What is Paper Chromatography?
- Paper chromatography is a technique which is used for separating mixture of dissolved chemical substances by making them travel at different rates on a piece of paper.
- A strip or sheet of cellulose-based paper is used as the stationary phase (often the moisture held in the paper acts as the actual stationary medium).
- A solvent (or mixture of solvents) acts as the mobile phase and moves by capillary action through the paper carrying the dissolved substances.
- The mixture to be separated is spotted near one end of the paper and then the paper is placed so that only the end touches the solvent, the solvent moves up (or sometimes down) carrying the components.
- Different components in the mixture move different distances because they have different affinities for the paper (stationary phase) versus the solvent (mobile phase).
- The term R ₑ (or R₍f₎) stands for the ratio of the distance moved by the compound divided by the distance moved by the solvent front; it is used to compare how far substances travelled.
- It is considered a cost-effective, simple analytical tool and it requires very small sample quantities, making it useful especially for educational purposes or when only little material is available.
- The principle underlying it can be partition chromatography (distribution of substance between two liquid phases) or sometimes adsorption chromatography (adsorption onto solid surface) depending on system.
- Although it is simple, the resolution (how cleanly components separate) may not be as high as more advanced methods (like TLC or HPLC) when complex mixtures are involved.
- The method is still used to separate things like pigments, amino acids, small molecules (for example in plants, food analysis) when high tech instrument isn’t available.
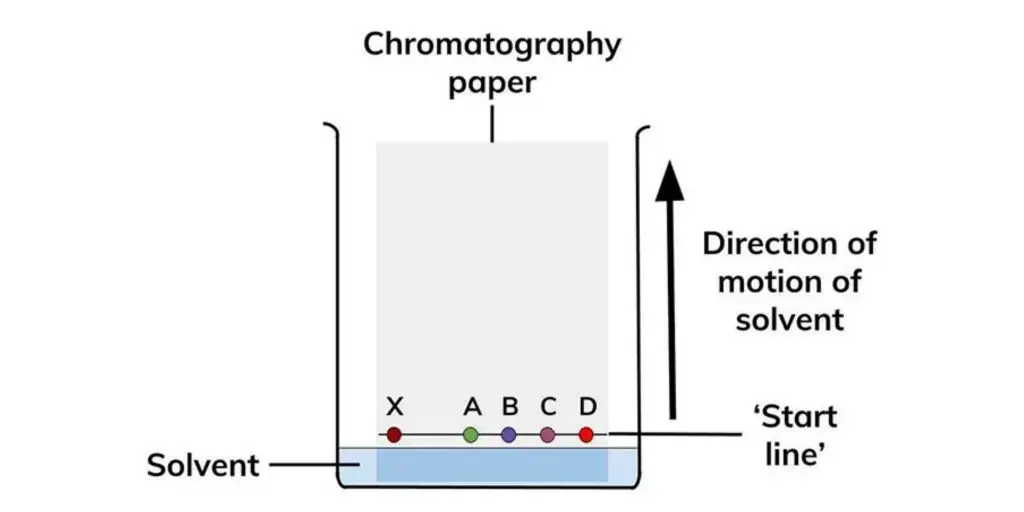
Types of Paper chromatography
1. Ascending paper chromatography – In this type the solvent is placed at bottom of paper and it is allowed to climb up by capillary action, the components are separated as they move upward.
2. Descending paper chromatography – In this variation the solvent moves downwards (by gravity + capillary) from top toward bottom of paper and the separation occurs during that downward flow.
3.Ascending-Descending (or combined) paper chromatography – A mixed method where initially solvent travels up then at some fold/rod it continues by going down, so that the paper gets two directional movement of mobile phase.
4. Radial (or circular) paper chromatography – A circular piece of paper is used, sample is spotted at centre, solvent is made to spread radially outward forming rings of separated components.
5. Two-dimensional (2-D) paper chromatography – Paper is developed in one solvent in one direction, then turned 90° and developed in another solvent (or same) so that substances with similar Rₑ moves are resolved.
6. Paper partition vs adsorption chromatography – Though not a separate “directional type”, the technique is classified by mechanism: in one the paper’s water (in fibres) acts as stationary phase (partition) and in another paper impregnated with silica/alumina acts as adsorbent (adsorption).
Principle of Paper chromatography
- The method is based on partitioning and sometimes on adsorption of mixture components between a stationary phase and a mobile phase.
- In this technique the stationary phase is the water held inside the pores of cellulose-paper and the paper fibres themselves act as support.
- The mobile phase is the solvent (or solvent mix) which moves by capillary action over/through the paper carrying the dissolved substances.
- The mixture to be separated is applied near one end of the paper and then the mobile phase is allowed to move, so components travel at different rates.
- The components with higher solubility in the mobile phase and lesser affinity for the stationary phase will move further.
- Conversely the substances with stronger attraction to the stationary phase (paper/ water) will move slower and will lag behind.
- The result is that separate spots (or bands) of components appear on the paper (chromatogram) because of different migration distances.
- The R₍f₎ value (retardation/retention factor) is used to quantify how far a component moved, relative to solvent front.
- The choice of paper type, solvent polarity, temperature (for example 20-30 °C or 25 °C) etc will influence separation.
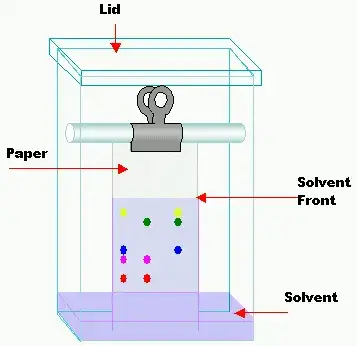
Instrumentation of Paper chromatography
1. Stationary phase – In paper chromatography the stationary phase is the water (or moisture) held within the pores and fibres of the cellulose-paper, and the paper support itself acts as the fixed medium.
2. Mobile phase – The mobile phase is the solvent (or mixture of solvents) which moves by capillary action along/through the paper carrying components of the mixture.
3. Chromatography paper (support medium) – A uniform, absorbent sheet of cellulose‐based paper is used to support the stationary phase and to allow the mobile phase to travel.
4. Sample Spot / Applied Mixture – The mixture to be separated is applied as a small spot or line on the paper (near one edge) so that when the mobile phase moves, the components will migrate.
5. Solvent front/Developing chamber – The boundary of how far the mobile phase has travelled (solvent front) and the container in which the paper is developed (often covered to saturate vapour) are also essential parts of the setup.
6. Detection / Visualisation system – After separation the components may be colour-spotted, stained, or visualised (for example with UV) so that the separated bands/spots can be seen and measured.
Steps in Paper Chromatography
- Preparation of paper – A special chromatography paper is selected and it is usually cut in rectangular shape. Sometimes the paper are washed by solvent to remove impurities that may interfere with the separation.
- Preparation of sample – The sample solution (like pigment extract or amino acid mixture) is prepared and then filtered. In many cases, dilution is also done for proper movement by capillary action.
- Marking of baseline – A light pencil line is drawn around 2 cm from bottom edge, it is called origin line or base line. This line is used for spotting the sample.
- Application / spotting of sample – The sample is applied carefully on the base line using capillary tube or micropipette. Spots must be small and dried properly before next step otherwise diffusion happen.
- Development of chromatogram – The paper strip is then placed into the chromatography chamber containing the mobile phase (solvent system). Solvent level must be below the origin line. The chamber is kept closed to maintain vapor saturation. As the solvent move upward by capillary action, components of mixture get separated depending upon their affinity toward stationary / mobile phase. Different types of development techniques can be used:
- Ascending Development – The conventional ascending method is used where solvent moves up against gravity, spots are kept at bottom portion of paper and the tank contains solvent at bottom, this is most common.
- Descending Development – A special chamber is used where solvent flows down from top to bottom, the spot is placed near top and solvent holder is at top, so separation is produced by solvent moving downward.
- Ascending–Descending Development – A hybrid method is employed when longer path is needed, first ascending then descending is allowed, length of separation is increased and resolution may improve.
- Circular / Radial Development – The sample is spotted at centre of a circular paper, solvent is introduced through a wick at centre and it spreads radially in all directions, uniform spreading is achieved, useful for specific set-ups.
- Monitoring Solvent Front – When solvent front nears edge, the paper is removed and the solvent front is immediately marked with pencil, delay causes error in measurements.
- Drying of the paper – After solvent front reach a certain height, the paper is removed and the solvent front is marked immediately with pencil. Then the paper is dried by air or in oven around 40°C( sometimes 60 °C depending type of solvent ).
- Detection of spots – The separated spots are visualized either by natural color or by using locating reagents (like ninhydrin for amino acids). Sometimes UV light is used for colorless compounds.
- Radiolabeled / Fluorescent Detection – Radioactive or fluorescently labeled analytes are detected by measuring radioactivity or fluorescence respectively, instrumentation (scanners, detectors) are used when sensitivity is required.
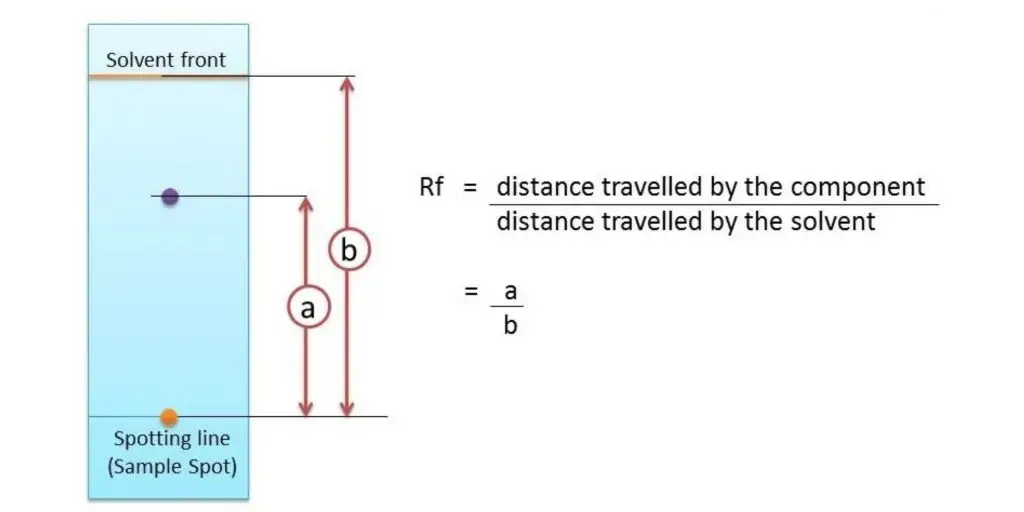
- Calculation of Rf value – Distance travelled by solute and solvent front are measured, and the Rf (retention factor) value is calculated by formula Rf = distance travelled by solute / distance travelled by solvent.
Rf values – Retention factors
- Rf value (Retention factor) in Paper Chromatography refers as ratio of the distance moved by a solute to distance moved by solvent front.
- It can define as – the measure of relative movement of a compound on the paper medium.
- In experiment, the Rf of each component is calculated by dividing the distance traveled by substance (spot) by the distance traveled by solvent front.
- Formula generally written as – Rf = Distance travelled by solute / Distance travelled by solvent front.
- Each compound shows different Rf values depending on its solubility and interaction by stationary phase (paper) and mobile phase (solvent).
- The greater the solubility of substance in mobile phase, higher distance it travels, giving larger Rf.
- If compound more strongly adsorbed on paper, its Rf value becomes smaller or lower.
- The Rf value always less than 1.0, since solute cannot move farther than solvent front.
- In some cases slight variation occur due to temperature, type of paper, solvent polarity etc.
- The same compound always gives same Rf under identical conditions, which help for identification of unknown substances.
- Sometimes it also called as ratio to front because it indicates how far component goes toward the solvent front.
- By comparing Rf of known and unknown spots, identification of components in mixture can be done easily.
- The Rf values provide qualitative data, not quantitative concentration information.
- Values usually expressed without unit since it’s a ratio number.
- For example, if a spot moves 3 cm and solvent moves 6 cm then Rf = 3/6 = 0.5, which mean the component moved half distance of solvent front.
- Rf values, though simple, are important analytical tool for purity checking / compound recognition etc.
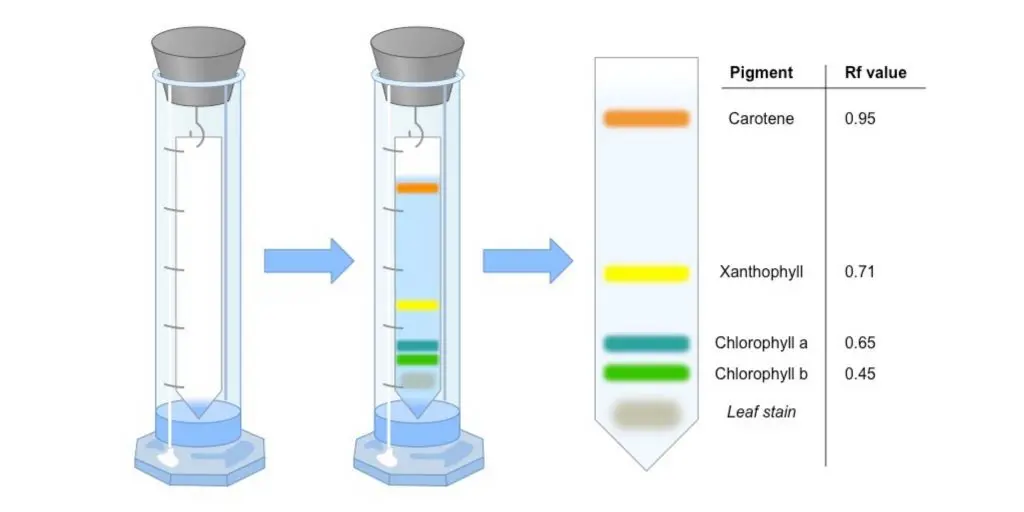
Applications of Paper Chromatography
- It is mainly used for identification of amino acids, proteins and sugars from mixtures, where spots are compared by their Rf values.
- In forensic science, paper chromatography is used for detecting of blood, ink, or drugs stains by separation on the paper sheet.
- The plant pigments like chlorophyll, carotenoids and xanthophylls are separated and studied by this method.
- The method is also applied for purity testing of several pharmaceutical substances; impurities get separated on paper.
- In food industry, it is utilized for detection of artificial colors, preservatives, and adulterants etc.
- Drugs analysis by chromatographic paper is often performed in hospitals or forensic labs for detecting toxic materials.
- Environmental chemists used it for determination of pollutants present in water / soil samples.
- Used in biochemical laboratories for qualitative analysis of organic compounds, particularly in metabolic studies.
- The method has been used for monitoring reactions, by observing how components change with time on paper strip.
- It is simple technique which can used in educational purpose / demonstration for explaining principle of chromatography to students.
Advantages of Paper Chromatography
- The method is simple and easy to perform without using any complicated or costly instruments.
- It can used for separation of very small quantity of mixture which is not possible by many other techniques.
- Only a small amount of solvent is needed, so the cost of analysis remain very low.
- The equipment required are minimal – only paper, solvent, and chamber – that’s why it’s easily carried out in school or small lab also.
- Both qualitative / quantitative analysis can done by this method depending on purpose of experiment.
- The technique is rapid, giving results within short time compared to column or gas chromatography.
- Samples like amino acids, sugars, pigments etc. can be separated without any prior treatment which save much time.
- It is portable, so field analysis or outdoor testing is possible with less effort.
- No complex skill is required, even beginners can perform with little training, though accuracy depend by handling.
- Method is non-destructive; the separated spots may be recovered or further analyzed by other techniques.
Limitations of Paper Chromatography
- The method is not suitable for volatile substances, since they evaporate before separation happen properly.
- Accuracy of results often affected by temperature and humidity, so conditions must be kept almost same but it’s hard to control always.
- Resolution between two closely related compounds, like isomers, is usually poor and overlapping spots may occur.
- Quantitative estimation not very reliable because spot intensity may vary by uneven absorption on paper.
- Speed of separation is slow compared to modern chromatographic techniques like HPLC or gas chromatography.
- Paper quality (thickness, texture, brand etc.) influences migration of solvent front, so reproducibility is difficult.
- The solvent system selection require trial and error, since no single solvent work for all types of mixtures.
- Samples having very high molecular weight or insoluble in chosen solvent can’t separated effectively.
- Detection of colorless compounds is difficult, often needs special reagents or UV lamp which increase time and cost.
- It is not suitable for industrial large scale work, mainly used for small / educational or research purposes only.
FAQ
What is paper chromatography?
Paper chromatography is a technique used to separate and analyze the components of a mixture based on their differential migration through a paper or cellulose matrix. It relies on the principles of partition and adsorption chromatography.
Can paper chromatography be used for quantitative analysis?
Paper chromatography is not typically used for quantitative analysis due to limitations in accuracy and reproducibility. It is more commonly employed as a qualitative technique for separation, identification, and comparison of components in a mixture.
What are the different development techniques in paper chromatography?
Different development techniques in paper chromatography include ascending development (solvent flows against gravity), descending development (solvent flows from top to bottom), ascending-descending development (a hybrid of ascending and descending techniques), and circular/radial development (solvent spreads uniformly in all directions from a central spot on a circular paper).
How can the components on a paper chromatogram be detected?
Components on a paper chromatogram can be detected using various methods. Colorless analytes can be visualized by treating the paper with reagents such as iodine vapor or ninhydrin, which produce color reactions. Radiolabeled or fluorescently labeled analytes can be detected by measuring radioactivity or fluorescence, respectively.
How can I ensure the best results in paper chromatography?
To achieve the best results in paper chromatography, it is important to carefully select the appropriate paper and mobile phase, ensure proper saturation of the chromatographic chamber with solvent vapor, apply the sample accurately and in small quantities, and optimize the development technique and conditions for the specific analyte or mixture being analyzed.
What are the limitations of paper chromatography?
Some limitations of paper chromatography include the inability to handle large sample quantities, lack of effectiveness in quantitative analysis, difficulty in separating complex mixtures, and lower accuracy compared to more advanced chromatographic techniques like high-performance liquid chromatography (HPLC) or high-performance thin-layer chromatography (HPTLC).
How is an Rf value calculated in paper chromatography?
The Rf (retention factor) value is calculated by dividing the distance traveled by a component from the application point to the distance traveled by the solvent front. It is a measure of the relative mobility of a component in the chromatogram and is constant under specific experimental conditions.
How does paper chromatography work?
In paper chromatography, a sample mixture is applied to a piece of filter paper, and the edge of the paper is immersed in a solvent or mobile phase. The solvent moves up the paper through capillary action, carrying the components of the mixture with it. As the solvent moves, the components separate based on their affinity for the stationary phase (paper) and the mobile phase (solvent), resulting in distinct bands or spots on the paper known as chromatograms.
What are the applications of paper chromatography?
Paper chromatography has a wide range of applications, including analyzing the purity of pharmaceuticals, detecting adulterants in food and drinks, studying ripening and fermentation processes, identifying drugs and doping substances in humans and animals, analyzing cosmetics, and examining reaction mixtures in biochemical labs. It can also be used to analyze specific classes of compounds such as amino acids, alkaloids, polysaccharides, proteins, pigments, and plant extracts.
What are the advantages of paper chromatography?
Paper chromatography offers several advantages, including its simplicity, rapidness, requirement of small sample quantities, low cost compared to other chromatography methods, ability to identify both organic and inorganic compounds, compact setup, and excellent resolving power.
- Skoog, D.A., West, D.M., Holler, F.J., Crouch, S.R. (2013). Fundamentals of Analytical Chemistry. Cengage Learning. Chapter 27: Paper Chromatography.
- Bhattacharya, S., Banerjee, R. (2018). Thin-Layer and Paper Chromatography: A Laboratory Handbook. CRC Press.
- Sherma, J., Fried, B. (2003). Handbook of Thin-Layer Chromatography. Marcel Dekker.
- Stahl, E. (1969). Thin-Layer Chromatography: A Laboratory Handbook. Springer.
- Smith, R.M. (1995). Introduction to Paper Chromatography. CRC Press.
- Text Highlighting: Select any text in the post content to highlight it
- Text Annotation: Select text and add comments with annotations
- Comment Management: Edit or delete your own comments
- Highlight Management: Remove your own highlights
How to use: Simply select any text in the post content above, and you'll see annotation options. Login here or create an account to get started.