modified Salt Broth
- Peptic digest of animal tissue (10 g per litre).
- Heart infusion or HMH peptone (10 g per litre).
- Glucose (1 g per litre).
- Sodium chloride (65 g per litre).
- Bromcresol purple (0.016 g per litre).
- The final pH is adjusted to 7.2 ± 0.2 at 25°C.
Preparation of Modified Salt Broth
- The required amount of medium powder is weighed, usually 86.01 g for 1000 mL of distilled water.
- The powder is suspended properly in the measured volume of distilled water.
- The mixture is heated gently and stirred until the medium is dissolved.
- The broth is dispensed into clean test tubes, commonly 5 mL each.
- The tubes are loosely capped or plugged with cotton.
- Autoclaving is done at 121°C for 15 minutes under 15 lbs pressure.
- After sterilization, the tubes are allowed to cool to about 40–45°C before inoculation.
Procedure of Salt Tolerance Test
A. Salt Tolerance Test using 6.5% NaCl Broth
- The broth medium containing 6.5% sodium chloride is allowed to attain room temperature before use.
- A fresh pure culture of the test organism (18–24 hours old) is selected for inoculation.
- Well isolated colonies are chosen from the culture plate.
- A sterile inoculating loop is used to pick a light inoculum (1–3 colonies).
- The selected inoculum is transferred gently into the 6.5% NaCl broth medium.
- Heavy inoculum is avoided as turbidity of inoculum itself can give false positive result.
- The tube is incubated aerobically at a temperature of 35–37°C.
- The cap of the tube is loosened slightly to allow proper gas exchange.
- After incubation for 18–24 hours the broth is observed for turbidity.
- Presence of turbidity indicates growth and shows salt tolerance of organism.
- If indicator is present colour change may be observed but turbidity alone is taken as positive result.
- If no visible growth is observed after 24 hours the tube is re-incubated and examined at 48 and 72 hours before reporting negative result.
B. Procedure using Mannitol Salt Agar (MSA)
- A Mannitol Salt Agar plate is taken and labeled properly with organism name.
- The surface of agar plate is inoculated aseptically using streak culture technique.
- The inoculated plate is incubated in inverted position at 35–37°C for 24–48 hours.
- After incubation the plate is examined for bacterial growth.
- Change in colour of medium from red to yellow indicates mannitol fermentation.
- Growth without colour change indicates salt tolerance without mannitol fermentation.
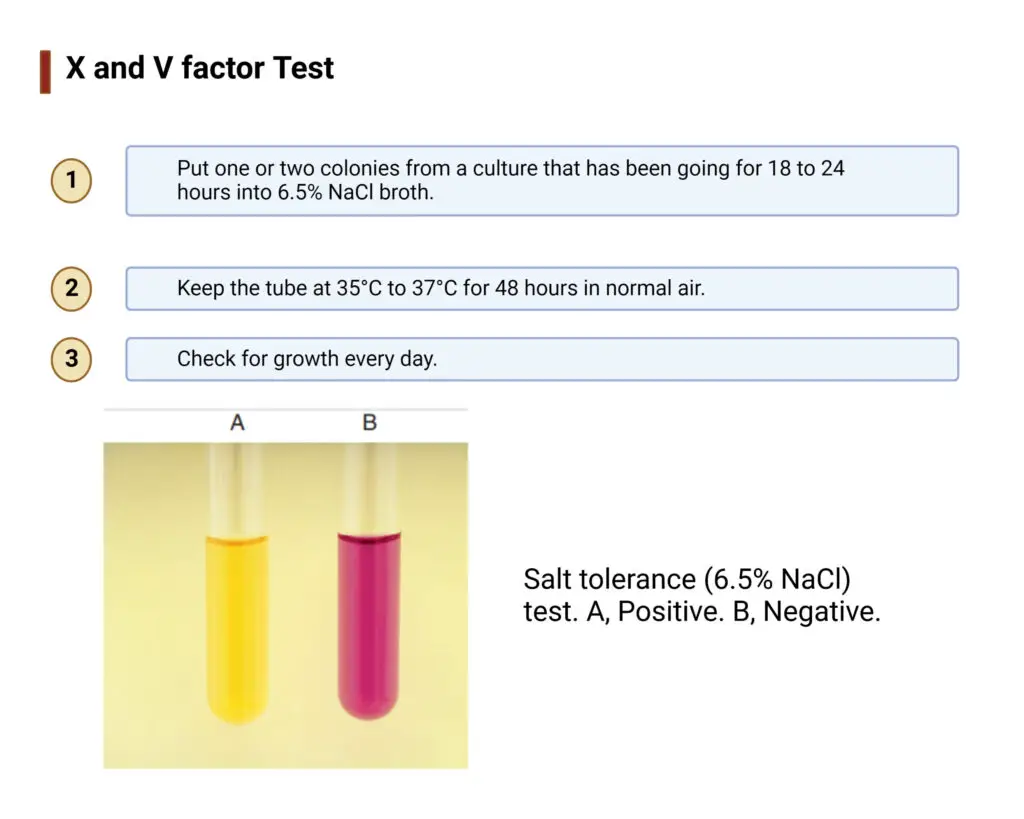
Results of Salt Tolerance Test

Positive Result
- A positive result indicates that the organism is salt tolerant and can survive in high salt concentration.
- Visible growth is observed in the broth medium in the form of turbidity.
- Turbidity indicates multiplication of bacterial cells and is taken as the main criteria for positivity.
- If pH indicator is present in the medium colour change may be seen due to acid production.
- The colour generally changes from purple to yellow when fermentation of sugar occurs.
- In some cases growth is seen without any colour change which is also considered positive.
- Turbidity alone is sufficient for reporting positive salt tolerance test.
- This type of growth without fermentation is seen in some strains of Enterococcus and Aerococcus.
- Common organisms showing positive result are Enterococcus faecalis, Staphylococcus species and Mycobacterium abscessus.
Negative Result
- A negative result indicates that the organism is salt intolerant and cannot survive in high salt medium.
- No visible growth is observed in the broth after incubation period.
- The broth remains clear and transparent similar to the uninoculated control tube.
- No change in colour of the medium is observed.
- The indicator retains its original colour such as purple or red-orange.
- Absence of turbidity confirms inability of organism to tolerate salt.
- Common organisms showing negative result include Streptococcus gallolyticus, Streptococcus pyogenes and Mycobacterium chelonae.
Organisms Showing Positive and Negative Result in Salt Tolerance Test
Positive
- Enterococcus species
- Enterococcus faecalis
- Enterococcus faecium
- Enterococcus durans
- Enterococcus avium
- Staphylococcus species
- Staphylococcus aureus
- Staphylococcus epidermidis
- Aerococcus species
- Aerococcus viridans
- Aerococcus urinae
- Mycobacterium species
- Mycobacterium abscessus
- Vibrio species
- Vibrio parahaemolyticus
- Vibrio alginolyticus
- Vibrio vulnificus
- Other salt tolerant bacteria
- Streptococcus agalactiae (Group B streptococcus)
- Pediococcus species
- Leuconostoc species
- Bacillus velezensis
- Bacillus pumilus
- Oceanobacillus species
- Halomonas species
Negative
- Streptococcus species (non-enterococcal)
- Streptococcus gallolyticus (formerly Streptococcus bovis)
- Streptococcus pyogenes
- Streptococcus equinus
- Mycobacterium species
- Mycobacterium chelonae
- Gram negative enteric bacteria
- Escherichia coli
- Other salt intolerant bacteria
- Stomatococcus species
- Helcococcus species
- Vibrio cholerae
| Result | Group | Organism |
|---|---|---|
| Positive | Enterococcus species | Enterococcus faecalis |
| Positive | Enterococcus species | Enterococcus faecium |
| Positive | Enterococcus species | Enterococcus durans |
| Positive | Enterococcus species | Enterococcus avium |
| Positive | Staphylococcus species | Staphylococcus aureus |
| Positive | Staphylococcus species | Staphylococcus epidermidis |
| Positive | Aerococcus species | Aerococcus viridans |
| Positive | Aerococcus species | Aerococcus urinae |
| Positive | Mycobacterium species | Mycobacterium abscessus |
| Positive | Vibrio species | Vibrio parahaemolyticus |
| Positive | Vibrio species | Vibrio alginolyticus |
| Positive | Vibrio species | Vibrio vulnificus |
| Positive | Other salt tolerant bacteria | Streptococcus agalactiae |
| Positive | Other salt tolerant bacteria | Pediococcus species |
| Positive | Other salt tolerant bacteria | Leuconostoc species |
| Positive | Other salt tolerant bacteria | Bacillus velezensis |
| Positive | Other salt tolerant bacteria | Bacillus pumilus |
| Positive | Other salt tolerant bacteria | Oceanobacillus species |
| Positive | Other salt tolerant bacteria | Halomonas species |
| Negative | Streptococcus species (non enterococcal) | Streptococcus gallolyticus |
| Negative | Streptococcus species (non enterococcal) | Streptococcus pyogenes |
| Negative | Streptococcus species (non enterococcal) | Streptococcus equinus |
| Negative | Mycobacterium species | Mycobacterium chelonae |
| Negative | Gram negative enteric bacteria | Escherichia coli |
| Negative | Other salt intolerant bacteria | Stomatococcus species |
| Negative | Other salt intolerant bacteria | Helcococcus species |
| Negative | Other salt intolerant bacteria | Vibrio cholerae |
Quality Control of Salt Tolerance Test
Before performing the test the medium is checked for correct pH colour depth and sterility. The broth should be free from contamination and show clear appearance before inoculation.
Positive Control
- Enterococcus faecalis (ATCC 29212) is used as the positive control organism.
- The organism is inoculated into 6.5% NaCl broth medium.
- After incubation growth should be observed in the form of turbidity.
- If bromcresol purple indicator is present colour may change from purple to yellow.
- Growth within 24 to 48 hours confirms proper performance of the medium.
Negative Control
- Streptococcus gallolyticus (ATCC 9809) is used as a negative control organism.
- After incubation no growth should be observed in the broth.
- The medium remains clear with no colour change.
- Streptococcus pyogenes (ATCC 19615) is also used as a negative control strain.
- Growth of the organism is inhibited in high salt medium.
- Streptococcus bovis (ATCC 33317) may be used as additional negative control.
- The broth remains clear even after extended incubation up to 72 hours.
Mannitol Salt Medium Quality Control
- Staphylococcus aureus (ATCC 25923) is used as positive control.
- The organism shows good growth with yellow colour change of medium.
- Staphylococcus epidermidis (ATCC 12228) is used as fermentation negative control.
- Growth is observed but the medium retains red colour.
- Escherichia coli (ATCC 25922) is used as inhibited control.
- No growth is observed confirming selective nature of the medium.
Precautions of Salt Tolerance Test
- A light inoculum should be used for inoculation of the broth.
- Heavy inoculum must be avoided as it can produce false turbidity and give false positive result.
- Fresh pure culture of organism (18–24 hours old) should be selected for test.
- Well isolated colonies are preferred for accurate inoculation.
- Inoculation should be done carefully and gently into the medium.
- Negative result should not be reported before 72 hours of incubation.
- Some organisms grow slowly and may show delayed growth.
- The tube should be gently shaken before observing the result.
- Settled growth at bottom may be missed if tube is not mixed properly.
- Correct salt concentration (6.5% NaCl) must be ensured while preparing the medium.
- Media should be stored in tightly closed container protected from light and moisture.
- Deterioration of indicator may occur if media is not stored properly.
- Caps of tubes should be loosened during incubation for proper aeration.
- This test should not be used as a single confirmatory test for identification.
- Other biochemical tests should be performed for confirmation.
- All media and cultures should be handled using standard laboratory safety precautions.
Uses of Salt Tolerance Test
- It is used for differentiation of Enterococcus species from non enterococcal group D streptococci.
- It helps in identification of Enterococcus faecalis from Streptococcus gallolyticus.
- The test is used for selective isolation of Staphylococcus species using high salt media.
- It helps in differentiation of Staphylococcus aureus from Staphylococcus epidermidis on mannitol salt agar.
- It is used to differentiate rapidly growing mycobacteria such as Mycobacterium abscessus and Mycobacterium chelonae.
- The test is useful in identification of Aerococcus species from other catalase negative cocci.
- It helps in characterization of halophilic Vibrio species based on salt requirement.
- Salt enriched media is used as enrichment step for screening of methicillin resistant Staphylococcus aureus.
- It is used in environmental and agricultural studies for isolation of halophilic bacteria.
- The test helps in general bacterial characterization to study salt tolerance property.
Advantages of Salt Tolerance Test
- It is a simple and reliable test for differentiation of Enterococcus species from non enterococcal group D streptococci.
- The test helps in distinguishing Enterococcus faecalis from Streptococcus gallolyticus.
- Correct identification of enterococci helps in selection of appropriate antibiotic therapy.
- It is useful in differentiation of rapidly growing mycobacteria such as Mycobacterium abscessus and Mycobacterium chelonae.
- When performed using mannitol salt agar it helps in selective isolation of Staphylococcus species.
- It aids in differentiation of pathogenic Staphylococcus aureus from non pathogenic Staphylococcus epidermidis.
- The test helps in characterization of halophilic Vibrio species based on salt requirement.
- It is useful in identification of Aerococcus species from other similar catalase negative cocci.
- Salt supplemented media is used as enrichment method for screening of methicillin resistant Staphylococcus aureus.
- The test is economical easy to perform and suitable for routine laboratory use.
Limitations of Salt Tolerance Test
- Heavy inoculation can produce turbidity of inoculum itself leading to false positive result.
- The test is not specific only for Enterococcus species.
- Other organisms such as Pediococcus, Leuconostoc and Aerococcus may also grow in high salt medium.
- Some beta hemolytic streptococci like Streptococcus agalactiae can show growth and give confusing results.
- The test is presumptive in nature and should not be used alone for identification.
- Confirmatory tests such as bile esculin test catalase test or molecular tests are required.
- Some organisms show turbidity without colour change making interpretation difficult.
- Growth may settle at the bottom of tube causing false negative result if not mixed properly.
- Very light inoculum may give delayed reaction or false negative result.
- Staphylococcus species can grow in high salt and may interfere with interpretation.
- Negative result cannot be reported early and requires incubation up to 72 hours or more.
- Salt containing media are sensitive to light and temperature and improper storage may affect results.
- Acharya, T. (2023). Salt tolerance test for Enterococcus species. Microbe Online. https://microbeonline.com/salt-tolerance-test-enterococcus-species-principle-procedure-results/
- Barajas González, J. A., Keller de la Rosa, Y. E., Carrillo-González, R., González-Chávez, M. C. A., Hidalgo Lara, M. E., Soto Hernández, R. M., & Herrera Cabrera, B. E. (2024). NaCl modifies biochemical traits in bacterial endophytes isolated from halophytes: Towards salinity stress mitigation using consortia. Plants, 13(12), 1626. https://doi.org/10.3390/plants13121626
- Biochemical foundations and clinical applications of the salt tolerance test in modern microbiology. (n.d.).
- Bruins, M. J., Juffer, P., Wolfhagen, M. J. H. M., & Ruijs, G. J. H. M. (2007). Salt tolerance of methicillin-resistant and methicillin-susceptible Staphylococcus aureus. Journal of Clinical Microbiology, 45(2), 682–683. https://doi.org/10.1128/JCM.02417-06,,
- Conville, P. S., & Witebsky, F. G. (1998). Variables affecting results of sodium chloride tolerance test for identification of rapidly growing mycobacteria. Journal of Clinical Microbiology, 36(6), 1555–1559. https://doi.org/10.1128/jcm.36.6.1555-1559.1998,
- Dalynn Biologicals. (2018). Salt tolerance broths [Technical insert]. https://www.dalynn.com/dyn/ck_assets/files/tech/TS27.pdf,
- Feng, Y., Ming, T., Zhou, J., Lu, C., Wang, R., & Su, X. (2022). The response and survival mechanisms of Staphylococcus aureus under high salinity stress in salted foods. Foods, 11(10), 1503. https://doi.org/10.3390/foods11101503,,
- Gallardo, K., Candia, J. E., Remonsellez, F., Escudero, L. V., & Demergasso, C. S. (2016). The ecological coherence of temperature and salinity tolerance interaction and pigmentation in a non-marine Vibrio isolated from Salar de Atacama. Frontiers in Microbiology, 7, 1943. https://doi.org/10.3389/fmicb.2016.01943,
- Hardy Diagnostics. (2020). Sodium chloride (NaCl) 6.5% media [Instructions for use]. https://hardydiagnostics.com/media/assets/product/documents/NaCl65Media.pdf,
- Hardy Diagnostics. (2020). Tryptic soy broth (TSB) with 6.5% sodium chloride [Instructions for use]. https://hardydiagnostics.com/media/assets/product/documents/TSBwith6_5NaCl.pdf,
- Hartline, R. (2023, February 18). 1.29: Mannitol salt agar. Biology LibreTexts. https://bio.libretexts.org/Bookshelves/Microbiology/Microbiology_Laboratory_Manual_(Hartline)/01%3A_Labs/1.29%3A_Mannitol_Salt_Agar,
- HiMedia Laboratories. (2011). Salt broth, modified [Technical Data]. https://exodocientifica.com.br/_technical-data/M1290.pdf,
- InformationBoxTicket Lifestyles. (n.d.). Salt tolerance test in microbiology | Principle, procedure, and applications explained! [Video]. YouTube. https://www.youtube.com/watch?v=0uhwATb6AuU,
- Kunin, C. M., & Rudy, J. (1991). Effect of NaCl-induced osmotic stress on intracellular concentrations of glycine betaine and potassium in Escherichia coli, Enterococcus faecalis, and staphylococci. Journal of Laboratory and Clinical Medicine, 118(3), 217–224.,
- Li, F., Xiong, X.-S., Yang, Y.-Y., Wang, J.-J., Wang, M.-M., Tang, J.-W., Liu, Q.-H., Wang, L., & Gu, B. (2021). Effects of NaCl concentrations on growth patterns, phenotypes associated with virulence, and energy metabolism in Escherichia coli BW25113. Frontiers in Microbiology, 12, 705326. https://doi.org/10.3389/fmicb.2021.705326,
- Micromaster Laboratories. (n.d.). Mannitol salt broth (DM161) [Product Specification Sheet]. https://www.micromasterlab.com/wp-content/uploads/bsk-pdf-manager/DM161-_PSS_717.pdf,
- Rapid Test Methods Ltd. (2019, October 22). Vibrio species detection and identification in seafood. rapidmicrobiology. https://www.rapidmicrobiology.com/test-method/detection-and-identification-of-vibrio-species-in-food,
- Remel. (2008). Brain heart infusion broth w/ 6.5% NaCl [Instructions for use]. https://assets.fishersci.com/TFS-Assets/LSG/manuals/IFU60286.pdf,
- Remel. (2012). Tryptic soy broth w/ 6.5% NaCl [Instructions for use]. https://documents.thermofisher.com/TFS-Assets/LSG/manuals/IFU65030.pdf,
- Schwan, W. R., & Wetzel, K. J. (2016). Osmolyte transport in Staphylococcus aureus and the role in pathogenesis. World Journal of Clinical Infectious Diseases, 6(2), 22–27. https://doi.org/10.5495/wjcid.v6.i2.22,
- Siegrist, J. (n.d.). Vibrio detection. Sigma-Aldrich. https://www.sigmaaldrich.com/US/en/technical-documents/technical-article/microbiological-testing/pathogen-and-spoilage-testing/vibrio-detection,
- Solheim, M., La Rosa, S. L., Mathisen, T., Snipen, L. G., Nes, I. F., & Brede, D. A. (2014). Transcriptomic and functional analysis of NaCl-induced stress in Enterococcus faecalis. PLoS ONE, 9(4), e94571. https://doi.org/10.1371/journal.pone.0094571,
- Watson, R. (n.d.). Summary of biochemical tests. University of Wyoming. https://www.uwyo.edu/molb2210_lect/medmicro/info/biochemical_tests.htm,
- Whitaker, W. B., Parent, M. A., Naughton, L. M., Richards, G. P., Blumerman, S. L., & Boyd, E. F. (2010). Modulation of responses of Vibrio parahaemolyticus O3:K6 to pH and temperature stresses by growth at different salt concentrations. Applied and Environmental Microbiology, 76(14), 4720–4729. https://doi.org/10.1128/AEM.00474-10,