The Peroxide Value test is the process used to estimate the extent of primary oxidation in fats and oils. It is the measure of hydroperoxides formed at the initial stage of lipid oxidation, and it is expressed as milliequivalent of active oxygen per kilogram of oil. It is the major indicator of freshness because these peroxides are the earliest products formed when the oil begins to deteriorate.
In this test the oil sample is dissolved in an acidic solvent mixture, and potassium iodide (KI) is added. It is the hydroperoxides present in the oil which oxidise the iodide ions, releasing free iodine in the solution. The liberated iodine is then titrated with sodium thiosulfate using starch as an indicator. It is the amount of thiosulfate used that shows the peroxide value of that oil.
A low peroxide value indicates fresh oil, generally below 10 meq/kg for edible oils. A high value shows that oxidation has progressed and rancidity has started. It is important that hydroperoxides are unstable and later break down into secondary oxidation products. This process occurs when the oil is already highly degraded and sometimes results in a low peroxide value again. Thus careful interpretation is required for testing the quality of oils.
Objectives of Peroxide Value Test
- It is used to measure the amount of hydroperoxides formed during initial oxidation of fats and oils. This helps in quantifying the active oxygen present in the sample.
- It is carried out to assess the freshness and quality of oils and fats. Lower peroxide value indicates better quality while higher value shows deterioration.
- It is used to detect the early stage of rancidity before the development of unpleasant smell and taste. This is referred to as primary oxidative rancidity.
- It is applied to predict the shelf life and oxidative stability of oils during storage and processing. The test helps in estimating the time period of safe usage.
- It is used to ensure safety of food products as excessive peroxide formation may be harmful for consumption. It also helps in checking compliance with prescribed safety standards.
- It is useful in quality control during production by monitoring raw materials and processing conditions to avoid rancid products.
- It helps in evaluating nutritional quality as oxidation leads to destruction of essential fatty acids and fat-soluble vitamins.
Principle of Peroxide Value Test
The principle of the Peroxide Value test is based on an iodometric titration where the hydroperoxides present in the fat or oil oxidise the iodide ions. It is the hydroperoxides formed during the early stage of lipid oxidation that act as oxidising agents in this method. The sample is first dissolved in an acidic solvent mixture (acetic acid with an organic solvent). In this condition, potassium iodide reacts with the hydroperoxides and free iodine is released.
It is the iodine liberated in this step that is measured. The iodine formed is titrated with standard sodium thiosulfate solution using starch as an indicator. The dark blue colour produced by iodine–starch complex disappears at the end point. The amount of thiosulfate required in titration is directly related to the peroxide content of the sample and it is expressed as milliequivalent of active oxygen per kilogram of the sample.
It is important that hydroperoxides are unstable intermediates. These compounds later decompose into aldehydes and other secondary oxidised products. This process occurs when oxidation is advanced and sometimes gives a low peroxide value again. Because of this, the peroxide value alone cannot always show the full condition of the oil and additional tests may be required for proper oxidation assessment.
Requirements for Peroxide Value Test
Apparatus and Equipment
- Glass stoppered Erlenmeyer flask of about 250 ml capacity is required to carry out the reaction and to prevent loss of iodine.
- Analytical balance is used for accurate weighing of oil or fat sample. The balance should measure up to 0.0001 g.
- Burette of suitable capacity (10 ml or 25 ml) is required for titration of iodine with sodium thiosulfate solution.
- Pipettes of different volumes are used for transferring potassium iodide solution and other reagents.
- Stopwatch or timer is required to maintain fixed reaction time during iodine liberation.
- Magnetic stirrer along with stirring bar is used for proper mixing of the reaction mixture.
Reagents and Chemicals
- Solvent mixture consisting of glacial acetic acid and chloroform or isooctane is required for dissolving fat sample.
- Potassium iodide solution (saturated) is used for reaction with peroxides present in oil or fat.
- Sodium thiosulfate solution of known normality is required for titration of liberated iodine.
- Starch indicator solution is used to detect the end point of titration by disappearance of blue colour.
- Distilled water free from dissolved oxygen is required for dilution of reaction mixture.
- Primary standard chemicals are used for standardization of sodium thiosulfate solution.
Sample Preparation and Conditions
- Required amount of oil or fat sample is weighed accurately depending upon expected peroxide value.
- The test is carried out under controlled light conditions and direct sunlight should be avoided.
- Blank determination is performed using all reagents except the fat sample to correct reagent error.
- Sample should be properly homogenized without aeration and solid fats are melted gently before analysis.
Procedure of Peroxide Value Test
- Accurately weigh required quantity of oil or fat sample (generally about 5 g) into a clean dry glass stoppered Erlenmeyer flask.
- Add measured volume of solvent mixture containing glacial acetic acid and chloroform or isooctane to dissolve the sample completely. Swirl the flask gently.
- Add fixed volume of freshly prepared saturated potassium iodide solution to the flask. The flask is immediately closed with stopper.
- Allow the mixture to stand for exactly one minute. During this period the flask is shaken vigorously so that peroxides react with iodide ions.
- After one minute add required volume of distilled water to stop the reaction and dilute the mixture.
- Titrate the liberated iodine with standard sodium thiosulfate solution while shaking continuously until the yellow colour becomes pale.
- Add few drops of starch indicator solution. The solution turns blue in colour.
- Continue titration dropwise with constant shaking until the blue colour just disappears and solution becomes colourless.
- Carry out a blank determination using all reagents except the oil or fat sample under same conditions.
- Note the volume of sodium thiosulfate used for sample and blank and calculate the peroxide value of the sample.
Calculation of Peroxide Value Test
The calculation of Peroxide Value (PV) is done by iodometric titration method. It is based on the amount of iodine liberated from potassium iodide by peroxides present in fat or oil sample and titrated against sodium thiosulfate solution.
Formula used
Peroxide Value (PV) is calculated by the following formula–
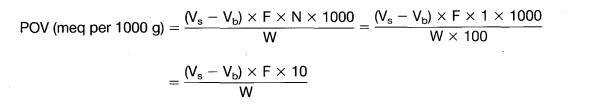
Where
- V – Volume of sodium thiosulfate used for sample titration (mL).
- V0 – Volume of sodium thiosulfate used for blank titration (mL).
- N – Normality of sodium thiosulfate solution.
- W – Weight of oil or fat sample taken (g).
- 1000 – Conversion factor to express result per kilogram of sample.
Unit– Peroxide Value is expressed as milliequivalents of active oxygen per kilogram of fat (meq/kg).
Sample Calculation
The calculation is explained using experimental values obtained during peroxide value test.
- Weight of oil sample taken (W) – 5.0921 g.
- Volume of sodium thiosulfate used for sample (V) – 1.09 mL.
- Volume of sodium thiosulfate used for blank (V₀) – 0.90 mL.
- Corrected volume (V – V₀) – 0.19 mL.
- Normality of sodium thiosulfate (N) – 0.1003 N.
Calculation
Peroxide Value = 3.74 meq/kg.
Expression in SI unit
- The value obtained in meq/kg can be converted to mmol/kg.
- Since valency of oxygen is 2, the value is divided by 2.
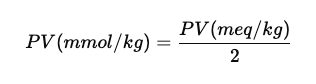
- Therefore, Peroxide Value in mmol/kg = 1.87 mmol/kg.
Note
- Higher peroxide value indicates higher degree of lipid oxidation.
- Blank correction is necessary to eliminate error due to reagents.
- Accuracy of PV depends on correct standardization of sodium thiosulfate solution.
Result of Peroxide Value Test
- The result is obtained as a numerical value representing the amount of hydroperoxides present in oil or fat sample. It is expressed as milliequivalents of active oxygen per kilogram of sample (meq O₂/kg).
- The end point of the test is indicated by disappearance of blue colour after addition of starch indicator during titration. This shows complete reaction of iodine.
- A low peroxide value indicates fresh oil with minimum primary oxidation. Freshly refined oils generally show very low values.
- A high peroxide value indicates higher degree of oxidation and development of rancidity in oil or fat sample.
- The obtained value is compared with standard permissible limits to assess quality and suitability of oil for consumption.
- The final result is calculated on the basis of volume of sodium thiosulfate used for sample and blank titration along with weight of sample.
Uses of Peroxide Value Test
- It is used to measure the primary oxidation of fats and oils by estimating hydroperoxides formed during oxidation.
- It is used to assess the freshness of edible oils and fats where low peroxide value indicates fresh sample.
- It is used in quality control of oils fats and fat containing food products during processing storage and distribution.
- It is used to detect the early stage of oxidative rancidity before development of unpleasant odour and taste.
- It is used to predict the shelf life of oils and fat rich food products based on their oxidative condition.
- It is used to ensure food safety by preventing consumption of oxidized fats which may produce toxic compounds.
- It is used for regulatory compliance to check whether oils meet prescribed standards and limits.
- It is used to verify the quality of raw materials such as crude oils nuts and oil seeds before processing.
- It is used to monitor the effect of heating frying and other processing conditions on oil stability.
- It is used to evaluate the effectiveness of antioxidants added to fats and oils.
- It is also used in cosmetic and pharmaceutical industries to check stability of lipid based products.
Advantages of Peroxide Value Test
- It is used to detect early stage of lipid oxidation by measuring hydroperoxides which are primary oxidation products.
- It is a standard and widely accepted method used by different regulatory bodies for quality evaluation of fats and oils.
- It is a simple and rapid test which can be performed easily by iodometric titration method.
- It is a cost effective method as it requires minimum laboratory apparatus and chemicals.
- It is useful in assessing food safety by detecting oxidized fats which may produce harmful compounds.
- It is helpful in predicting shelf life of oils and fat rich food products during storage.
- It shows good sensitivity under controlled conditions and gives reliable results.
- It is applicable to different types of fats and oils such as animal fats vegetable oils and margarines.
Limitations of Peroxide Value Test
- It measures only primary oxidation products and does not indicate secondary oxidation of fats and oils.
- It may show low value even in highly oxidized oil due to decomposition of hydroperoxides.
- It is affected by atmospheric oxygen which can oxidize iodide and give higher results.
- Unsaturated fatty acids present in oil may absorb iodine leading to underestimation of peroxide value.
- It does not give complete information about past oxidative history of the sample.
- The results depend on experimental conditions such as time temperature and sample weight.
- It is difficult to detect end point clearly in dark or highly coloured oils.
- Presence of antioxidants and other interfering substances may affect accuracy of the test.
- It requires relatively large sample and use of organic solvents which may cause safety problems.
- Abeyrathne, E. D. N. S., Nam, K., & Ahn, D. U. (2021). Analytical methods for lipid oxidation and antioxidant capacity in food systems. Antioxidants, 10(10), 1587.
- Bakota, E. (2014). Peroxide value method. Protocols.io. https://dx.doi.org/10.17504/protocols.io.cjeujd
- Barriuso, B., Astiasarán, I., & Ansorena, D. (2013). A review of analytical methods measuring lipid oxidation status in foods: A challenging task. European Food Research and Technology, 236, 1-15.
- CDR FoodLab. (n.d.). Peroxide value (POV) test in oils and fats.
- Codex Alimentarius Commission. (1999). Codex standard for edible fats and oils not covered by individual standards (CODEX STAN 19-1981, Rev. 2-1999).
- Codex Alimentarius Commission. (2024). Standard for fish oils (CXS 329-2017).
- Comprehensive technical analysis of peroxide value determination: Chemical kinetics, standardized methodologies, and analytical rigor in lipid quality assessment. (n.d.).
- Dunford, N. T. (2016). Edible oil quality (FAPC-197). Oklahoma State University Extension.
- Fiveable Content Team. (2025, September). Peroxide value. Fiveable.
- Gordon, V. C., Rainey, C. C., & Studmire, W. C. (2021). Validation of the SafTest peroxide test kit for the measurement of the peroxide content of oils, tallows, meat meals, potato chips, and grain-based snacks: AOAC Performance Tested MethodSM 101001. Journal of AOAC International, 104(2), 325-338.
- Gotham, J. P., Li, R., Tipple, T. E., Lancaster, J. R., Jr., Liu, T., & Li, Q. (2020). Quantitation of spin probe-detectable oxidants in cells using electron paramagnetic resonance spectroscopy: To probe or to trap? Free Radical Biology and Medicine, 154, 84–94.
- Gotoh, N., Miyake, S., Takei, H., & Sasaki, K. (2011). Simple method for measuring the peroxide value in a colored lipid. Food Analytical Methods, 4(4).
- Hanna Instruments. (n.d.). Has your food joined the dark side? Test for peroxide value to find out! [Blog post].
- Ichu, C., & Nwakanma, H. (2019). Comparative study of the physicochemical characterization and quality of edible vegetable oils.
- International Fragrance Association. (2019). IFRA analytical method: Determination of the peroxide value.
- International Organization for Standardization. (2017). Animal and vegetable fats and oils — Determination of peroxide value — Iodometric (visual) endpoint determination (ISO Standard No. 3960:2017).
- Ivanova, V. V., Pimpilova, M. G., Stoyanova, M. K., & Dimcheva, N. D. (2024). Electrochemical method for the assay of organic peroxides directly in acetonitrile [Preprint]. Preprints.org.
- Lea, C. H. (2007). The determination of the peroxide values of edible fats and oils: The iodimetric method. Journal of the Society of Chemical Industry, 65(10), 286-291. (Original work published 1946).
- Ottaway, J. M., Chance Carter, J., Adams, K. L., Camancho, J., Lavine, B. K., & Booksh, K. S. (2021). Comparison of spectroscopic techniques for determining the peroxide value of 19 classes of naturally aged, plant-based edible oils. Applied Spectroscopy, 75(7).
- Pasicaran, J. P. (n.d.). Peroxide value determination [Uploaded document]. Scribd.
- Ruiz-Medina, A., Ayora Cañada, M. J., & Lendl, B. (2001). A rapid method for peroxide value determination in edible oils based on flow analysis with Fourier transfer infrared spectroscopic detection. The Analyst, 126(2), 242-246.
- SI Analytics. (n.d.). Determination of peroxide value (POV) in fats and oils [Application Report]. Xylem Analytics.
- Sutton, A. T., & Rustandi, R. R. (2024). Determining the oxidation mechanism through radical intermediates in polysorbates 80 and 20 by electron paramagnetic resonance spectroscopy. Pharmaceuticals, 17(2), 233.
- Suzen, S., Gurer-Orhan, H., & Saso, L. (2017). Detection of reactive oxygen and nitrogen species by electron paramagnetic resonance (EPR) technique. Molecules, 22(1), 181.
- Swartz, H. M., Khan, N., & Khramtsov, V. V. (2007). Use of electron paramagnetic resonance spectroscopy to evaluate the redox state in vivo. Antioxidants & Redox Signaling, 9(10), 1757–1771.
- Taylor & Francis. (n.d.). Peroxide value. Taylor & Francis Knowledge.
- Vitas. (n.d.). Quantification of peroxide value (PV) in oil (AM-014).
- Wikipedia. (2024, December). Peroxide value. In Wikipedia.
- YesWeLab. (2024, October 31). Laboratory analysis of peroxide value [Blog post].
- Yildiz, G., Wehling, R. L., & Cuppett, S. L. (2003). Comparison of four analytical methods for the determination of peroxide value in oxidized soybean oils. Journal of the American Oil Chemists’ Society, 80(2), 103-107.
- Yildiz, Y., Duchaine, Y., & Ergin, Z. N. (2025). Analytical methods for determining the peroxide value of hemp seed (Cannabis sativa L.) oil. European Journal of Applied Sciences, 13(04), 364-369.
- YSI. (n.d.). Determination of peroxide value (POV) in fats and oils [Application Note XA00078].
- Yu, X., Li, Q., Sun, D., Dong, X., & Wang, T. (2014). Determination of peroxide value of edible oils by FTIR spectroscopy using polyethylene film. Analytical Methods, 7(5).