The Optochin Susceptibility Test is a laboratory test that is used in microbiology for the identification of Streptococcus pneumoniae. It is mainly used to differentiate pneumococcus from other alpha hemolytic streptococci, especially viridans group streptococci. These organisms shows similar hemolytic pattern on blood agar but differs in their response to optochin.
It is the test in which optochin (ethylhydrocupreine hydrochloride) is used as the chemical agent. Optochin is a quinine derivative which inhibits the growth of Streptococcus pneumoniae by interfering with the ATP synthesis system of the cell. This inhibition is due to the binding of optochin to F₀F₁-ATPase enzyme complex, which results in failure of energy production and growth inhibition.
In this test, a filter paper disc containing optochin (5 µg) is placed on the surface of sheep blood agar plate previously inoculated with the test organism. The plate is incubated at 35–37°C for 18–24 hours in presence of CO₂. After incubation, the plate is observed for a clear zone of inhibition around the disc. A zone diameter of 14 mm or more indicates optochin sensitivity and the organism is identified as Streptococcus pneumoniae, whereas absence of zone or growth up to the disc indicates optochin resistance.
Objective of Optochin Susceptibility Test
- It is used for the presumptive identification of Streptococcus pneumoniae.
- It is the process of differentiating S. pneumoniae from other alpha-hemolytic streptococci that shows resistance to optochin.
- It is done to determine whether the organism is susceptible or resistant to ethylhydrocupreine hydrochloride (optochin).
- It helps in distinguishing organisms showing similar alpha-hemolytic colonies on blood agar.
- It is used as a diagnostic aid for confirming pneumococcal infections from clinical specimens.
Principle of Optochin Susceptibility Test
The principle of Optochin susceptibility test is based on the selective sensitivity of Streptococcus pneumoniae to optochin (ethylhydrocupreine hydrochloride), which helps in its differentiation from other alpha-hemolytic streptococci. Optochin is a quinine derivative and it inhibits the growth of pneumococci by interfering with the ATP synthase enzyme present in the bacterial cell membrane. This inhibition blocks the essential ion transport mechanism and ATP synthesis, due to which the cell is unable to survive.
In this test, a paper disc impregnated with optochin is placed on blood agar medium inoculated with the test organism. As the optochin diffuses into the agar, growth of Streptococcus pneumoniae is inhibited and a clear zone of inhibition is formed around the disc. Other alpha-hemolytic streptococci are resistant to optochin and hence they grow up to the edge of the disc without forming a zone. This differential response forms the basis of the optochin susceptibility test.
Requirements of Optochin Susceptibility Test
Reagents and Media
- Optochin disk is required (6 mm, 5 µg ethylhydrocupreine hydrochloride) and it is stored at low temperature to preserve its activity.
- Blood agar plate prepared on trypticase soy agar with 5% sheep blood is the recommended medium for this test.
- A 0.5 McFarland turbidity standard is required for adjusting the inoculum to proper density.
Sample Requirements
- A pure culture is required, usually 18–24 hours old, showing alpha-hemolysis on blood agar.
- The inoculum density is adjusted to 0.5 McFarland so that the lawn of growth is uniform on the plate.
Equipment and Supplies
- Sterile loop or sterile cotton swab is used to streak the organism on the agar surface.
- Sterile forceps is used to place the optochin disk gently on the agar.
- A millimeter scale is needed to measure the inhibition zone diameter.
- An incubator maintaining 35°C to 37°C is required.
- A CO₂ source (5–10%) is needed as some pneumococci do not grow well in normal air.
Quality Control
- A positive control strain (S. pneumoniae ATCC 49619 or ATCC 6305) is used and it shows a clear zone of inhibition.
- A negative control strain (S. mitis ATCC 49456 or S. pyogenes ATCC 19615) is used and it grows up to the disk.
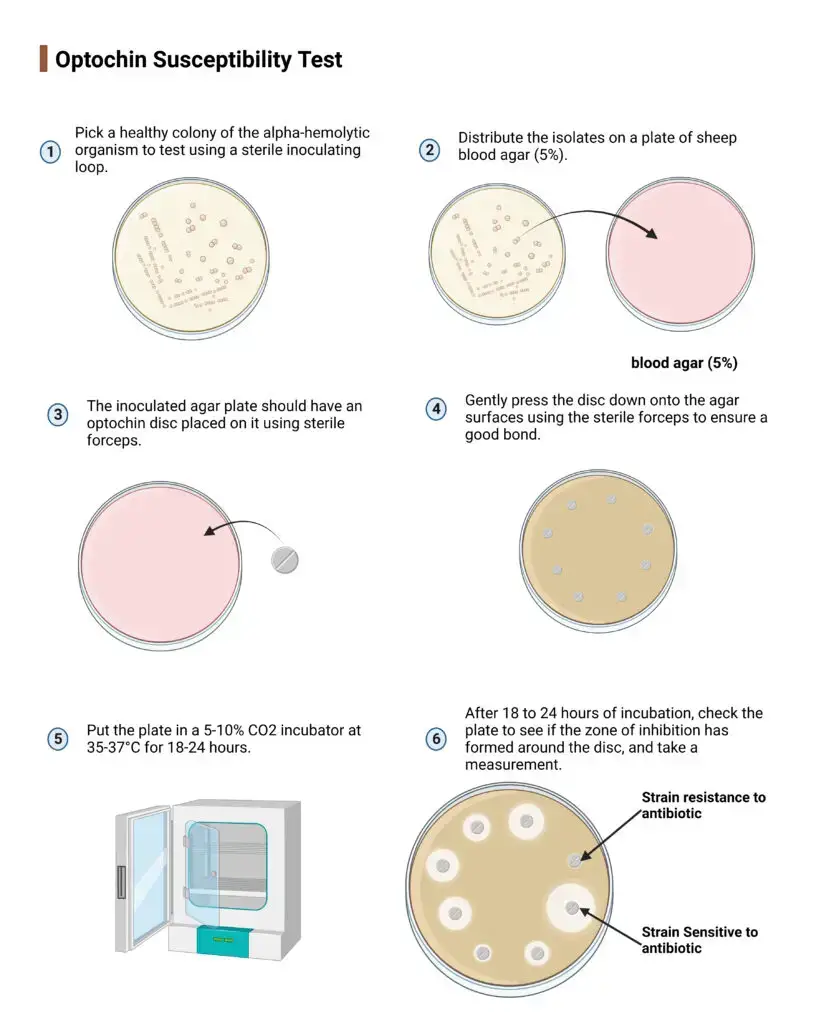
Procedure of Optochin Susceptibility Test
- Preliminary Identification and Preparation
- It is important to confirm the organism before the test is started.
- Gram staining is performed to observe Gram-positive diplococci.
- Three to four well isolated α-hemolytic colonies is selected from a fresh culture (18–24 hour).
- The colonies is suspended in sterile broth (saline or Heart Infusion Broth) and the turbidity is adjusted to 0.5 McFarland standard.
- It is the step where heavy inoculum may reduce the inhibition zone and this cause wrong interpretation.
- Media Selection
- Blood agar plate is used for the test.
- TSA or Columbia agar with 5% sheep blood is used as these are giving better inhibition zone.
- Some of the main features are that TSA produce larger zone and less false negative results.
- Inoculation
- A sterile swab is dipped into the suspension and the surface of agar is streaked in three directions to make a uniform lawn.
- In this step some laboratories streak one half with test culture and the other half with known positive control.
- Disk Application
- A 6 mm filter paper disk containing 5 µg optochin is placed in the center of inoculated area with sterile forceps.
- The disk is gently pressed so that proper contact with the agar surface is maintained.
- Incubation
- The plates is incubated at 35°C–37°C.
- Incubation is done in CO₂ enriched condition (5–10% CO₂) or in a candle jar.
- Duration is 18–24 hours for proper reading.
- Reading and Interpretation
- The zone of inhibition around the disk is observed and its diameter is measured including the disk.
- If the diameter is 14 mm or more, it is referred to as susceptible indicating Streptococcus pneumoniae.
- A zone between 7–13 mm is equivocal and these isolates is tested again using bile solubility.
- A zone of 6 mm (growth up to the disk) is resistant which is common in viridans streptococci.
Result and Interpretation of Optochin Susceptibility Test
After incubation, the zone around the optochin disk is examined. It is measured including the disk diameter. The growth pattern around the disk help in determining whether the organism is Streptococcus pneumoniae or other α-hemolytic streptococci.
Interpretation
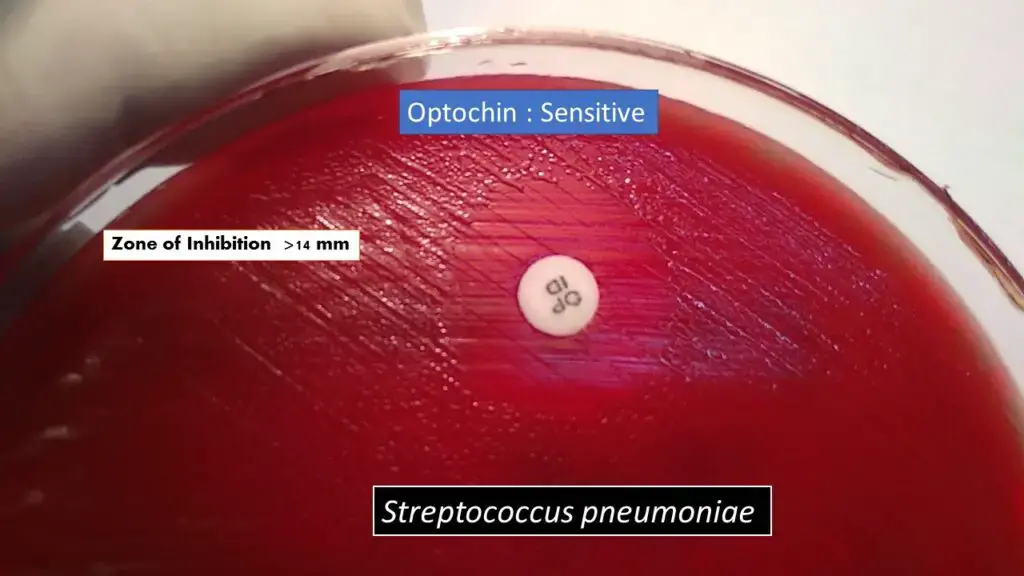
1. Susceptible Result (Positive for S. pneumoniae)
- A clear inhibition zone of 14 mm or more is observed.
- It is the indication that the organism is susceptible to optochin.
- The organism is presumptively identified as Streptococcus pneumoniae.
- In this condition the bacterial lawn fails to grow around the disk due to the effect on membrane surface tension.
2. Resistant Result (Negative)
- A zone of 6 mm (only the disk) or very small inhibition zone is present.
- These isolates is considered resistant to optochin.
- The result show growth up to the edge of the disk.
- It is commonly seen in viridans group streptococci such as Streptococcus mitis.
3. Equivocal or Indeterminate Result
- A zone of 7 mm to 13 mm is formed.
- These are uncertain results and cannot confirm S. pneumoniae.
- It is required to perform confirmatory tests (bile solubility test is usually used).
4. Exceptions and Important Considerations
- When a 10 mm disk is used instead of a 6 mm disk, the susceptible zone must be 16 mm or more.
- Some S. pneumoniae strains may be truly resistant due to ATP synthase mutations and in such case no inhibition zone is found, so molecular or bile solubility testing is needed.
- Plates incubated in air may show larger zones, but S. pneumoniae might not grow well in air, so CO₂ incubation is used and the 14 mm standard zone is based on this condition.
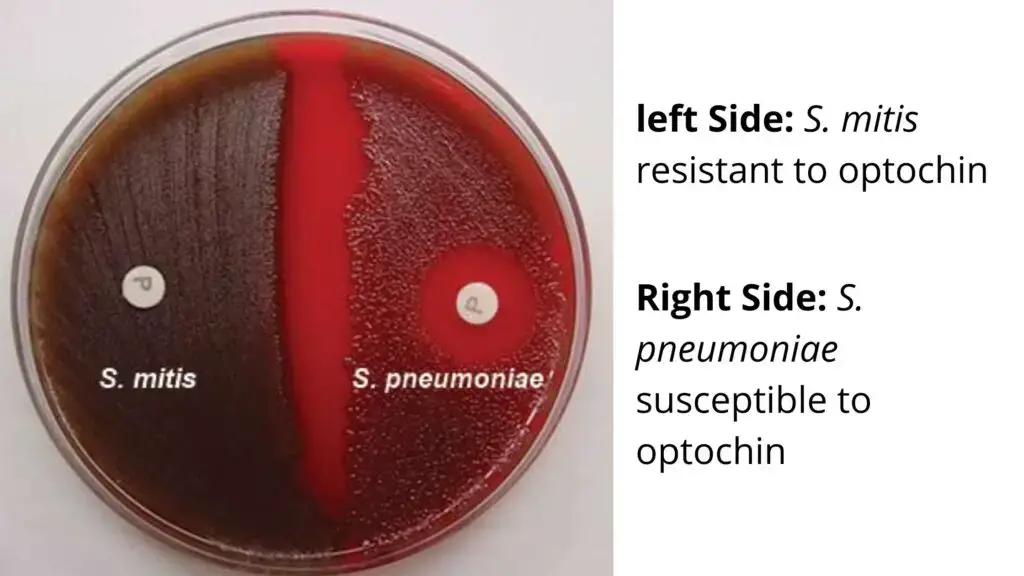
To exclude the possibility that other strains of Streptococcus pneumoniae are present, further tests (bile solubility or serology) should be conducted if the zone of inhibition is less than 14 mm.
Uses of Optochin Susceptibility Test
- It is used for presumptive identification of Streptococcus pneumoniae as the organism is susceptible to optochin.
- It is used to differentiate S. pneumoniae from other α-hemolytic streptococci such as viridans group which is resistant.
- Optochin disks is used directly on primary clinical specimens (blood, sputum, pleural fluid, lung aspirate, urine) to observe pneumococcal growth inhibition.
- It is used as a supporting test along with automated identification systems to reduce misidentification among Streptococcus mitis group.
- Historically it was used as a therapeutic compound for pneumococcal infection but discontinued due to toxicity.
- The compound is also used in research studies as it act as a Gallus gallus taste receptor agonist and has shown activity against Plasmodium falciparum.
Advantages of Optochin Susceptibility Test
- It is useful for differentiation of Streptococcus pneumoniae from other alpha hemolytic streptococci.
- The test is simple to perform and does not require complex procedure.
- It is an inexpensive and cost effective laboratory test.
- The test shows high reliability and good sensitivity for pneumococcal identification.
- It provides rapid presumptive identification as compared to bile solubility test.
- Optochin discs can be applied directly on primary culture plates from clinical samples.
- The test shows good specificity due to selective action of optochin on Streptococcus pneumoniae.
Limitations of Optochin Susceptibility Test
- It is a presumptive test only and final confirmation is required by other tests such as bile solubility or serological methods.
- Some strains of Streptococcus pneumoniae may show resistance to optochin and can be misidentified as viridans streptococci.
- Certain non-pneumococcal alpha hemolytic streptococci may show partial or intermediate sensitivity to optochin.
- The size of zone of inhibition is affected by type of blood agar medium used in the test.
- Variation in inoculum density may alter the zone size and give false sensitive or false resistant result.
- Incubation conditions especially increased CO₂ concentration may reduce the inhibition zone.
- Different serotypes of Streptococcus pneumoniae may produce variable zones of inhibition.
- Optochin discs are sensitive to light and moisture and improper storage may reduce its activity.
- The test requires overnight incubation and hence results are not obtained rapidly.
- Acharya, T. (n.d.). Optochin sensitivity test: Principle, procedure, results. Microbe Online. https://microbeonline.com/optochin-test-principle-procedure-expected-results-and-quality-control/
- Burckhardt, I., Panitz, J., Burckhardt, F., & Zimmermann, S. (2017). Identification of Streptococcus pneumoniae: Development of a standardized protocol for optochin susceptibility testing using total lab automation. BioMed Research International, 2017, 4174168. https://doi.org/10.1155/2017/4174168
- Collins, C. H., Lyne, P. M., Grange, J. M., & Falkinham III, J. O. (2004). Collins and Lyne’s microbiological methods (8th ed.). Arnold.
- Dalynn Biologicals. (2014). Optochin disks [Product Information]. http://www.dalynn.com/dyn/ck_assets/files/tech/DO60.pdf
- Fernández MacLoughlin, G. J., Gomez Barreto, D., de la Torre, C., Pinetta, E. A., del Castillo, F., & Palma, L. (1996). Cefpodoxime proxetil suspension compared with cefaclor suspension for treatment of acute otitis media in paediatric patients. Journal of Antimicrobial Chemotherapy, 37(3), 565–573. https://doi.org/10.1093/jac/37.3.565
- Gardam, M. A., & Miller, M. A. (1998). Optochin revisited: Defining the optimal type of blood agar for presumptive identification of Streptococcus pneumoniae. Journal of Clinical Microbiology, 36(3), 833–834. https://doi.org/10.1128/jcm.36.3.833-834.1998
Marín, M., Cercenado, E., Sánchez-Carrillo, C., Ruiz, A., Gómez González, Á., Rodríguez-Sánchez, B., & Bouza, E. (2017). Accurate differentiation of Streptococcus pneumoniae from other species within the Streptococcus mitis group by peak analysis using MALDI-TOF MS. Frontiers in Microbiology, 8, 698. https://doi.org/10.3389/fmicb.2017.00698 - MedChemExpress. (n.d.). Ethylhydrocupreine hydrochloride (Optochin hydrochloride). Retrieved from https://www.medchemexpress.com/ethylhydrocupreine-hydrochloride.html
Millipore. (2018). 74042 Optochin Disks [Product Information]. Sigma-Aldrich. https://www.sigmaaldrich.com/deepweb/assets/sigmaaldrich/product/documents/181/445/74042dat.pdf - MonenusKedir. (2023). Microbiology of the respiratory system 2023.pdf [PowerPoint slides]. Slideshare.
- Moore, H. F. (1915). The action of ethylhydrocuprein (optochin) on type strains of pneumococci in vitro and in vivo, and on some other microorganisms in vitro. The Journal of Experimental Medicine, 22(3), 269–285. https://doi.org/10.1084/jem.22.3.269
- National Center for Biotechnology Information. (n.d.). PubChem compound summary for CID 16219340, Ethylhydrocupreine hydrochloride. https://pubchem.ncbi.nlm.nih.gov/compound/Ethylhydrocupreine-hydrochloride
- Pikis, A., Campos, J. M., Rodriguez, W. J., & Keith, J. M. (2001). Optochin resistance in Streptococcus pneumoniae: Mechanism, significance, and clinical implications. The Journal of Infectious Diseases, 184(5), 582–590. https://doi.org/10.1086/322803
- VirtualUnknown: Microbiology. (n.d.). Optochin susceptibility test. VUMIE. https://vumicro.com/docs/optochin-susceptibility-test/
- Vohrnová, S., Kozáková, J., & Honskus, M. (2025). Comparison of Streptococcus pneumoniae isolates occurring in optochin-susceptible and optochin-resistant variants by analyzing whole-genome sequencing data. Microbiology Spectrum, 13(4), e01939-24. https://doi.org/10.1128/spectrum.01939-24
- Wikipedia. (n.d.). Optochin. Retrieved from https://en.wikipedia.org/wiki/Optochin