Microtubules Definition (What are microtubules?)
- Microtubules are microscopic, hollow tubes made of alpha and beta tubulin that are a part of the cell’s cytoskeleton.
- Microtubules extend throughout the cell providing it with proper shape and keeping the organelles in place.
- They are the largest structures in the cytoskeleton and are about 24 nm thick.
- They facilitate cell movement, cell division, and transportation of materials within the cells.
- They are also involved in the division of chromosomes during the process of mitosis and in locomotion.
- Robertis and Franchi initially detected microtubules in the axoplasm of myelinated nerve fibres (1953). They were known as neurotubules.
- Using glutarldehyde as a fixative in electron microscopy, Sabatini, Bensch, and Barnett (1963) shed light on the precise nature of microtubules.
- Ledbetter and Porter were the first to characterise in detail the microtubules of plant cells (1963).
Occurrence of Microtubules
- Microtubules are present in all eukaryotic cells, with the exception of human erythrocytes, either free in the cytoplasm or as components of centrioles, cilia, and flagella.
- The vertebrate brain is the most abundant source of microtubules for biochemical research; nerve cell axons and dendrites contain significant microtubule density. In the cytoplasm of animal and plant cells, microtubules are present at the seven places listed below:—
- cilia and flagella.
- centrioles and basal bodies.
- nerve processes.
- the mitotic machinery.
- The outermost layer of meristematic plant cells.
- elongating cells such as during lens development or spermatogenesis in certain insects.
- The axostyle of parasitic flagellates, the axoneme of Echinosphaerium, the fibre networks of Stentor, and the cytopharyngeal basket of Nassula are examples of chosen structures in Protozoa.
- The stabilities of various microtubules vary. Cytoplasmic and spindle microtubules are very unstable structures, but cilia and flagella microtubules are more robust to many treatments.
Microtubules Structure
- Microtubules are a morphologically and chemically similar family of filamentous rods found in both plant and animal cells.
- A microtubule consists of long, unbranched, hollow tubules with a diameter of 24–25 nm, a length of several micrometres, and a wall thickness of 6 nm with 13 subunits or protofilaments.
- Thus, the microtubule wall comprises of 13 separate linear or spiralling filamentous structures with a diameter of around 5 nm, which are comprised of tubulin.
- This protofilament’s centre-to-centre distance is 4.5 nm.
- Negative staining techniques have demonstrated that microtubules have a 14-nm-wide lumen and a protofilament or subunit structure in their walls.
Chemical Composition of Microtubules
- Biochemically, a microtubule protofilament is composed of the protein tubulin.
- Tubulin has a molecular weight of 55,000 and a sedimentation constant of 6S; it is an acidic protein.
- It exists in two forms, -tubulin and -tubulin, each of which contains around 450 amino acids.
- The amino acid sequences of these two proteins are unique, albeit closely related, and are believed to have evolved from a single ancestral protein.
- From the lowest to the highest eukaryotes, the two proteins diverge extremely little; for example, the -tubulins of sea urchin flagella and chick brain cells differ by only one amino acid.
- This similarity suggests that the majority of mutations affect the functioning of microtubules and are therefore deadly and eliminated by natural selection.
- Tubulin polymerizes into microtubules in the form of dimers (rather, heterodimers of – and – tubulins; each having 115,000 MW; see Berns, 1983).
- Thus, heterodimers of tubulins assemble to produce linear “protofilaments” with – tubulin of one dimer contacting – tubulin of the following dimer.
- Due to the parallel alignment of all 13 protofilaments with the same polarity, microtubules are polar structures with a plus or fast-growing end and a minus or slow-growing end.
- In cells, the minus ends of cytoplasmic microtubules are securely attached to microtubule organising centres (MTOCs), where their assembly or polymerization begins.
- MTOCs also prevent the disintegration of the microtubules’ negative ends. Typically, the plus ends of microtubules terminate at the cell borders and are safeguarded by capping proteins.
Intracellular Organization of Microtubules
- Microtubules form a structural network in the cytoplasm.
- Microtubule cytoskeleton functions include chromosomal separation, transport, motility, and mechanical support.
- Due to the existence of motor proteins that enable the transport of cellular components and other materials through microtubules, it can either contract or expand to generate energy.
- The configurations of microtubules are cell-type dependent.
- So that the transport of organelles, vesicles, and proteins down the apical-basal axis of the cell might be facilitated easily.
- They also play a crucial function in cell migration.
Microtubule-Associated Proteins (MAPs)
- Recently, a number of proteins that connect with the surface of microtubules have been found; these proteins are known as microtubule-associated proteins (MAPs).
- Along with microtubules, the following two primary classes of MAPs have been identified from brain:
- High molecular weight (HMW) proteins have molecular weights of 200,000 to 300,000 or greater.
- Molecular weights between 40,000 and 60,000 are associated with tau proteins. Both kinds of proteins contain two domains, one of which binds to microtubules. Because this domain binds to several unpolymerized tubulin molecules concurrently, these MAPs tend to accelerate the nucleation step of tubulin polymerization in vitro.
- It is hypothesised that the second domain links the microtubule to other cellular components.
- Antibodies to HMW and tau proteins reveal that both proteins bind microtubules over their full length.
Microtubule Organizing Centres (MTOCs)
- The microtubules are not dispersed randomly throughout the cell, but rather are arranged in specialised patterns to perform certain functions. As observed in vitro, spontaneous nucleation presumably does not occur in vivo.
- Rather, assembly is initiated at microtubule organising centres (MTOCs). Thus, MTOCs are nucleating centres that serve as tubulin polymerization templates.
- MTOCs exist in basal bodies (e.g., Chlamydomonas), in centrioles (e.g., most animal cells), at the poles of mitotic spindles in dividing cells that lack centrioles (e.g., most plant cells), on chromosomes (i.e., kinetochore), in membranes, and possibly in numerous other locations.
- Recent research has shown that the majority of cytoplasmic microtubules do not originate directly from centrioles, but rather from a heavily stained pericentriolar substance that surrounds the centriole.
- At various stages of a cell’s life, the activation and deactivation of these organising centres for microtubule assembly are likely regulated by one or more of the following factors: changes in nucleation centres, changes in Ca2+ concentration, and the involvement of MAPs.
Assembly and Disassembly of Microtubules
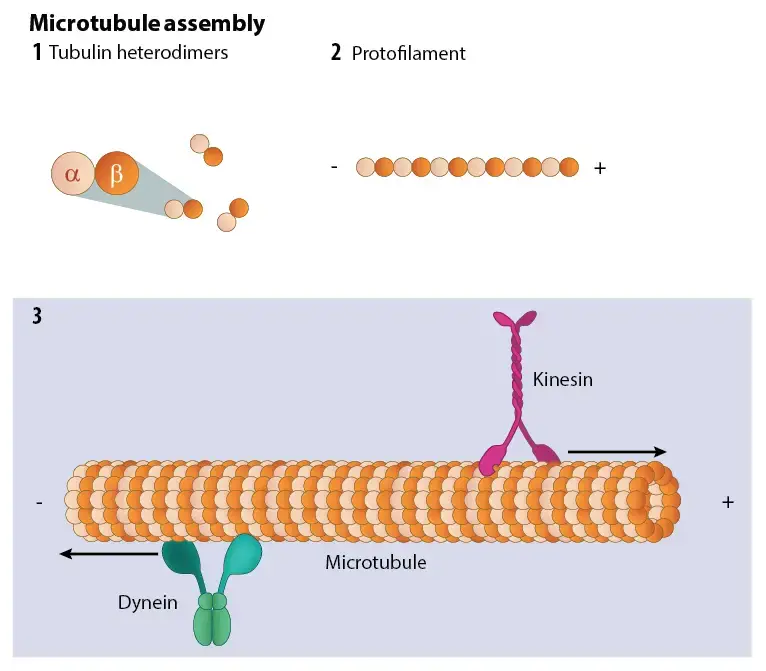
- Microtubules in the cytoplasm are extremely dynamic structures, constantly forming and dissolving in response to cellular processes.
- As with microfilaments, they expand through the addition of subunits in a reversible manner, accompanied by nucleotide (GTP) hydrolysis and conformational change.
- The process of microtubule polymerization (assembly) and depolymerization (disassembly) appears to be an instance of selfassembly.
- The assembly of microtubules from tubulin dimers is a controlled and carefully directed process.
- MTOCs are the orientation sites in the cell from which polymerization is directed.
- During interphase (cytoplasmic microtubules) and metaphase (spindle microtubules), the amount of polymerized tubulin is high, whereas it is minimal during prophase and anaphase.
- Microtubules and free tubulin are in equilibrium within the cell. A cyclic AMP-dependent kinase promotes the polymerization of tubulin by phosphorylating its monomers.
- There is a correlation between cell shape, the number and orientation of microtubules, and cAMP.
- The tubulin assembly and disassembly process is a polarised phenomenon. At one end of a microtubule, tubulin dimers are assembled, while disintegration is common at the other end.
- If a cell is treated with specific medications such as colchicine, vincristine, or vinblastine, the assembly of microtubules is hindered while the disassembly occurs, resulting in the microtubule’s disarray.
- In addition, the assembly is accompanied by the hydrolysis of guanosine triphosphate (GTP) to guanosine diphosphate (GDP), and the absence of GTP terminates the assembly.
- Ca2+ and the calcium-binding protein calmodulin regulate the assembly and disassembly of tubulin intracellularly. The presence of Ca2+ decreases the polymerization of tubulin, and the addition of calmodulin enhances this action.
- The in vivo mechanism involved in the self-assembly of microtubules is poorly understood; nevertheless, in vitro research have uncovered a number of fascinating facts about it.
- In a classic work employing isolated tubulin from bovine brain, Weingarten et al. (1975) revealed that tubulin alone was insufficient to induce microtubule formation in vitro.
- Under normal circumstances, if brain microtubules are isolated and depolymerized into tubulin subunits, the tubulin molecules can be reassembled into microtubules by adding Mg2+ and GTP (an energy source).
- Nevertheless, according to King (1986), in vitro assembly of microtubules is possible in the presence of low calcium concentration, MAPs, GTP, and a threshold concentration of free tubulin monomers.
- Evidently, in vitro polymerization consists of two different steps, initiation and elongation.
- The initiation process appears to include the development of a multimeric “nucleating” centre, after which the fast addition of additional subunits occurs during elongation.
- Consequently, during in vitro microtubule polymerization, – and – tubulins interact to create heterodimers.
- The heterodimers assemble to form multimeric rings, spirals, and other intermediate structures, which eventually unfold into filaments or protofilaments.
- Assembling protofilaments side-by-side generates sheet-like structures that coil to form a tube. This small cylinder is elongated by the direct addition of heterodimers at one end of the tubule (i.e., the plus end of tubule).
- During anaphase, it is hypothesised that the addition of dimers to one end of a microtubule coincides with the removal of dimers from the other end.
Function of Microtubules
Microtubules serve multiple purposes in eukaryotic cells, including the following:
1. Mechanical function
- There is a correlation between the direction and distribution of microtubules and the shape of the cell (e.g., non-mammalian vertebrate red blood cells) and cell processes or protuberances such as axons and dendrites of neurons, microvilli, etc.
2. Morphogenesis
- During cell differentiation, the form of developing cells is determined by the mechanical function of microtubules.
- During spermiogenesis, for instance, the elongation of the nucleus of the spermatid is accompanied by the creation of an ordered array of microtubules that are wrapped around the nucleus in a double helix configuration.
- The development of many microtubules also accompanies the elongation of cells during the formation of the lens placode in the eye.
3. Cellular polarity and motility
- Microtubules are also involved in the determination of the inherent polarity of specific cells.
- The microtubules are responsible for the directional gliding of cultured cells.
4. Contraction
- Microtubules are involved in the contraction of the spindle, the movement of chromosomes and centrioles, and the motion of cilia and flagella.
5. Circulation and transport
- Microtubules are involved in the intracellular transport of macromolecules, granules, and vesicles.
- Examples:
- Within the protist Actinosphaerium’s (Heliozoa) long, thin pseudopodia, cytoplasmic particles travel back and forth. These pseudopodia contain up to 500 microtubules arranged in a helical pattern.
- In the protozoan Nassula, the food in the gullet is propelled by microtubules.
- Different stimuli cause melanin granules to migrate centrifugally and centripetally in melanocytes. It has been found that these granules move between microtubule-created channels in the cytoplasmic matrix.
- Between the microtubules in the erythrophores of fish scales, pigment granules may travel at a rate of 25 to 30 m per second.
- They play a function in the transport of proteins, glycoproteins, and enzymes along the axoplasm.
FAQ
What are microtubules?
Microtubules are long, thin, tube-like structures that are part of the cytoskeleton of eukaryotic cells. They are made up of protein subunits called tubulin, and are involved in many cellular processes, such as cell division, cell shape and movement, and intracellular transport.
How are microtubules formed?
Microtubules are formed from the polymerization of tubulin protein subunits. The tubulin subunits bind together to form long chains, which then assemble into the tubular structure of the microtubule.
What is the function of microtubules?
Microtubules have many functions in the cell, including providing structural support, helping to maintain cell shape and organization, aiding in cell division, and facilitating intracellular transport.
How do microtubules facilitate cell division?
Microtubules play a critical role in cell division by forming the mitotic spindle, a structure that separates the chromosomes during cell division.
What is the relationship between microtubules and cilia/flagella?
Cilia and flagella are specialized structures that extend from the surface of some eukaryotic cells and are involved in cell movement. Both cilia and flagella contain microtubules as part of their structure, which help to power their movement.
References
- Cooper GM. The Cell: A Molecular Approach. 2nd edition. Sunderland (MA): Sinauer Associates; 2000. Microtubules. Available from: https://www.ncbi.nlm.nih.gov/books/NBK9932/
- Parker, A. L., Kavallaris, M., & McCarroll, J. A. (2014). Microtubules and Their Role in Cellular Stress in Cancer. Frontiers in Oncology, 4. doi:10.3389/fonc.2014.00153
- https://www.mechanobio.info/cytoskeleton-dynamics/what-is-the-cytoskeleton/what-are-microtubules/
- https://www.ruf.rice.edu/~bioslabs/studies/invertebrates/microtubules.html
- https://www.nature.com/scitable/content/microtubules-the-basics-14673338/
- https://micro.magnet.fsu.edu/cells/microtubules/microtubules.html
- https://biologydictionary.net/microtubule/