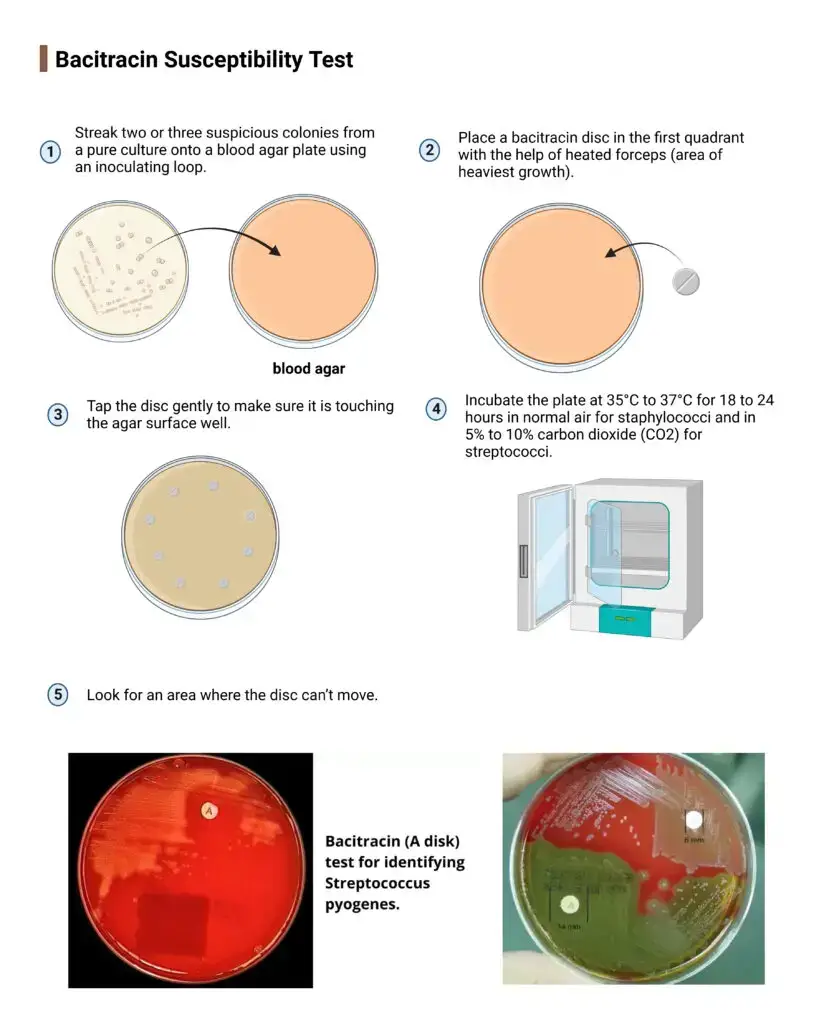
Bacitracin Susceptibility Test
- The Bacitracin susceptibility test is one of the antimicrobial disc tests used to distinguish between group A and other -hemolytic Streptococci.
- The majority of antimicrobial susceptibility tests are performed to distinguish between species within the same genus.
- To evaluate the susceptibility of microorganisms to particular antibiotics, antimicrobial discs are utilised.
- Bacitracin is a bactericidal agent used to treat superficial skin infections and is rarely administered systemically by injection.
- Bacillus subtilis produces the polypeptide antibiotic bacitracin. It inhibits bacterial growth by interfering with the formation of peptidoglycans.
- It prevents bactoprenol from transferring NAM and NAG sugars across the cell membrane, hence reducing peptidoglycan production.
- Bacitracin inhibits the growth of Group A -hemolytic Streptococci but not other -hemolytic Streptococci. This facilitates the separation of the two groups of organisms.
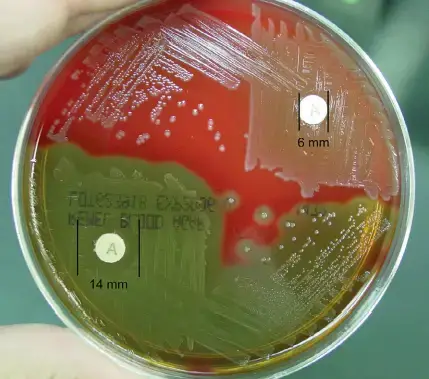
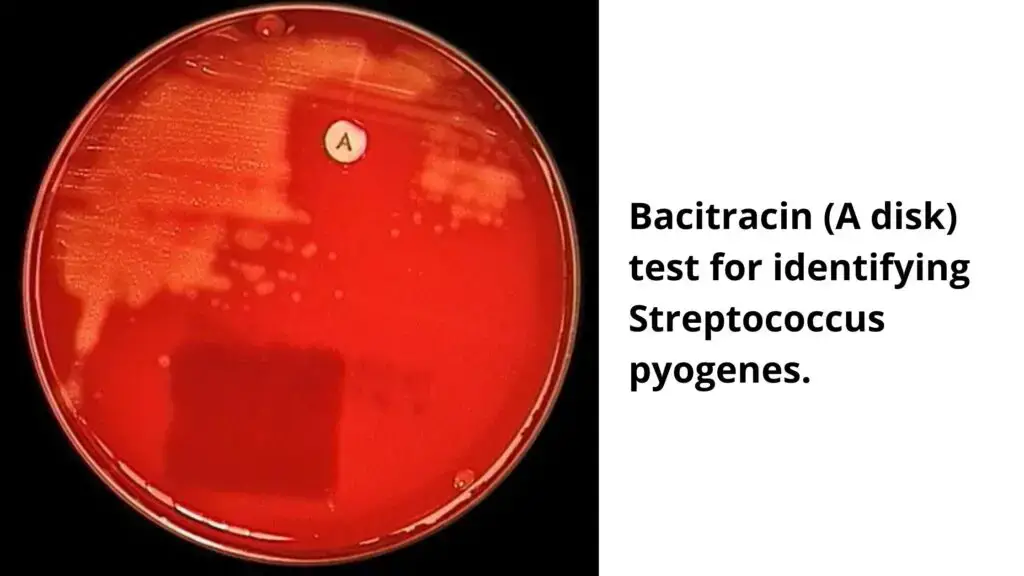
- Bacitracin discs are mostly utilised against Streptococcus pyogenes since it inhibits the organism’s growth.
- Bacitracin produces an approximately 12-millimeter zone of inhibition against S. pyogenes, which is utilised as a positive control when testing against other groups of organisms.
- Levinson and Frank, who utilised Bacitracin-impregnated filter paper discs for this purpose, determined that a significant number of sensitive beta-haemolytic streptococci were of Group A.
- Comparing Bacitracin disc, fluorescent antibody technique, and Lancefield precipitin technique, Steamer et al discovered that the Bacitracin disc approach was the most practical for ordinary clinical laboratory use.
- In order to separate Aerococcus viridans and S. milleri from enterococci and Streptococcus mitis, the Bacitracin sensitivity test, in conjunction with the Furacin and Optochin tests, is valuable.
Objective of Bacitracin Susceptibility Test
- Bacitracin Susceptibility Test Discs are used to identify and distinguish Group A streptococci (particularly S.pyogenes) from other beta-haemolytic streptococci.
Principle of Bacitracin Susceptibility Test
- The use of 0.04 units of Bacitracin disc significantly reduces the number of Group A beta-haemolytic streptococci that develop on blood agar.
- At 0.04 units disc, micrococci and streptococci are suppressed but coagulase-negative staphylococci are resistant.
- Susceptibility test discs for Bacitracin are circular pieces of filter paper soaked in 0.04 units of the antibiotic. After being impregnated, discs are placed on agar to allow the antimicrobial to spread into the medium and stunt microbiological development.
- After incubation, the test results are analysed based on the size of the inhibitory zone surrounding the discs.
- When growth is observed all the way to the disk’s rim, the organism is considered resistant; when a circular zone forms around the disc, the organism is considered inhibited and susceptible.
- If bacitracin discs are employed as a preliminary test prior to serological grouping, a lot of resources (time, manpower, and supplies) can be conserved.
- Evidence suggests that Group A Streptococci are more susceptible to Bacitracin than other beta-hemolytic strains.
- Antimicrobial discs for testing Bacitracin susceptibility have been proposed as a fast diagnostic tool for Group A Streptococci.
Requirements
- Media: Blood agar or Mueller Hinton Agar
- Other: Bacitracin 0.04U disks, Swabs, Sterile forceps, Broth for inoculation
Composition of Mueller Hinton Agar (MHA)
| Ingredients | Gms/Litre |
| Beef extract | 2.0 |
| Acid hydrolysate of casein | 17.5 |
| Starch | 1.5 |
| Agar | 17.0 |
Final pH 7.3 +/- 0.1 at 25ºC.
Blood Agar Composition
| Ingredients | Gram/liter |
| Peptone | 10.0 |
| Tryptose | 10.0 |
| Sodium chloride | 5.0 |
| Agar | 15.0 |
Procedure of Bacitracin test
The Bacitracin Susceptibility test can be performed in two ways, with either of two nutritional mediums. Pure cultures of an organism obtained through subculturing or from clinical samples can be used for testing. The following are instructions for doing either of two Bacitracin susceptibility tests:
Hebert’s method using Blood Agar plates
- Streak two or three suspicious colonies from a pure culture onto a blood agar plate using an inoculating loop.
- Place a bacitracin disc in the first quadrant with the help of heated forceps (area of heaviest growth).
- Tap the disc gently to make sure it is touching the agar surface well.
- Incubate the plate at 35°C to 37°C for 18 to 24 hours in normal air for staphylococci and in 5% to 10% carbon dioxide (CO2) for streptococci.
- Look for an area where the disc can’t move.
Note: Use chocolate agar for bacitracin on direct sputum culture plates and blood agar for optochin. When bacitracin disc (not Taxo A) is added to chocolate agar, it stops the growth of microbiota in the upper respiratory tract and makes it easier to find Haemophilus influenzae.
Mueller Hinton Agar method
- Mueller Hinton agar can also be used to see how resistant organisms that grow quickly are.
- The organism is grown in a culture for one night, and then a 0.5 McFarland suspension of the organism is made.
- Then, sterile swabs are used to put the suspension on the MHA plates so that bacterial lawns can grow on the agar.
- After the antibiotic discs have dried for about 10 minutes, they are put on the agar plates with sterile forceps, leaving about 10 mm between each disc.
- To make sure the discs stick to the plates, they are tapped with a clean stick.
- The upside-down plates are then kept at 35–37°C for 24 hours.
- Watch and measure the zone of inhibition. Serological testing can provide even more proof.

Result of Bacitracin Susceptibility Test
- Positive: Any zone of inhibition greater than 10 mm; susceptible
- Negative: No zone of inhibition; resistant
Quality Control
Conduct CLSI-recommended sterility and performance tests using blood agar and/or chocolate agar plates. After receiving or purchasing a batch of bacitracin discs, conduct a test to ensure their efficacy using the relevant test organism.
- Positive: Streptococcus pyogenes (ATCC19615)—susceptible Micrococcus luteus (ATCC10240)—susceptible
- Negative: Streptococcus agalactiae (ATCC27956)—resistant Staphylococcus aureus (ATCC25923)—resistant
Limitations of Bacitracin Susceptibility Test
- No bactericidal zone is evident around staphylococci when a 0.04-U disc of bacitracin is placed on BAP. If Micrococcus is not incubated for the full 24 hours if MH agar is used, a zone of inhibition larger than 7 mm but smaller than the 10-mm threshold may be produced.
- Therefore, adequate inoculum leading to confluent development should be used to avoid the possibility of a misleading zone of inhibition caused by insufficient inoculum.
- Dried blood agar plates should not be utilised for susceptibility testing because antibiotic diffusion is reduced on such agar, which could lead to false-negative results.
- The use of sensitivity discs is not recommended when testing for Bacitracin since the zone of inhibition size may vary depending on the concentration employed. Instead, differential discs (0.04 U) should be used (10 U).
- Serological testing, including latex agglutination tests, should be conducted for additional confirmation.
Uses of Bacitracin Susceptibility Test
- Bacitracin susceptibility patterns among various organisms can be studied using this method.
- A bacitracin susceptibility test is most commonly used to distinguish Group A beta-hemolytic Streptococci from other beta-hemolytic Streptococci.
- Since most Micrococcus and Rothia species are susceptible to penicillin while most coagulase-negative staphylococci are not, bacitracin differentiation may be effective for penicillin-susceptible strains.
References
- Biochemical Tests for the Identification of Aerobic Bacteria. (2016). Clinical Microbiology Procedures Handbook, 3.17.1.1–3.17.48.3. doi:10.1128/9781555818814.ch3.17.
- Tille, P. M., & Forbes, B. A. (2014). Bailey & Scott’s diagnostic microbiology (Thirteenth edition.). St. Louis, Missouri: Elsevier.
- https://microbiologynote.com/mueller-hinton-agar/
- https://microbiologynote.com/blood-agar/
- https://microbenotes.com/bacitracin-susceptibility-test-principle-procedure-and-result-interpretation/
- https://histogene.co/bacitracin-susceptibility-test/
- https://himedialabs.com/TD/DD015.pdf
- https://www.vumicro.com/vumie/help/VUMICRO/Bacitracin_Susceptibility.htm
- https://www.onlinebiologynotes.com/bacitracin-test-principle-requirements-procedure-results-interpretations-limitations/
- https://microbiologyinfo.com/bacitracin-susceptibility-test/