What are Monoclonal Antibodies?
- Monoclonal antibodies are synthetic antibodies created in a laboratory setting. They are generated from a single cell clone by combining B-lymphocytes, which are immunological cells, with myeloma cells, which are malignant cells that can divide indefinitely. This fusion process produces hybridomas, which are immortalized cell lines that produce a type of antibody known as monoclonal antibodies.
- Unlike antibodies produced naturally by the body, which may recognize a wide range of antigens, monoclonal antibodies are specifically developed to target and attach to antigens located on the surface of cells. Antigens might be receptors or other foreign proteins found on normal or malignant cells. Because they are produced from a single B-cell clone and target a single epitope, which is a unique binding spot on the antigen, monoclonal antibodies are highly selective in their binding.
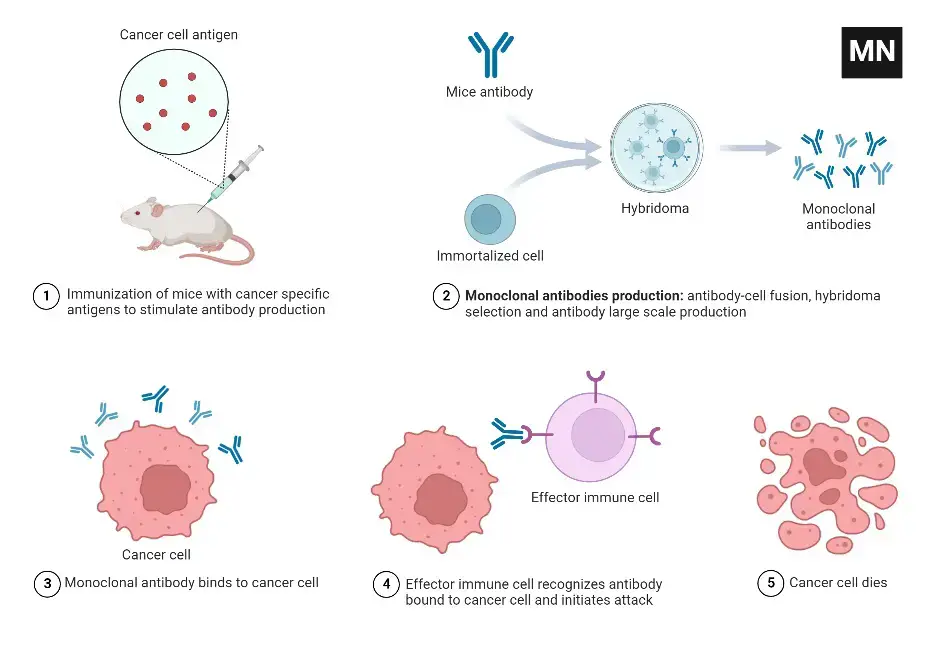
- Georges Kohler and Cesar Milstein invented the process of manufacturing monoclonal antibodies in 1975. It entails immunizing an animal, usually a mouse, and then harvesting B cells from its immune system. These B cells merge with myeloma cells to form hybridomas. The hybridomas are screened to find the clone that produces the desired specificity of antibodies. The antibodies produced by this selected clone are then collected as monoclonal antibodies.
- Monoclonal antibodies have numerous medical applications, including the treatment of disorders such as cancer. Because of their selectivity, they can specifically bind to cancer cells and can be combined with cytotoxic agents such as powerful radioactive chemicals. The cytotoxic agent attempts to eradicate cancer cells while causing minimal harm to healthy cells.
- Because of their selectivity and capacity to target specific antigens, monoclonal antibodies are significant agents in medicine. They have transformed several fields of study and therapy, enabling new methods of diagnosing and treating diseases.
Definition of Monoclonal Antibodies
Monoclonal antibodies are artificial antibodies produced in a laboratory by fusing immune cells with cancer cells. They are highly specific in targeting and binding to specific antigens on cells, making them valuable tools in medical research and treatment of diseases, including cancer.
Types of monoclonal antibodies based on composition
Monoclonal antibodies are synthetic proteins that function similarly to human antibodies within the immune system. There are four various techniques to make them, and their names reflect the materials used.
1. Murine monoclonal antibodies
- Murine monoclonal antibodies refer to a specific type of monoclonal antibodies that were the first to be produced on a laboratory scale using hybridoma technology in 1975. They earned the name “murine” because they originated from rodent hosts, specifically mice and rats belonging to the Muridae family.
- Murine monoclonal antibodies have been instrumental in the advancement of antibody production techniques and the exploration of their potential applications in therapeutics and analytical fields. They have been studied in preclinical trials for various purposes, including their potential use in the treatment of Cryptococcus neoformans, a fungal infection. Additionally, they have been employed as a foundation for antibody engineering techniques such as chimerization, humanization, and the development of bispecific antibodies using antibody fragments called single-chain antibody fragments (scFvs).
- Furthermore, murine monoclonal antibodies have been investigated in preclinical trials aimed at enhancing the therapeutic activity of amphotericin B administration by targeting the capsular polysaccharide. In the field of antibody-based therapeutics, the names of treatments involving murine monoclonal antibodies often end with “-omab,” indicating their origin and composition.
- Overall, murine monoclonal antibodies have played a crucial role in the development of modern antibody production techniques and have been investigated for their therapeutic potential in various preclinical trials.
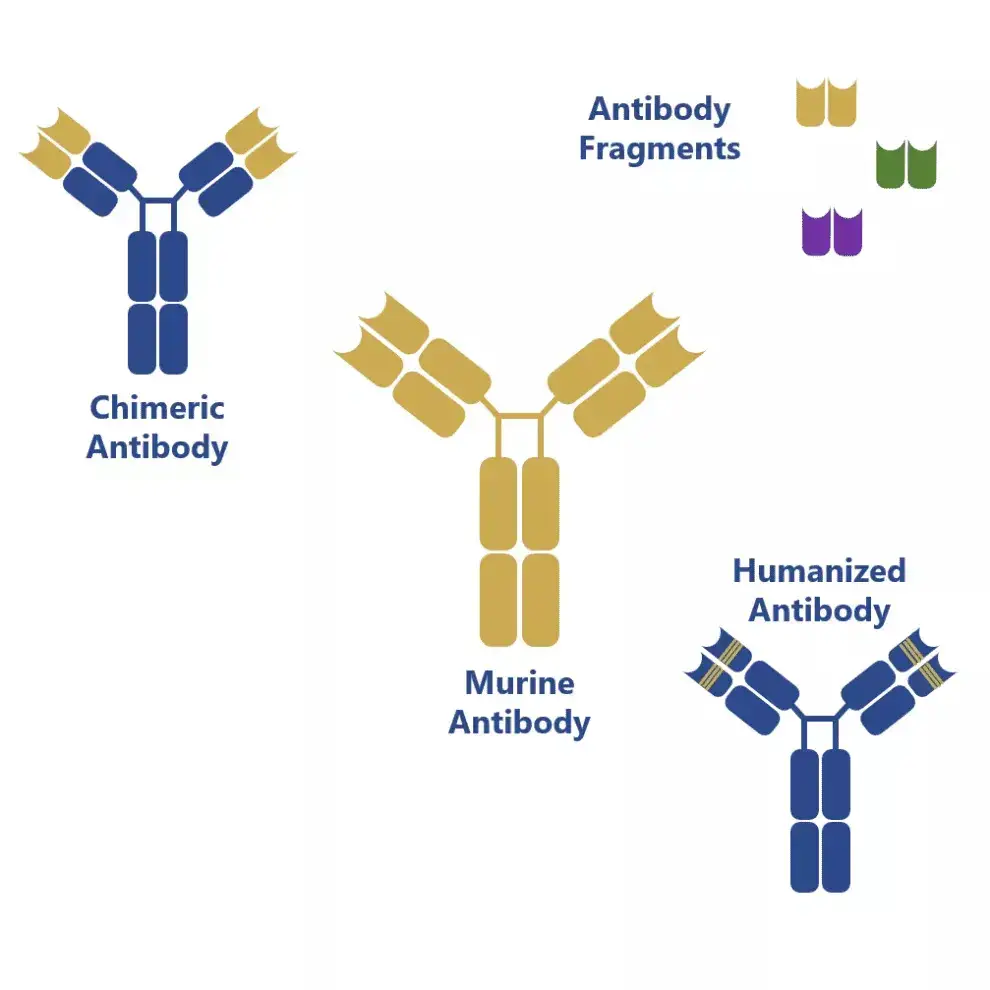
2. Chimeric monoclonal antibodies
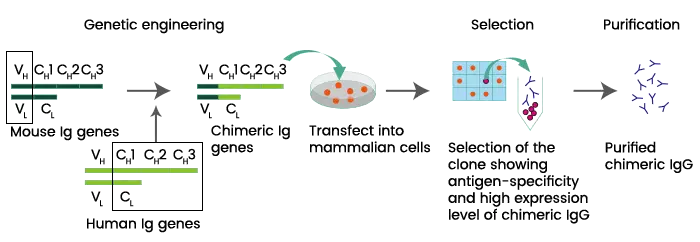
- Chimeric monoclonal antibodies are a specific type of monoclonal antibodies that are created by combining parts from two different species, typically mice and humans. This fusion involves joining the variable regions, responsible for antigen recognition, from one species (such as mice) with the constant regions, which determine the antibody’s effector functions, from the other species (such as humans).
- The purpose of creating chimeric monoclonal antibodies is to address concerns regarding immunogenicity, which is the potential for an immune response against foreign substances. By incorporating human components, these antibodies are designed to reduce the likelihood of triggering an immune response when used for therapeutic purposes. Additionally, chimeric monoclonal antibodies are engineered to have an increased serum half-life, meaning they stay in the bloodstream for a longer duration, which can enhance their therapeutic effectiveness.
- Despite the structural modifications, chimeric monoclonal antibodies retain the original antibody’s antigen specificity and affinity. This means they can still recognize and bind to the target antigen with the same level of precision as the original mouse monoclonal antibody.
- In the field of antibody-based therapeutics, treatments involving chimeric monoclonal antibodies are often denoted by names ending with “-ximab.” This naming convention helps identify the origin and composition of the therapeutic antibody.
- In summary, chimeric monoclonal antibodies are created by combining mouse and human components to reduce immunogenicity and extend their presence in the bloodstream. These antibodies retain the specificity and affinity of the original antibody and are commonly used in therapeutic applications, as indicated by the “-ximab” naming convention.
3. Humanized monoclonal antibodies
- Humanized monoclonal antibodies are a type of monoclonal antibody that is an extension of chimeric monoclonal antibodies. In humanized antibodies, all regions of the mouse antibody found in chimeric monoclonal antibodies are replaced with human counterparts, except for the complementarity-determining regions (CDRs). The CDRs are the specific amino acid sequences responsible for directly contacting and binding to the antigen.
- The purpose of humanizing monoclonal antibodies is to further minimize immunogenicity and increase the compatibility with the human immune system. By replacing the non-essential regions with human components, humanized monoclonal antibodies closely resemble human antibodies, reducing the likelihood of an immune response when used for therapeutic purposes.
- The CDRs, which play a critical role in antigen recognition and binding, are retained from the original mouse antibody. These small parts of the mouse protein are attached to the human protein framework, allowing the humanized antibody to maintain its specificity and affinity for the target antigen.
- In the nomenclature used for naming therapeutic antibodies, humanized monoclonal antibodies are often identified by names ending with “-zumab.” This naming convention helps distinguish them as humanized antibodies and also indicates their therapeutic application.
- In summary, humanized monoclonal antibodies are modified versions of chimeric antibodies in which all regions of the mouse antibody, except the CDRs, are replaced with human counterparts. This modification aims to minimize immunogenicity while maintaining the antigen specificity of the original mouse antibody. Humanized monoclonal antibodies are denoted by names ending with “-zumab” in the field of therapeutic applications.
4. Human monoclonal antibodies
- Human monoclonal antibodies are a type of monoclonal antibody that consists of fully human proteins. These antibodies are created through molecular biological techniques that involve modifying the amino acid sequences of the antibodies.
- By manipulating the amino acid sequences, the specificity, affinity, or biological functions of the antibodies can be altered. This allows for the acquisition of sequences that are not naturally present in the human antibody repertoire. These modifications can enhance the antibody’s binding capacity or confer new functionalities, making them valuable tools in therapeutic applications.
- Unlike chimeric or humanized monoclonal antibodies, which contain some non-human components, human monoclonal antibodies are composed entirely of human proteins. This minimizes the risk of immunogenicity and improves compatibility with the human immune system, making them highly desirable for therapeutic use.
- In the naming convention for therapeutic antibodies, treatments involving human monoclonal antibodies are typically identified by names ending with “-umab.” This naming convention helps distinguish them as fully human antibodies and indicates their therapeutic application.
- In summary, human monoclonal antibodies are fully human proteins that have been modified using molecular biological techniques to alter their amino acid sequences. These modifications can enhance specificity, affinity, or introduce new functionalities. Human monoclonal antibodies have names ending with “-umab” and are highly valuable in therapeutic applications due to their human origin and reduced immunogenicity.
Types of monoclonal antibodies based on functions
1. Naked monoclonal antibodies
Naked monoclonal antibodies are a type of monoclonal antibodies that do not have any drug or radioactive agent attached to them. They are the most commonly used monoclonal antibodies in cancer treatment. Naked monoclonal antibodies can attach to antigens on cancer cells, as well as non-cancerous cells or free-floating proteins.
These naked monoclonal antibodies function in different ways depending on their target and mechanism of action. Some of their functions include:
- Boosting immune response: Naked monoclonal antibodies can attach to cancer cells and act as markers for the body’s immune system to identify and destroy them. For example, Alemtuzumab (Campath®) is used to treat Chronic lymphocytic leukemia (CLL) by binding to the CD52 antigen found on lymphocytes, including leukemic cells. This attachment attracts immune cells to the cancer cells, leading to their destruction.
- Targeting immune system checkpoints: Some naked monoclonal antibodies work by targeting immune system checkpoints. These antibodies bind to proteins on immune cells, such as PD-1 or CTLA-4, that regulate immune responses. By blocking these checkpoints, the antibodies enhance the immune response against cancer cells.
- Blocking antigens on cancer cells: Naked monoclonal antibodies can attach to antigens on cancer cells that facilitate their growth and spread. For example, trastuzumab (Herceptin) is a monoclonal antibody designed to target the HER2 protein found in breast and stomach cancer cells. By binding to HER2, trastuzumab blocks its activation and inhibits the growth of cancer cells.
Overall, naked monoclonal antibodies play a vital role in cancer treatment by directly interacting with cancer cells or modulating the immune response against them. These antibodies can serve as markers, immune system activators, or inhibitors of cancer cell growth, depending on their specific targets.
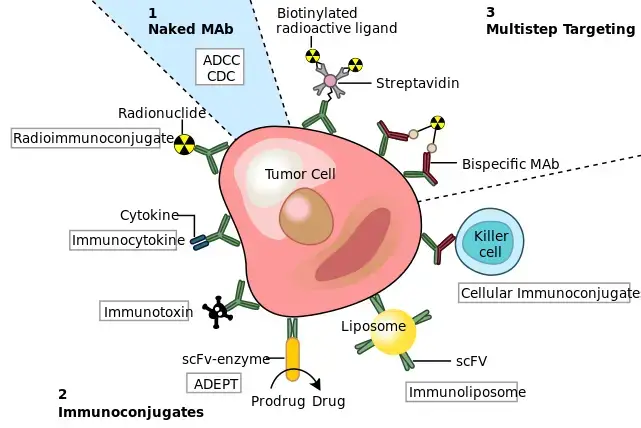
2. Conjugated monoclonal antibodies
- Conjugated monoclonal antibodies refer to a specific type of monoclonal antibodies that are combined or conjugated with chemotherapy drugs or a radioactive agent. The purpose of conjugating these antibodies with therapeutic agents is to enhance their effectiveness in targeting and delivering treatment directly to cancer cells.
- Conjugated monoclonal antibodies act as homing devices, circulating freely throughout the body until they find and attach to the specific target antigen on cancer cells. Once attached, they act as a vehicle to deliver the conjugated chemotherapy drug or radioactive agent directly to the cancer cells. This targeted delivery helps to concentrate the therapeutic agent at the site of the tumor, increasing its efficacy while reducing potential side effects on normal cells in other parts of the body.
- The conjugated monoclonal antibodies function by attaching or “hooking” onto the target antigen, which can be found on the surface of cancer cells. Once attached, the antibodies facilitate the internalization of the conjugated therapeutic agent into the cancer cells. This internalization can lead to various mechanisms of action, such as inducing cell death or interfering with cellular functions necessary for cancer cell survival.
- One of the key advantages of conjugated monoclonal antibodies is their ability to minimize the risks of damaging normal cells in other parts of the body. By specifically targeting cancer cells, the conjugated antibodies help to reduce off-target effects and improve the therapeutic index of the treatment. This targeted approach increases the likelihood of eliminating cancer cells while minimizing harm to healthy tissues.
- In summary, conjugated monoclonal antibodies are monoclonal antibodies that are combined or conjugated with chemotherapy drugs or radioactive agents. They serve as homing devices, delivering the therapeutic agents directly to cancer cells. This targeted approach reduces the risks of damaging normal cells in other body parts and improves the efficacy of the treatment.
a. Radiolabeled antibodies
- Radiolabeled antibodies, also known as radioimmunotherapy (RIT), are a type of monoclonal antibodies that have a small radioactive particle attached to them. This combination of a drug along with a radioactive agent allows for targeted delivery of radiation directly to the target cells, primarily affecting the target cells and to some extent neighboring cells.
- The radiolabeled antibodies function by specifically recognizing and binding to a specific antigen present on the target cells. By attaching a radioactive particle to the antibody, the radiation can be delivered directly to the cancer cells, enhancing the therapeutic effect.
- One example of a radiolabeled antibody is ibritumomab tiuxetan, commercially known as Zevalin. This antibody targets the CD20 antigen found on B-lymphocytes. It consists of two components: a monoclonal antibody drug called rituximab and a radioactive agent called Yttrium-90. Rituximab recognizes and binds to CD20 antigen on B-lymphocytes, while Yttrium-90 emits radiation that can effectively kill the cancer cells.
- The advantage of radiolabeled antibodies is that they deliver the radioactivity directly to the cancer cells, minimizing damage to healthy cells. This targeted approach allows for a higher concentration of radiation at the site of the tumor, increasing the efficacy of treatment while reducing potential side effects.
- Radiolabeled monoclonal antibody treatment, or radioimmunotherapy (RIT), holds promise in the field of cancer treatment. By combining the specific targeting abilities of monoclonal antibodies with the localized radiation delivery, RIT offers a unique approach to combating cancer cells.
- In summary, radiolabeled antibodies are monoclonal antibodies that have a small radioactive particle attached to them. They deliver radioactivity directly to cancer cells, primarily affecting the target cells and to some extent neighboring cells. One example is ibritumomab tiuxetan, which combines a monoclonal antibody drug with a radioactive agent. Radiolabeled monoclonal antibody treatment, known as radioimmunotherapy (RIT), shows potential in targeted cancer treatment.
b. Chemolabeled antibodies
- Chemolabeled antibodies, also known as antibody-drug conjugates (ADCs), are monoclonal antibodies that have potent chemotherapeutic or other medicines attached to them. These conjugates combine the targeting ability of monoclonal antibodies with the cytotoxic effects of chemotherapy drugs, allowing for targeted delivery of the drug to specific cells.
- One example of a chemolabeled antibody is Brentuximab vedotin, commercially known as Adcetris. This antibody is attached to the chemotherapy medication MMAE (monomethyl auristatin E). Brentuximab vedotin specifically targets the CD30 antigen found on certain cells, such as those in Hodgkin lymphoma or anaplastic large cell lymphoma. When the antibody binds to the CD30 antigen on the surface of these cells, it delivers the attached MMAE directly to the cancer cells, resulting in cell death.
- Another example is Ado-trastuzumab emtansine, commercially known as Kadcyla or TDM-1. This antibody targets the HER2 protein, which is often overexpressed in breast cancer cells. Attached to Ado-trastuzumab emtansine is the chemotherapy medication DM1. Upon binding to HER2-positive cancer cells, the conjugate delivers DM1 directly to the cancer cells, leading to their destruction.
- The advantage of chemolabeled antibodies is that they combine the specificity of monoclonal antibodies with the cytotoxic effects of chemotherapy drugs. This targeted delivery allows for higher concentrations of the drug at the site of the tumor, while reducing potential side effects on healthy tissues.
- Chemolabeled antibodies have shown promise in cancer treatment by increasing the effectiveness of chemotherapy while minimizing systemic toxicity. These conjugates provide a way to specifically deliver potent drugs to cancer cells, improving the therapeutic index and reducing the impact on healthy cells.
- In summary, chemolabeled antibodies, or antibody-drug conjugates (ADCs), combine monoclonal antibodies with potent chemotherapeutic or other medicines. Examples include Brentuximab vedotin, which is attached to the chemotherapy medication MMAE, and Ado-trastuzumab emtansine, which is attached to the chemotherapy medication DM1. These conjugates allow for targeted delivery of the drugs to specific cells, enhancing the effectiveness of treatment while minimizing side effects.
3. Bispecific monoclonal antibodies
- Bispecific monoclonal antibodies are a type of monoclonal antibody drug that are composed of two different monoclonal antibodies attached to each other. This unique structure allows them to simultaneously bind to two different target proteins or antigens, bringing them together.
- An example of a bispecific monoclonal antibody drug is blinatumomab, commercially known as Blincyto. Blinatumomab consists of two parts: one part is designed to attach to the CD19 protein found on leukemia and lymphoma cells, while the other part attaches to the CD3 protein found on immune T-cells. By binding to both CD19 and CD3, blinatumomab bridges the cancer cells and the immune T-cells.
- The mechanism of action of bispecific monoclonal antibodies like blinatumomab is to bring the cancer cells and immune cells in close proximity. This close interaction facilitates the activation of immune cells, such as T-cells, and enables them to recognize and attack the cancer cells more effectively. In the case of blinatumomab, it helps to activate T-cells and redirect their cytotoxic activity towards the cancer cells expressing CD19, leading to the destruction of the cancer cells.
- Bispecific monoclonal antibodies have the potential to enhance the body’s immune response against cancer. By bringing together immune cells and cancer cells, they enable a more targeted and efficient immune attack on the cancer cells. This approach can be particularly beneficial in cases where the cancer cells have mechanisms to evade the immune system.
- In summary, bispecific monoclonal antibodies are drugs composed of two different monoclonal antibodies attached to each other. They facilitate the interaction between cancer cells and immune cells, allowing the immune system to recognize and attack cancer cells more effectively. An example is blinatumomab, which binds to CD19 on cancer cells and CD3 on immune T-cells, enabling immune-mediated destruction of cancer cells. Bispecific monoclonal antibodies offer a promising approach in cancer immunotherapy by enhancing immune responses against cancer cells.
Method of production of monoclonal antibodies
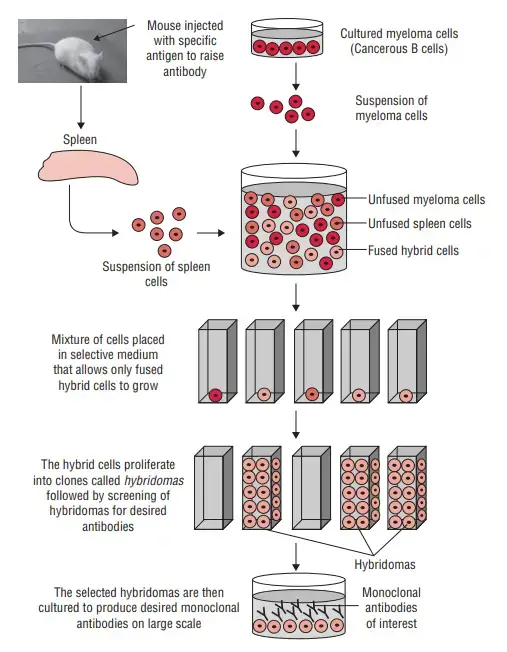
The production of monoclonal antibodies (mAbs) involves an in vitro process using tissue-culture techniques. Here is a step-by-step overview of the method:
- Antigen Identification and Animal Immunization: A specific antigen is identified, and an animal, often laboratory mice, is immunized with the antigen multiple times to stimulate an immune response.
- Removal of B-cells: B-cells, which produce antibodies, are extracted from the spleen of the immunized animal.
- Fusion with Myeloma Cells: The extracted B-cells are fused with myeloma cells, which are cancerous B-cells. Fusion is achieved by combining the cells and using polyethylene glycol to facilitate the fusion of their plasma membranes.
- Selective Medium: The fused cells, known as hybridoma cells, are placed in a selective medium called Hypoxanthine Aminopetrin Thymidine (HAT). This medium contains substances like hypoxanthine, aminopterin, and thymidine. It allows the growth of the hybridoma cells, while the myeloma cells do not grow, and the infused B-cells die off.
- Screening and Culture: Hybridoma cells, which have the ability to continuously produce antibodies, are screened to identify the desired monoclonal antibodies. Cells producing the specific mAbs are selected and transferred to tissue culture for further growth.
- Harvesting and Purification: The monoclonal antibodies are periodically harvested from the culture medium. The harvested antibodies then undergo purification processes to isolate and refine them, removing any contaminants.
- Large-Scale Production: To produce sufficient quantities of monoclonal antibodies for experimentation or treatment, the selected hybridoma cells are grown and harvested in large media quantities over several weeks. This process aims to generate millions of specific mAbs targeting the antigen used for immunization.
The method of production of monoclonal antibodies allows for the generation of large quantities of highly specific antibodies. These mAbs can be used for various purposes, such as experimental research or therapeutic applications. The ability to produce monoclonal antibodies in a controlled and standardized manner has revolutionized the fields of medicine and biotechnology, offering powerful tools for diagnosis, treatment, and research.
A typical monoclonal antibody production Steps
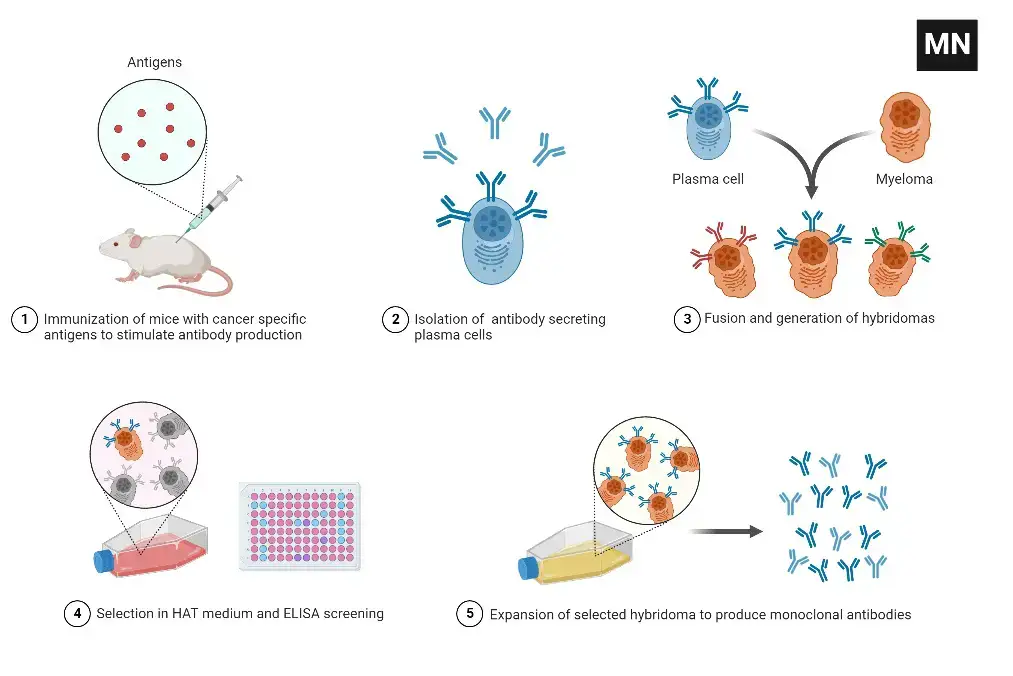
- Immunization of mice and isolation of splenocytes – Mice are inoculated with an antigen, and their blood is afterwards tested for antibody production. The splenocytes that produce antibodies are then extracted for in vitro hybridoma formation.
- Preparation of myeloma cells – Myeloma cells are immortalised cells that, when joined with spleen cells, can result in a hybridoma with limitless growth. The cells of myeloma are prepared for fusion.
- Fusion – Myeloma cells and isolated splenocytes are fused together to create hybridomas in the presence of polyehtylene glycol (PEG), which triggers the fusion of cell membranes.
- Clone screening and picking – Antigen specificity and immunoglobulin class are considered during the screening and selection of clones.
- Functional characterization – Confirm, confirm, and describe (by ELISA, for instance) each potentially high-producing colony.
- Scale up and wean – Scale up the clones that produce the appropriate antibodies and wean them off the selection agent (s).
- Expansion – Increase the number of clones yielding the necessary antibodies (e.g. bioreactors or large flasks).
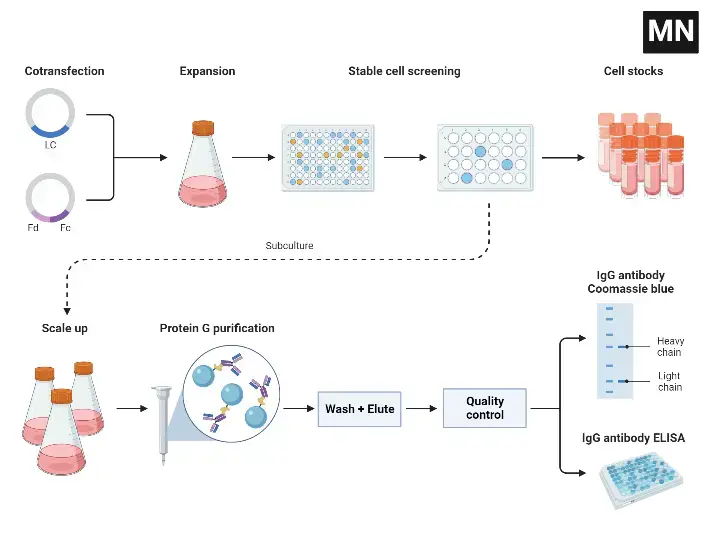
Recombinant Monoclonal Antibody Production
How do monoclonal antibody drugs work?
Monoclonal antibody drugs have various mechanisms of action that contribute to their therapeutic effects. Here is an overview of how monoclonal antibody drugs work:
- Flagging cancer cells: Monoclonal antibodies can coat cancer cells, making them more visible to immune system cells. This tagging helps the immune system identify and eliminate cancer cells more effectively.
- Triggering cell-membrane destruction: Some monoclonal antibodies can stimulate an immune response that leads to the destruction of a cancer cell’s outer membrane. This immune system response can help eliminate cancer cells.
- Blocking cell growth: Certain monoclonal antibodies interfere with the interaction between cancer cells and proteins that promote cell growth. By blocking these interactions, monoclonal antibodies inhibit the signaling pathways necessary for cancer cell growth and survival.
- Preventing blood vessel growth: Monoclonal antibody drugs can inhibit the formation of new blood vessels that supply nutrients to tumors. By blocking protein-cell interactions involved in blood vessel growth, these antibodies limit the tumor’s blood supply and impede its growth.
- Blocking immune system inhibitors: Proteins produced in the body can regulate immune cell activity and prevent overactivation of the immune system. Monoclonal antibodies can block these immune system inhibitors, allowing immune cells to effectively attack cancer cells without interference.
- Directly attacking cancer cells: Some monoclonal antibodies bind directly to cancer cells, triggering a series of events within the cell that leads to its self-destruction. These antibodies may induce cell death or disrupt essential cellular processes necessary for cancer cell survival.
- Delivering radiation treatment: Monoclonal antibodies with a high affinity for cancer cells can be coupled with small radioactive particles. By binding to cancer cells, the monoclonal antibody acts as a delivery vehicle, transporting the radiation treatment directly to the cancer cells. This targeted approach minimizes the side effects of radiation on healthy cells.
- Delivering chemotherapy: Similar to radiation treatment, certain monoclonal antibodies can be linked to chemotherapeutic medications. This allows the targeted delivery of chemotherapy drugs specifically to cancer cells, while sparing healthy cells and reducing the potential for systemic side effects.
- Binding cancer and immune cells: Some monoclonal antibody drugs combine two different monoclonal antibodies. One antibody binds to a cancer cell, while the other binds to a specific immune system cell. This dual binding can stimulate the immune system to recognize and attack cancer cells more effectively.
Monoclonal antibody drugs offer a versatile approach to cancer treatment, employing various mechanisms to target and eliminate cancer cells while minimizing damage to healthy cells. Their specific mechanisms of action depend on the particular monoclonal antibody and its intended target.
Side effects of Monoclonal Antibodies
Common side effects
- In general, the most frequent adverse effects of monoclonal antibody medicines are allergic reactions, such as hives and itching.
- Signs and symptoms of the flu, including chills, tiredness, fever, and muscular aches.
- Nausea, vomiting.
- Diarrhea.
- Skin rashes.
- Reduced blood pressure
Serious side effects
Serious yet uncommon adverse effects of monoclonal antibody treatment include:
- Infusion reactions: Extreme allergic reactions are possible and only rarely result in death. Before beginning monoclonal antibody therapy, you may be given medication to prevent an allergic reaction. Infusion responses typically occur during or shortly after treatment administration, so your healthcare team will constantly monitor you. You may be required to spend a few hours in the treatment centre for monitoring.
- Heart problems: Certain monoclonal antibodies augment the danger of hypertension, congestive heart failure, and heart attacks.
- Lung problems: Some monoclonal antibodies are connected with an increased likelihood of inflammatory lung disease.
- Skin problems: In some instances, skin sores and rashes can lead to dangerous infections. Additionally, serious sores can emerge on the tissue lining your cheeks and gums (mucosa).
- Bleeding: Some monoclonal antibody drugs are associated with an increased risk of internal bleeding.
Applications of Monoclonal antibodies
Monoclonal antibodies have a wide range of applications in the field of medicine and research. Here are some of the key applications:
- Cancer treatment: Monoclonal antibodies have revolutionized cancer treatment. They can target specific tumor antigens, stimulate immune responses, and deliver toxic substances directly to cancer cells. They are used in the treatment of various cancers, including breast cancer, lymphoma, and leukemia.
- Rheumatoid arthritis: Monoclonal antibodies that target Tumor Necrotic Factor-alpha (TNF-alpha) are used in the treatment of rheumatoid arthritis. TNF-alpha plays a role in the progression of the disease, and by blocking its action, these antibodies help reduce inflammation and joint damage.
- Diagnosis of diseases: Monoclonal antibodies are valuable tools for disease diagnosis. They can specifically bind to disease-specific antigens circulating in the body and are used in immunoassay techniques to detect and measure these antigens. This enables the diagnosis of various diseases, including infectious diseases and cancers.
- Immunotherapy: Monoclonal antibodies are an essential component of immunotherapy. They can stimulate the immune system to recognize and attack cancer cells or other disease-causing agents. They can also block immune checkpoints, enhancing the body’s immune response against diseases such as cancer.
- Targeted drug delivery: Monoclonal antibodies can be conjugated with drugs or toxins to specifically deliver them to target cells, such as cancer cells. This targeted drug delivery approach minimizes damage to healthy cells and improves the effectiveness of the treatment.
- Research and development: Monoclonal antibodies are extensively used in research and development for understanding disease mechanisms, studying specific proteins or pathways, and developing new therapies. They serve as valuable tools for investigating the underlying biology of diseases and testing potential treatments.
- COVID-19 treatment: Monoclonal antibodies have been studied for the treatment of COVID-19. They have shown promise in providing short-term protection against SARS-CoV-2, the virus causing COVID-19. Clinical trials have been conducted to evaluate their efficacy in reducing the severity of the disease and preventing hospitalizations.
Overall, monoclonal antibodies have diverse applications in the treatment of various diseases, diagnosis, targeted therapy, and research. Their specificity, ability to modulate immune responses, and targeted binding make them valuable tools in improving patient outcomes and advancing medical knowledge.
Uses of Monoclonal Antibodies
Monoclonal antibodies have a wide range of uses in various fields. Here are some key applications of monoclonal antibodies:
- Cell identification and classification: Monoclonal antibodies are used to identify and classify different cell types based on specific phenotypic markers. They have been instrumental in defining clusters of differentiation (CD) markers for various cell types, aiding in the characterization and classification of immune cells and other cell populations.
- Disease diagnosis: Monoclonal antibodies play a crucial role in the diagnosis of infectious and systemic diseases. They are used in immunoassays to detect specific antigens or antibodies present in blood, urine, or tissues. These assays are used for accurate and sensitive diagnosis of diseases, including viral infections, autoimmune disorders, and certain cancers.
- Tumor identification: Labeled monoclonal antibodies that bind to specific proteins expressed by tumor cells are utilized in staining histological tumor sections. This helps determine the tissue source of tumors, aiding in their identification and classification.
- Therapeutic applications: Monoclonal antibodies are used therapeutically to treat various diseases. Antibodies targeting specific molecules or cells involved in disease pathogenesis are employed in medical treatments. For example, monoclonal antibodies against cytokines like tumor necrosis factor (TNF) are used to treat inflammatory diseases such as rheumatoid arthritis. Antibodies against CD20 are used for B cell leukemia treatment, while antibodies against epidermal growth factor receptors and vascular endothelial growth factor are employed in targeting cancer cells.
- Functional analysis of molecules: Monoclonal antibodies are invaluable tools for functional analysis of cell surface and secreted molecules. They can bind to specific molecules and either stimulate or inhibit particular cellular functions, allowing researchers to study the roles and functions of these molecules. Monoclonal antibodies are also used to purify specific cell populations from complex mixtures, facilitating the study of their properties and functions.
These are just a few examples of the many applications of monoclonal antibodies. Their specificity, versatility, and ability to target specific molecules make them valuable tools in research, diagnosis, and therapy across various fields of medicine and biotechnology.
Limitations of Monoclonal Antibodies
While monoclonal antibodies have revolutionized medical treatments, they do have some limitations. Here are a few of the key limitations associated with monoclonal antibodies:
- Immunogenicity: Monoclonal antibodies produced using mice as the source of the antibody can elicit an immune response in patients. When patients are treated with mouse-derived antibodies, they may develop human anti-mouse antibodies (HAMA). These antibodies can interfere with the function of the injected monoclonal antibody and may lead to the clearance of the antibody from the body. In some cases, HAMA can also cause serum sickness, an immune-complex mediated disorder.
- Limited source of production: Monoclonal antibodies are most easily generated by immunizing mice. This limits the availability of antibodies, and large-scale production can be challenging. While genetic engineering techniques have expanded the usefulness of monoclonal antibodies, the initial reliance on mice for antibody production can be a limitation in terms of scalability.
- Foreignness in humans: Mouse-derived monoclonal antibodies can be recognized as foreign by the human immune system, leading to the development of an immune response against the antibody. This immune response can reduce the efficacy of the monoclonal antibody and potentially limit its therapeutic benefits.
- Humanization challenges: To mitigate the immunogenicity issue, genetic engineering techniques have been employed to create humanized monoclonal antibodies. However, the process of humanizing antibodies can be complex and challenging. Ensuring that the humanized antibodies retain their specificity and binding affinity while minimizing immunogenicity is a significant undertaking.
- Cost and accessibility: Monoclonal antibody therapies can be expensive, making them less accessible to certain patient populations. The high costs of production, development, and the need for specialized manufacturing facilities contribute to the overall cost of these therapies.
FAQ
What are monoclonal antibodies?
Monoclonal antibodies are laboratory-produced antibodies that are designed to target specific antigens or proteins in the body. They are produced from a single clone of cells and are highly specific in their binding.
How are monoclonal antibodies produced?
Monoclonal antibodies are produced through a process called hybridoma technology. This involves fusing antibody-producing cells from an immunized animal (usually mice) with immortal cancer cells to create hybridoma cells that can continuously produce the desired antibodies.
What are the therapeutic applications of monoclonal antibodies?
Monoclonal antibodies have various therapeutic applications, including the treatment of cancers, autoimmune diseases, and inflammatory disorders. They can be used to target specific cells or proteins, modulate immune responses, deliver drugs or radiation directly to target cells, and more.
Are monoclonal antibodies the same as vaccines?
No, monoclonal antibodies and vaccines are different. Monoclonal antibodies are laboratory-produced proteins that directly target specific antigens or proteins, while vaccines stimulate the body’s immune system to produce its own antibodies against a particular pathogen.
Are monoclonal antibodies safe?
Monoclonal antibodies are generally considered safe, but like any medication, they can have potential side effects. These side effects can vary depending on the specific antibody and the individual patient. Common side effects include infusion reactions, allergic reactions, and immunogenicity.
How are monoclonal antibodies administered?
Monoclonal antibodies can be administered through intravenous (IV) infusion, subcutaneous injection, or intramuscular injection, depending on the specific antibody and its intended use. The route of administration is determined by factors such as the target disease and the desired pharmacokinetics.
How long do monoclonal antibodies stay in the body?
The duration of monoclonal antibodies in the body can vary depending on factors such as the specific antibody, its half-life, and the rate of clearance. Some monoclonal antibodies may have a shorter duration of action and require repeated dosing, while others may have a longer duration and require less frequent dosing.
Can monoclonal antibodies be used to prevent or treat COVID-19?
Yes, monoclonal antibodies have been authorized or approved for emergency use in the prevention and treatment of COVID-19. They can be used in certain high-risk individuals to help prevent severe disease or as a treatment option for those who have already been infected with the SARS-CoV-2 virus.
Can monoclonal antibodies replace other treatments like chemotherapy?
Monoclonal antibodies are not meant to replace other treatments like chemotherapy. They are often used as complementary therapies or targeted treatments that work in combination with other approaches, including chemotherapy, radiation therapy, and immunotherapy, depending on the specific condition and treatment plan.
Are monoclonal antibodies available to everyone?
Monoclonal antibodies are approved for specific indications and are available by prescription. Availability may vary depending on the country, healthcare system, and regulatory approvals. Access to monoclonal antibodies can also be influenced by factors such as cost, reimbursement policies, and healthcare provider recommendations.
References
- Bayat AA, Yeganeh O, Ghods R, Zarnani AH, Ardekani RB, Mahmoudi AR, Mahmoudian J, Haghighat-Noutash F, Jeddi-Tehrani M. Production and characterization of a murine monoclonal antibody against human ferritin. Avicenna J Med Biotechnol. 2013 Oct;5(4):212-9. PMID: 24285995; PMCID: PMC3838765.
- Casadevall A, Cleare W, Feldmesser M, Glatman-Freedman A, Goldman DL, Kozel TR, Lendvai N, Mukherjee J, Pirofski LA, Rivera J, Rosas AL, Scharff MD, Valadon P, Westin K, Zhong Z. Characterization of a murine monoclonal antibody to Cryptococcus neoformans polysaccharide that is a candidate for human therapeutic studies. Antimicrob Agents Chemother. 1998 Jun;42(6):1437-46. doi: 10.1128/AAC.42.6.1437. PMID: 9624491; PMCID: PMC105619.
- Holzlöhner P, Hanack K. Generation of Murine Monoclonal Antibodies by Hybridoma Technology. J Vis Exp. 2017 Jan 2;(119):54832. doi: 10.3791/54832. PMID: 28117810; PMCID: PMC5407676.
- Yokoyama, W. M., Christensen, M., Santos, G. D., Miller, D., Ho, J., Wu, T., … Neethling, F. A. (2013). Production of Monoclonal Antibodies. Current Protocols in Immunology, 102(1), 2.5.1–2.5.29. doi:10.1002/0471142735.im0205s102
- Mallbris L, Davies J, Glasebrook A, Tang Y, Glaesner W, Nickoloff BJ. Molecular Insights into Fully Human and Humanized Monoclonal Antibodies: What are the Differences and Should Dermatologists Care? J Clin Aesthet Dermatol. 2016 Jul;9(7):13-5. Epub 2016 Jul 1. PMID: 27672407; PMCID: PMC5022998.
- Cornacoff, J.B., Giles-Komar, J. (2005). Humanized Monoclonal Antibodies. In: Vohr, HW. (eds) Encyclopedic Reference of Immunotoxicology. Springer, Berlin, Heidelberg. https://doi.org/10.1007/3-540-27806-0_699
- https://absoluteantibody.com/our-technology/formats-we-have-made/chimeric-antibodies/
- http://www.receptabio.com.br/en/pesquisa-desenvolvimento/anticorpos-monoclonais-recepta-detem-potencial-para-tratar-diversos-tipos-de-cancer/
- https://www.nature.com/articles/d41586-019-02840-w
- https://www.moleculardevices.com/applications/monoclonal-antibody-production#gref
- https://www.medicinenet.com/hybridoma/definition.htm
- https://www.medicinenet.com/monoclonal_antibodies/drug-class.htm
- https://www.mayoclinic.org/diseases-conditions/cancer/in-depth/monoclonal-antibody/art-20047808
- https://www.intechopen.com/chapters/51512
- https://www.genscript.com/how-to-make-monoclonal-antibodies.html
- https://www.cancer.org/treatment/treatments-and-side-effects/treatment-types/immunotherapy/monoclonal-antibodies.html
- https://www.sinobiological.com/resource/antibody-technical/chimeric-monoclonal-antibody
- https://www.proteogenix.science/scientific-corner/antibody-production/murine-monoclonal-antibodies-and-their-use-in-modern-medicine/
- https://www.prospecbio.com/monoclonal_antibodies
- https://www.nuventra.com/resources/blog/monoclonal-antibodies-past-present-and-future/
- https://www.nih.gov/news-events/news-releases/clinical-trials-monoclonal-antibodies-prevent-covid-19-now-enrolling
- https://combatcovid.hhs.gov/what-are-monoclonal-antibodies
- https://www.cancerresearchuk.org/about-cancer/cancer-in-general/treatment/immunotherapy/types/monoclonal-antibodies