What is Phytoremediation?
- Phytoremediation refers to the technologies that utilise plants to clean up chemically contaminated land, air, and water.
- In addition to being a cost-effective technology for environmental remediation, phytoremediation has proven to be a fantastic solution for all type of environmental problems.
- Phytoremediation is a cost-effective plant-based method that capitalises on plants’ ability to concentrate environmental components and chemicals and metabolise diverse substances in their tissues.
- It refers to the natural ability of certain plants to bioaccumulate, decompose, or render pollutants in soil, water, or air harmless. Heavy metal toxins and organic contaminants are the focus of phytoremediation.
- Phytoremediation is typically used to stationary contaminated soil or water settings. Examples include the rehabilitation of abandoned metal mine workings and sites where polychlorinated biphenyls were deposited during the manufacturing process, as well as the mitigation of an existing coal mine by lowering the impact of toxins in soils, water, or air.
- In phytoremediation initiatives around the world, contaminants such as metals, herbicides, solvents, explosives, and crude oil and its derivatives have been reduced.
- At toxic waste sites, many plants, including mustard plants, alpine pennycress, hemp, and pigweed, have shown effective at hyperaccumulating pollutants.
- Due to physiological variations, not all plants are capable of accumulating heavy metals or organic contaminants. Even within the same species, cultivars have variable capacities to accumulate contaminants.
Types of Phytoremediation
There are three varieties of phytoremediation:
1. In situ phytoremediation
- For the aim of remediation, in situ phytoremediation involves the placing of living plants in contaminated surface water, contaminated soil or sediment, or contaminated soil or sediment in contact with contaminated ground water.
- The polluted material is not removed prior to phytoremediation in this method. If the phyto process consists merely of uptake and accumulation of a pollutant, as opposed to transformation, the plants may be harvested and removed from the site after remediation for disposal or recovery of the contaminants.
- The contamination must be physically accessible to the plant’s roots for the in-situ method to be applicable.
- Typically, this is the least expensive phytoremediation method.
Advantages of In situ phytoremediation
- This procedure is occurring in situ.
- Passive method.
- Solar driven.
- 10–20% of the cost of mechanical therapies.
- More rapidly than natural attenuation
- High public approval.
- Less air and water pollution.
- Conserves natural resources.
Disadvantages of In situ phytoremediation
- Only shallow groundwater, soils, and sediments are considered.
- High quantities of hazardous substances can be poisonous to plants and the animals that eat them.
- Mass transfer restrictions present in other biotreatments.
- Slower than a mechanical approach.
- Effective only for moderately hydrophobic substances.
- Unknown are the toxicity and bioavailability of biodegradation products.
- It is possible for contaminants to be moved into groundwater.
- influenced by the site’s soil and climate circumstances.
2. In-vivo phytoremediation with relocated contaminants
- For locations where the contaminant is inaccessible to plants, such as toxins in deep aquifers, an alternative phytoremediation technique is conceivable.
- In this method, the pollutant is mechanically removed, then transferred to a temporary treatment area where it can be exposed to plants selected for effective phytoremediation.
- The treated water or soil can be restored to its original place, and if necessary, the plants can be harvested for disposal.
- In general, this approach would be more expensive than the more passive method outlined above.
- Treatment could take place either at the site of contamination or elsewhere.
3. In vitro phytoremediation
- In the first two phytoremediation strategies, living plants are utilised. Components of living plants, such as extracted enzymes, are a third type of phytoremediation application.
- Theoretically, this method might be implemented in situ in certain circumstances, such as the application of plant extracts to a contaminated pond or wetland or the employment of an enzyme-impregnated porous barrier in a contaminated ground water plume.
- Probably, this strategy might also be applied to contaminated materials that have been moved to a temporary treatment area.
- Theoretically, this approach would be the most expensive method of phytoremediation due to the costs of preparing/extracting the plant enzymes; however, in some plants, such as tarragon (Artemisia dracunculus var satiya), exudates are released in response to stress, which could reduce production costs.
- The length of time that the enzymes remain active for degrading pollutants is a key consideration for this strategy.
Processes Of Phytoremediation
Phytoremediation is based on certain natural processes carried out by plants, such as:
- The uptake of metals and certain organic compounds (i.e., moderately water soluble, log Kow=0.5 to 3, such as BTEX) from soil and water; the photosynthesis of organic compounds; and the transpiration of carbon dioxide.
- Accumulation or processing of these compounds through lignification, volatilization, metabolism, and mineralization (transformation into carbon dioxide and water);
- Utilization of enzymes to convert complex organic molecules to simpler molecules (ultimately CO2 and water);
- Through the release of chemicals (exudates) and decomposition of root tissue, increasing the carbon and oxygen content of soil near roots (therefore boosting microbial/fungal activity);
- Utilization of groundwater (even contaminated groundwater) for plant activities.
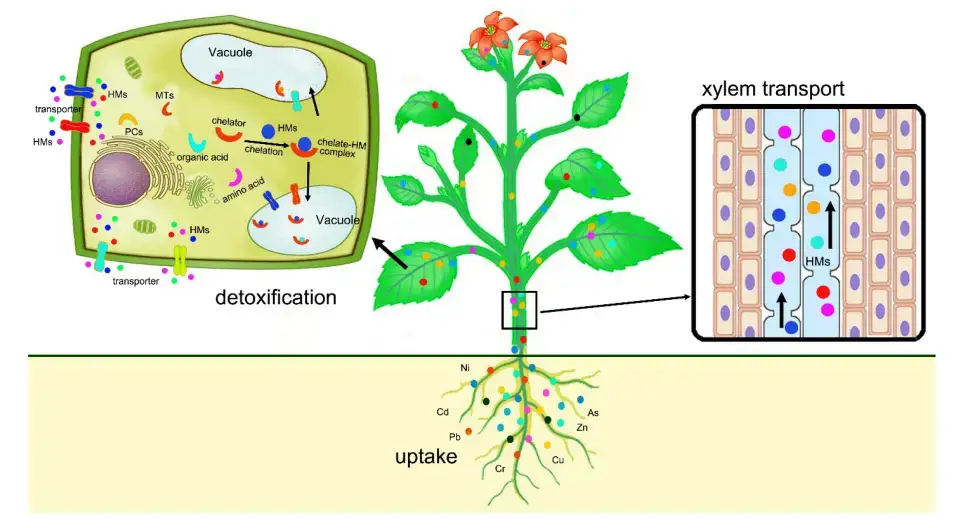
Mechanisms of phytoremediation
For the remediation of heavy metal-contaminated soils, a number of phytoremediation techniques are available, including:
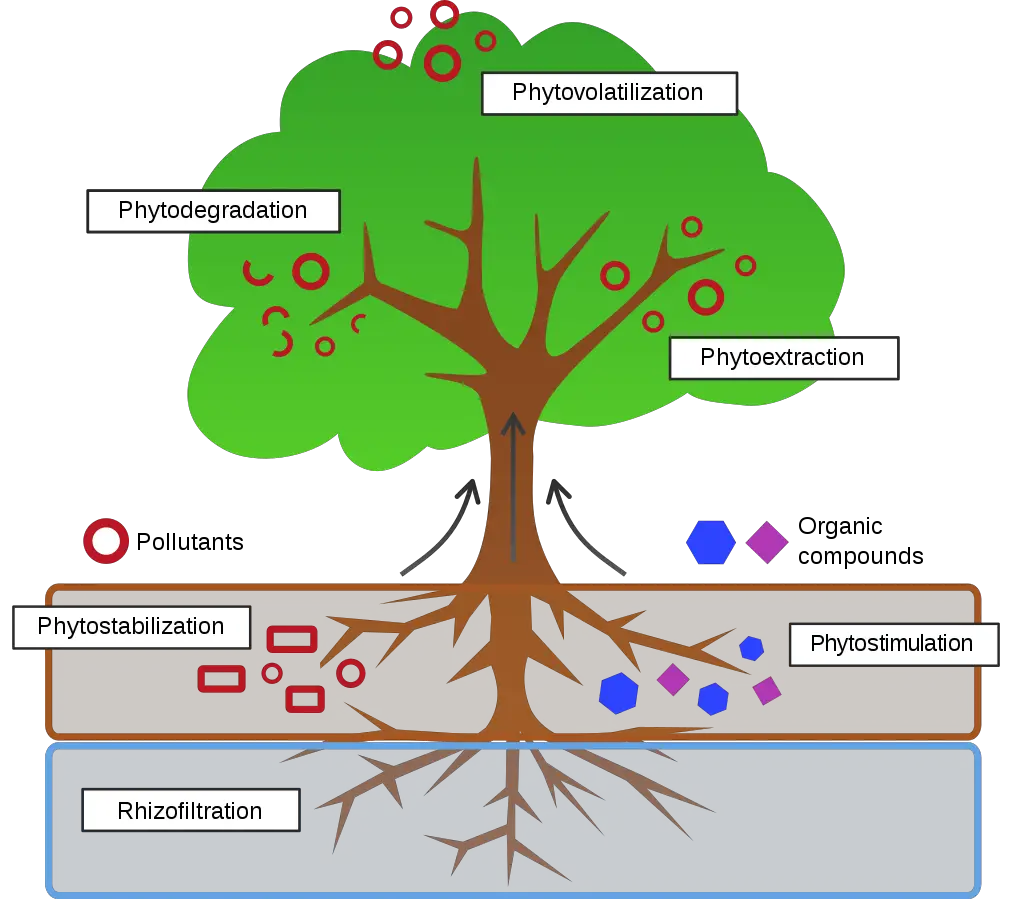
1. Phytostabilization/Phytosequestration
Phytostabilization is the absorption and precipitation of pollutants, primarily metals, by plants, which reduces their mobility and prevents their migration to groundwater (leaching) or air (wind transport) or entry into the food chain.
- There are a variety of mechanisms that come within this category, which is also known as phytostabilization.
- They may involve absorption by roots, adsorption to the surface of roots, or the creation of biochemicals by a plant that are released into the soil or groundwater in the immediate area of the roots and can sequester, precipitate, or immobilise adjacent pollutants in other ways.
- Phytostabilization is the application of metal-tolerant plant species to immobilise heavy metals belowground and reduce their bioavailability, hence preventing their migration into the environment and decreasing the risk of metals entering the food chain.
- Phytostabilization can take place by precipitation of heavy metals or reduction in metal valence in the rhizosphere, absorption and sequestration within root tissues, or adsorption onto root cell walls.
- In places affected by heavy metals, plant development aids in the preservation of soil health. In addition to stabilising heavy metals underground and minimising their leaching into groundwater, the developed plant cover limits the wind-borne dispersion of soil particles containing heavy metals.
- In contrast to phytoextraction, phytostabilization does not necessitate the removal of potentially hazardous material.
Advantages
- There is no need for hazardous biomass disposal.
- Plant reduces soil erosion and water availability in the system.
Disadvantages
- Pollutants continue to exist in places.
- Regular surveillance is required.
2. Phytoextraction/phytoaccumulation
- It is the absorption of pollutants from polluted locations (soil, water, sediments) by plant roots, as well as their transport and accumulation in aboveground plant tissues.
- Additionally known as phytomining and biomining.
- For this, plants with the genetic capacity to tolerate larger amounts of undesirable pollutants are utilised.
- These plants are cultivated for a period of time on contaminated locations, harvested, and then burned to reprocess the toxins.
- To reduce the concentration of these contaminants in contaminated soil to an acceptable level, many cycles of cultivation, harvesting, and combustion have been utilised.
- Following requirements for the disposal of hazardous waste, the ash from incineration is used in landfills.
- Using plants to translocate metal pollutants from contaminated places is a time-tested and effective method.
- The plant species that will be used for phytoextraction must possess certain essential characteristics, such as a high translocation factor, a high bioconcentration factor, a high tolerance to contaminants, rapid growth, high biomass production, a good root system, a high assimilation rate, and an easy harvesting process.
- The Asteraceae, Brassicaceae, Lamiaceae, Euphorbiaceae, and Scrophulariaceae plant families are better appropriate for phytoextraction.
- As phytoextraction is a biological process done by plants, it eliminates toxins from contaminated places without affecting the soil, water, or sediment qualities.
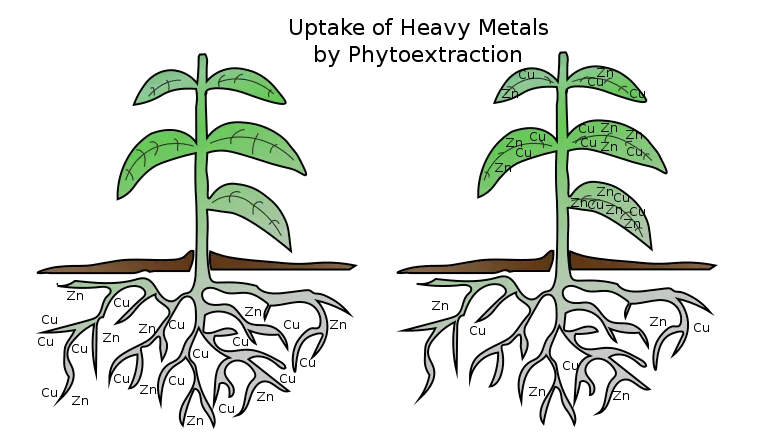
Types of Phytoextraction
Phytoextraction is widely categorised as chelate-assisted phytoextraction (induced phytoextraction) and long-term phytoextraction (continuous phytoextraction), of which chelate-assisted phytoextraction is more acceptable and is being implemented commercially at the present time.
a. Chelate-Assisted Phytoextraction
- Chelate-assisted phytoextraction entails the employment of metal chelating agents in conjunction with non-accumulator plants with a high biomass potential so as to increase the soluble metal fraction.
- Transport of metals to the harvestable shoot during discharge of soil solution constitutes the additive function of two fundamental processes.
- It has been noted that the adoption of fast-growing tree species satisfies the necessity for chemically assisted high biomass production in order to obtain high metal accumulation.
- Chelates such as ethylenediaminetetraacetic acid (EDTA) are credited with the ability to promote the production of soluble metal-EDTA in plants, so facilitating the transfer of metals from the root system to the shoot, where they accumulate as metal–EDTA complexes.
- Transport of metal–chelate complexes from the xylem to the shoots seems to play a significant role in the accumulation of chelate-assisted metal complexes in plants.
- In addition to an efficient capillary plumbing system, the movement of metal–chelate complexes within plants is dependent on a high-surface-area collection system given by the roots.
- Movement of metals as a metal–chelate complex to shoots is followed by retention of the metal–chelate complex when transpiration stream water evaporates.
b. Continuous Phytoextraction
- The uptake of metal chelates by plants is associated to stress, which is occasionally followed by plant demise. It is unclear, however, whether stress is required for induction or only reflects the accumulation of high concentrations of synthetic chelate within the plant.
- It has been discovered that chemical additives (such as EDTA) are not only phytotoxic, but also exert their toxic effects on beneficial soil microorganisms known to play crucial roles in plant growth and development.
- In an alternative method, metal buildup can be accomplished by utilising the unique physiological processes that occur during the entire plant growth cycle.
- Continuous phytoextraction, unlike forced metal uptake, is done by the genetic and physiological aptitude of plants designed to accumulate, translocate, and tolerate high metal concentrations.
- There have been reports of continuous phytoextraction removing metals such as zinc, cadmium, and nickel as well as arsenic and chromium from soils rich in heavy metals by hyperaccumulating plants.
- Understanding the biological mechanisms by which plants become superior for the remediation of soils can be achieved by dissecting the method that is used to create metal hyperaccumulation.
- As a form of management technique for metal-contaminated sediment, phytoextraction is believed to address efficiency, duration, leaching, contamination of the food chain, and economic benefits.
- However, its implementation is limited by low biomass output and inadequately high metal uptake into plant tissue, as well as a lack of knowledge regarding the minimal additions (e.g., compost, irrigation) required to enable long-term plant establishment.
Advantages
- The price is quite affordable.
- Pollutants are eliminated permanently from locations.
Disadvantages
- The majority of hyperaccumulators have modest growth rates, poor biomass, and shallow root systems.
- Harvested biomass must be disposed of properly.
3. Phytodegradation
- The word phytodegradation refers to the enzymatic breakdown of pollutants into more simple or less harmful compounds by plants, either in the rhizosphere before to their absorption or sometimes in the root after their uptake and subsequent synthesis.
- It is a significant detoxification method that is also known as phytotransformation. If photodegradation happens in the rhizosphere, plant roots will release the necessary enzymes.
- Several root-associated bacteria also contribute to this process.
- Alternatively, if degradation occurs within the plant, it is essential that the plant be able to absorb the pollutants and translocate them to the site of transformation in their original state, without causing cell death.
- Numerous toxins, including herbicides, insecticides, pesticides, chlorinated solvents, munitions, and inorganic contaminants, can be metabolised.
- The plant enzymes utilised in this process are also recognised; dehalogenase, nitrilase, phosphatase, nitroreductase, and oxidoreductase are notable examples.
- These produce the best outcomes for lowly polluted soils.
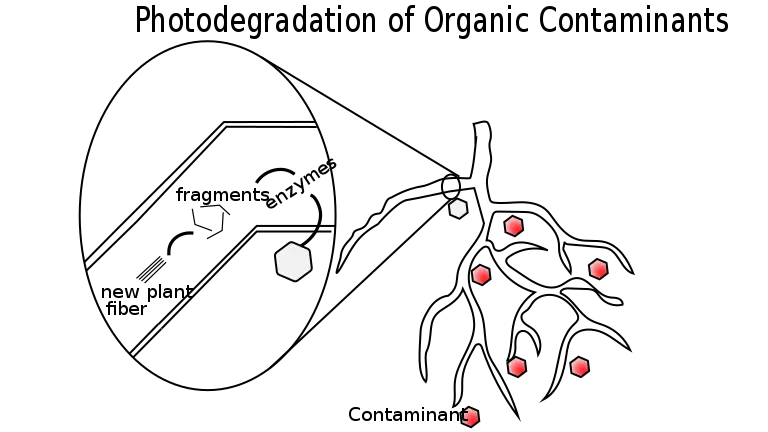
Advantages
- It is not dependent on rhizosphere-associated bacteria.
- Plant enzymes play a role in decomposition.
Disadvantages
- It is restricted to just degrading organic contaminants.
- It is ineffective against deep pollution but effective against minor contamination.
4. Rhizofiltration
- Rhizofiltration is the process of pollutants in the rhizosphere being absorbed into the root system of plants.
- This remediation method uses aquatic or terrestrial plants to detoxify aquatic habitats.
- Plants are grown either on the contaminated site (in situ) or in an ex situ environment during this process.
- The roots of plants absorb contaminants till saturation, after which the plants are removed together with their roots.
- Due to the accumulation of toxins in the roots without transfer to the shoots, the likelihood of air contamination is quite low.
- Rhizofiltration can be used to remove metals (Cr, Cd, Co, Cu, Ag, Hg, Ni, Mn, Pb, Zn, Mo), metalloids (As, Se), and radioactive materials (137Cs, 239Pu, 90Sr, 234U).
- Important plants utilised in this process include Indian mustard, rye, sunflower, tobacco, and spinach. Due of their quick development and fibrous roots, terrestrial plants are frequently used in this method.
- In in situ conditions, these plants are utilised as floating rafts, whereas in ex situ conditions, they are grown in engineered tank or pipe systems.
- It has also been observed that the introduction of microorganisms into the rhizosphere stimulates the uptake of pollutants.
Advantages
- Primarily aquatic plants are utilised, although occasionally terrestrial species are as well.
- Heavy metals are not required to be translocated to shots.
Disadvantages
- It must maintain an optimal pH level.
- Growing plants in a nursery before moving them to the cleanup site.
5. Phytovolatilization
- Phytovolatilization is the process by which plants collect toxins from contaminated places via their roots, transform the more harmful elements into less dangerous ones within the plant, and release them into the atmosphere via their leaves.
- This technique, which is based on the evapotranspiration process, is mostly employed for mercury, selenium, and organic solvent pollution.
- Tritium (3H), a radioactive isotope, has also been reported to be remedied using this technique with woody plants.
- The efficacy of phytovolatilization is affected by the environment of remediation locations and the genetic capability of the utilised plant species.
- Moreover, the absorption, transport, and chemical fate of these pollutants are highly reliant on their concentration at polluted areas.
- According to the United States Environmental Protection Agency, Alfalfa, Black locust, Canola, and Indian mustard are effective phytovolatilizing plants.
- Despite reports that this procedure eliminates a number of dangerous pollutants, it does not resolve the issue entirely.
- It simply extracts pollutants from the soil, water, and/or sediments and releases them into the atmosphere. We are aware that the concentration of the emitted substance is low and frequently less harmful, but this does not prevent it from upsetting the natural equilibrium of the atmosphere.
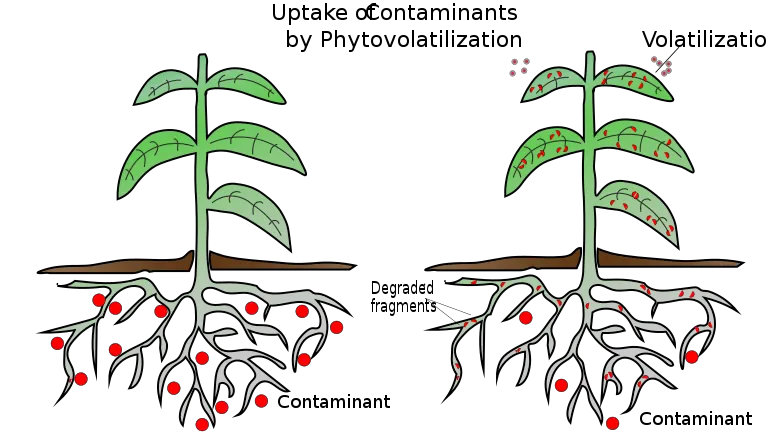
Advantages
- Pollutants could be converted into a less hazardous form.
- Pollutants released into the atmosphere are significantly diluted and less harmful.
Disadvantages
- Vegetation can accumulate pollutants.
- There have been reports of low amounts of contaminants in plant tissue.
Hyperaccumulation
- Hyperaccumulation is the capacity of plants to absorb heavy metals from contaminated soil and accumulate them to a greater degree than in the polluted soil.
- This buildup should be at least 100 times greater, but in the majority of plants used for this purpose, it is between 50 and 500 times more.
- Approximately 500 plant species have been identified as potentially capable of hyperaccumulation.
- According to the most recent studies, the defence mechanism may be a viable explanation for why plants have this unique ability.
- In reality, these plants protect themselves from various pathogens and/or herbivores by collecting naturally toxic substances in their tissues.
- The accumulation capacity of hyperaccumulators varies depending on the surrounding environment, the existence of relevant genes, and the control and expression of those genes.
- Hyperaccumulation begins with the uptake of metals from the soil, followed by the development of a metallocomplex with the aid of certain proteins (to detoxify the toxic effects of the metals), their transfer from plant roots to shoots via the apoplast or symplast, distribution and sequestration of these metals at the cellular level within the tissues, and finally the accumulation of these metals in the metabolically less active cells of the tissue.
- In general, it is a well-known fact that plants with the potential to hyperaccumulate grow slowly and produce less biomass; these are two of the most significant disadvantages of natural hyperaccumulators.
- Other genes from the HMA, MATE, YSL, and MTP families have also been identified to be involved in this process. Transport of metals after metalloprotein production is a crucial stage for hyperaccumulation and is primarily mediated by the ZIP gene family.
- As the several gene families responsible for this overall process have been discovered, they can now be transferred to a fast-growing plant to speed the accumulation process.
Examples of Hyperaccumulation

Best Plants For Phytoremediation
Indian mustard (Brassica juncea L.)
- According to the International Journal of Molecular Sciences, heavy metals harm not just industrial sites but also agricultural land, posing a threat to human health.
- Brassicaceae species are exceptionally useful for accumulating specific metals while also producing large amounts of biomass, and Indian mustard is the star of this group.
- It may remove three times as much Cd as conventional treatments, as well as 28% of Pb and up to 48% of Se, and is also efficient against Zn, Hg, and Cu.
- Unknown is the fact that Indian mustard also removed radioactive Cs137 from Chernobyl in the 1980s (Phytoremediation of Radiocesium-Contaminated Soil in the Vicinity of Chernobyl, Ukraine).
Willow (Salix species). (White Willow)
- Water-loving plants beautify landscapes, but their value is not limited to aesthetics alone.
- In addition, they have an intriguing application in phytoremediation: their roots have exhibited survivability, collecting fewer amounts of heavy metals than Brassicaceae, and they cope with Cd, Ni, and Pb, as well as areas contaminated with diesel fuel and combined heavy metals.
- Perhaps you recall Westergasfabriek Park in Amsterdam, which LAN discussed in the article Westergasfabriek Park Transforms from a Polluted Gas Factory to an Award-Winning Design by Gerard De Silva. Through ponds and water gardens, it demonstrates the recreational and remedial benefits of willows.
- Like the Swedish efforts detailed in Willows for electricity and phytoremediation in Sweden, large-scale systems for urban waste water are likewise beneficial.
Poplar tree (Populus deltoides).
- The beneficial impact of poplar trees on land and water has also been extensively investigated.
- Their secret is a naturally well-designed root structure that absorbs vast amounts of water.
- According to study from the National Institute of Environmental Health, hybrid poplars are better able to withstand chlorinated solvents such as trichloroethylene and the well-known carcinogenic carbon tetrachloride (95% of material eliminated).
- In addition, the Canadian database for bioremediation methods, PhytoPet(Bioremediation of Aquatic and Terrestrial Ecosystems), notes that poplar trees can digest petroleum hydrocarbons such as benzene, toulene, and o-xylene. However, they are uncommon in public gardens.
Indian grass (Sorghastrum nutans)
- Researchers examined how this native plant of the Midwestern United States improves the surrounding soil and ground water.
- The ability of Indian grass to detoxify common agrochemical residues, such as atrazine and metalochlor-related pesticides and herbicides, goes unnoticed by the majority of people who see it growing along roadside verges.
- Indian grass is one of nine graminae family members recognised by PhytoPet(Bioremediation of Aquatic and Terrestrial Ecosystems) as capable of remediating petroleum hydrocarbons. The list also contains grasses such as Common buffalo grass and Western wheatgrass, which rank highest.
Sunflower (Helianthus Annuus L.)
- Experiments such as Influence of the sunflower rhizosphere on the biodegradation of PAHs in soil demonstrate that sunflowers effectively remove PAHs from soil, but what is truly astonishing is the wide variety of contaminants they can acquire.
- Heavy metals such as Pb, Zn (Heavy Metals Extraction Potential of Sunflower (Helianthus annuus) and Canola (Brassica napus)), N, P, K, Cd, Cu, or Mn (Capability Of Heavy Metals Absorption By Corn, Alfalfa, And Sunflower Intercropping Date Palm) appear to be its food, which is good news because sunflowers have a fast growth rate and can begin producing quickly.
- In fact, one-month-old plants removed more than 95% of uranium in 24 hours (SUNFLOWER (Helinathus annuus L.) – A POTENTIAL CROP FOR THE ENVIRONMENTAL INDUSTRY), demonstrating their ability to remove radioactive metals, such as Cs and Sr, from shallow subsurface water.
- Numerous areas, such as waste mining sites, appear to benefit greatly from the combination of sunflowers and other plants.
Advantages of Phytoremediation
- In theory, plants that engage in phytoremediation of harmful components may be collected, eliminating the toxins from the contaminated location.
- The plants are plainly observable.
- Possibility of recovering and reusing precious metals (by firms specialising in “phytomining”).
- Because it uses naturally occurring organisms and retains the ecosystem in a more natural state, it is perhaps the least destructive option.
- It maintains the soil’s fertility by preserving the topsoil.
- It improves soil health, crop yield, and phytochemicals in plants.
Disadvantages of Phytoremediation
- Phytoremediation merely relocates hazardous heavy metals; it does not eliminate them from the site.
- The extent of phytoremediation is restricted to the root surface area and depth.
- Slow growth and low biomass demand a commitment over the long term.
- It is not possible to totally avoid the leaching of toxins into the groundwater using plant-based remediation techniques. The viability of plants is influenced by the toxicity of the contaminated land and the general state of the soil.
- Sometimes, when plants absorb heavy metals, the metal is linked to the organic matter of the soil, making it inaccessible for absorption.
References
- Yan, A., Wang, Y., Tan, S. N., Mohd Yusof, M. L., Ghosh, S., & Chen, Z. (2020). Phytoremediation: A Promising Approach for Revegetation of Heavy Metal-Polluted Land. Frontiers in Plant Science, 11. doi:10.3389/fpls.2020.00359
- Pandey, V. C., & Bajpai, O. (2019). Phytoremediation. Phytomanagement of Polluted Sites, 1–49. doi:10.1016/b978-0-12-813912-7.00001-6
- McCutcheon, S. C., & Jørgensen, S. E. (2008). Phytoremediation. Encyclopedia of Ecology, 2751–2766. doi:10.1016/b978-008045405-4.00069-0
- Peuke AD, Rennenberg H. Phytoremediation. EMBO Rep. 2005 Jun;6(6):497-501. doi: 10.1038/sj.embor.7400445. PMID: 15940279; PMCID: PMC1369103.
- Jan, A. T., Ali, A., & Rizwanul Haq, Q. M. (2015). Phytoremediation. Soil Remediation and Plants, 63–84. doi:10.1016/b978-0-12-799937-1.00003-6
- Chandra, R., & Hoduck, K. (2018). Phytoremediation and Physiological Effects of Mixed Heavy Metals on Poplar Hybrids. In H. E. M. Saleh, & R. F. Aglan (Eds.), Heavy Metals. IntechOpen. https://doi.org/10.5772/intechopen.76348
- Arthur, Ellen & Rice, Pamela & Rice, Patricia & Anderson, Todd & Baladi, Sadika & Henderson, Keri & Coats, Joel. (2005). Phytoremediation—An Overview. Critical Reviews in Plant Sciences. 24. 10.1080/07352680590952496.
- Susarla, Sridhar & Medina, Victor & Mccutcheon, Steven. (2002). Phytoremediation: An Ecological Solution to Organic Chemical Contamination. Ecological Engineering. 18. 647-658. 10.1016/S0925-8574(02)00026-5.
- https://www.geoengineer.org/education/web-class-projects/cee-549-geoenvironmental-engineering-winter-2013/assignments/phytoremediation
- https://land8.com/5-best-plants-for-phytoremediation/
- https://www.igi-global.com/chapter/introduction-to-phytoremediation/241164
- https://frtr.gov/matrix2/section4/4-3.html
- https://www.thoughtco.com/six-types-of-phytoremediation-375529
- https://clu-in.org/download/toolkit/phyto_o.pdf
- https://www.nature.com/scitable/knowledge/library/phytoremediation-17359669/#:~:text=Phytoremediation%20basically%20refers%20to%20the,cost%2Deffective%20environmental%20restoration%20technology.
- https://en.wikipedia.org/wiki/Phytoremediation
- http://www.cpeo.org/techtree/ttdescript/phytrem.htm