What is Antigen Processing, And Presentation?
- Antigen processing and presentation are essential steps in the immune response, particularly in the adaptive immune system. They involve the breakdown of antigens (foreign substances) into smaller fragments, their association with specialized proteins, and their subsequent presentation to immune cells known as T cells.
- Antigen processing refers to the intracellular mechanisms by which antigens are degraded into smaller peptide fragments. This process occurs within antigen-presenting cells (APCs), such as macrophages, dendritic cells, and B cells. The antigens can be derived from various sources, including intracellular pathogens, extracellular pathogens that have been engulfed by phagocytosis, or endocytosed antigens.
- During antigen processing, the antigens are broken down into peptide fragments through enzymatic degradation. This typically occurs within cellular compartments such as lysosomes or endosomes, where proteolytic enzymes break down the proteins. The resulting peptide fragments are usually 8-20 amino acids in length.
- Following antigen processing, the peptide fragments associate with specialized proteins called major histocompatibility complex (MHC) molecules. MHC molecules are of two types: MHC class I and MHC class II. MHC class I molecules present peptides derived from intracellular antigens, while MHC class II molecules present peptides from extracellular antigens.
- In MHC class I antigen presentation, the processed peptide fragments are loaded onto MHC class I molecules within the endoplasmic reticulum (ER) of the antigen-presenting cell. The MHC class I-peptide complex is then transported to the cell surface, where it can be recognized by CD8+ T cells. This interaction triggers an immune response against infected or abnormal cells.
- In MHC class II antigen presentation, the processed peptide fragments associate with MHC class II molecules within endosomes or lysosomes. MHC class II molecules are produced in the ER and associate with an invariant chain to prevent premature binding of self-peptides. In the endosomal compartments, the invariant chain is degraded, allowing the peptide fragments to bind to the MHC class II molecules. The MHC class II-peptide complex is then transported to the cell surface, where it can be recognized by CD4+ T cells, leading to immune activation.
- The process of antigen processing and presentation is crucial for the immune system to distinguish between self and non-self antigens. It allows T cells to recognize and mount immune responses against foreign pathogens, infected cells, or abnormal cells. Understanding antigen processing and presentation is vital for studying immune responses, vaccine development, and the treatment of various diseases.
Major histocompatibility complex
- MHC-encoded proteins were first identified in the 1930s during studies of tissue rejection in transplantation experiments.
- Therefore, these proteins were given the name histocompatibility (histo meaning “tissue” and compatibility meaning “getting along”).
- The genes influencing the histocompatibility of tissue transplantation have been mapped to a wide genomic area with several loci, hence the word “complex.”
- Moreover, it was discovered that the proteins generated by these genes had profound effects on histocompatibility. To differentiate these proteins from others (encoded elsewhere in the genome) that had very minimal impacts on histocompatibility, these molecules were dubbed “major” histocompatibility molecules.
- Thus, the genes encoding these proteins were designated as MHC genes (major histocompatibility complex genes). Soon later, it was shown that MHC-controlled rejection of transplanted tissue was caused by the recipient’s immunological reaction to the donor cells.
- Although this observation suggested that MHC gene products were directly engaged in immune responses, it took immunologists several more decades to establish the physiological role of MHC-encoded proteins in presenting antigenic peptides to T cells.
- MHC class I and MHC class II molecules are the MHC-encoded proteins involved in the majority of antigen recognition by T lymphocytes. TCRs of CD8+ T cells identify MHC class I-bound peptides, whereas TCRs of CD4+ T cells recognise MHC class II-bound peptides.
- Additionally, the CD8 coreceptor of CD8+ T cells attaches to MHC class I, whereas the CD4 coreceptor of CD4+ T cells binds to MHC class II. The MHC class I protein is a heterodimer composed of a long transmembrane α chain that is non-covalently connected to β2-microglobulin (β2m).
- The MHC class I α chain, but not β2m, is encoded within the MHC. Class II MHC protein consists of a α chain and a somewhat smaller β chain, both of which are transmembrane proteins encoded by MHC genes.
- With the exception of the peptide-binding groove, the tertiary structures of MHC class I and class II molecules are extremely similar despite this change in composition.
- While virtually all nucleated cells express MHC class I, only a few cell types that serve as APCs (such as DCs, macrophages, and B cells) express MHC class II.
- Consequently, virtually any cell can serve as a target cell and provide antigen to CTLs produced from CD8+ Tc cells, whereas only APCs can activate CD4+ Th cells.
Major histocompatibility complex I (MHC Class I)
- The Major Histocompatibility Complex I (MHC Class I) is an important component of the immune system. It is the first class of the MHC molecule and is responsible for encoding glycoproteins that are expressed on the surface of nearly all nucleated cells in the body, with the exception of cells in the retina and brain.
- The primary function of MHC Class I molecules is to present antigen-processed peptides to T-cytotoxic cells, also known as CD8+ T cells. This presentation occurs through the cytosolic pathway, where intracellular proteins are broken down into smaller peptide fragments. These peptides are then loaded onto MHC Class I molecules and transported to the cell surface for recognition by CD8+ T cells. This interaction is crucial for the immune system to identify infected or abnormal cells and mount an appropriate immune response, such as destroying the infected cells.
- In humans, the MHC Class I protein is encoded by three genes known as HLA-A, HLA-B, and HLA-C. These genes are highly polymorphic, meaning they have many different alleles within the population. This diversity allows the immune system to recognize a wide range of pathogens and adapt to new infectious agents.
- MHC Class I molecules are composed of two chains. The first chain is a transmembrane glycoprotein with a molecular weight of approximately 45,000. The second chain is a non-MHC-encoded polypeptide called β2-microglobulin, with a molecular weight of around 12,000. The association between these two chains is noncovalent, meaning they are not linked by a chemical bond. Together, they form the functional MHC Class I molecule.
- The expression of MHC Class I molecules is widespread throughout the body, with the exception of cells in the retina and brain. This broad distribution ensures that infected or abnormal cells can be recognized and targeted by the immune system. By presenting antigenic peptides on their surface, MHC Class I molecules play a vital role in immune surveillance and defense against pathogens.
- Understanding the structure and function of MHC Class I molecules is crucial in various areas of immunology and medicine, including transplantation, autoimmune diseases, and vaccine development. By studying these molecules, researchers can gain insights into the immune response and develop strategies to modulate or enhance immune reactions for therapeutic purposes.
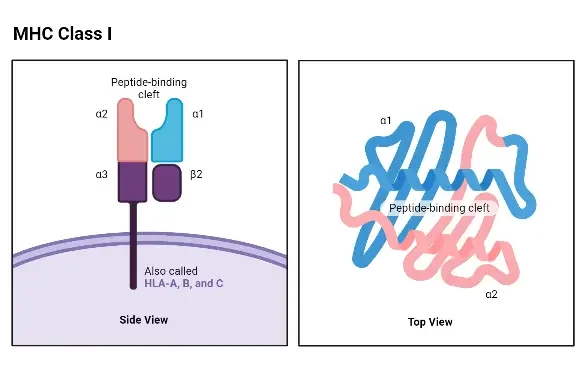
Structure of MHC Class I
- The structure of MHC Class I molecules is composed of two polypeptide chains, which differ significantly in size. In both humans and mice, the larger chain, known as the α chain, has a molecular weight of approximately 44 kDa in humans and 47 kDa in mice. This α chain is encoded by an MHC Class I gene. The smaller chain, called β-2 microglobulin, has a molecular weight of around 12 kDa in both species and is encoded by a nonpolymorphic gene located outside of the MHC complex.
- The structure of the α chain is highly conserved among different loci. There are no known structural differences between the α chains encoded by the HLA-A, HLA-B, and HLA-C loci in humans, or between the α chains encoded by the H-2K, H-2D, and H-2L loci in mice.
- The α chain of MHC Class I molecules can be divided into several regions or domains. These include the peptide-binding domain, the immunoglobulin-like domain, the transmembrane domain, and the cytoplasmic domain. The peptide-binding domain, located at the N-terminal end of the α chain, is the region where allelic differences in the amino acid sequence can be found. This domain contains the site where antigenic peptides bind. The presence of allelic differences in the peptide-binding domain affects the ability of MHC Class I molecules to accommodate peptides, which, in turn, influences the magnitude of the T-cell response.
- X-ray crystallography studies have revealed that the peptide-binding site of MHC Class I molecules forms a cleft with a “floor” and two “walls” created by spiral-shaped portions of the α chain known as alpha 1 and alpha 2. The closed “floor” of the cleft restricts the accommodation of relatively small peptides consisting of 9 to 11 amino acid residues.
- The immunoglobulin-like domain of the α chain is structurally conserved and resembles a domain found in the constant region of antibodies (C-region). This domain contains the binding site for the T-cell accessory molecule CD8.
- The transmembrane and cytoplasmic domains of the α chain ensure that it spans the cell membrane and is properly expressed by the cell. The β-2 microglobulin chain is essential for the proper expression of the α chain. Mutant lymphoid cell lines, such as Daudi, fail to express MHC Class I molecules due to defects in the β-2 microglobulin gene.
Major histocompatibility complex II (MHC Class II)
- Major Histocompatibility Complex II (MHC Class II) molecules are essential components of the immune system involved in antigen presentation. They are encoded by the class II MHC genes, primarily expressed on antigen-presenting cells such as macrophages, dendritic cells, and B cells. These MHC Class II molecules play a crucial role in presenting processed antigenic peptides to CD4+ T helper (TH) cells.
- The genes responsible for encoding class II MHC proteins are located within the HLA-D region. Within this region, there are three families of molecules known as DP, DQ, and DR-encoded molecules. These different families contribute to the diversity of MHC Class II molecules, enabling the immune system to recognize and respond to a wide range of antigens.
- The MHC Class II molecules play a vital role in regulating immune responsiveness. The allelic forms of the HLA-D genes confer variations in the ability to mount an immune response against specific antigens. This diversity in MHC Class II alleles is essential for the immune system’s capacity to recognize and respond to a diverse array of pathogens.
- The proteins encoded by the HLA-D locus consist of two noncovalently associated transmembrane glycoproteins. The molecular weight of these chains is approximately 33,000 and 29,000, respectively. The expression of MHC Class II molecules is not ubiquitous but rather has a restricted tissue distribution. They are primarily found on macrophages, dendritic cells, B cells, and other antigen-presenting cells. However, under the influence of interferon-gamma (IFN-γ), MHC Class II molecules can also be expressed on other cell types such as endothelial cells and epithelial cells. This induction of MHC Class II expression expands the antigen-presenting capability to a broader range of cells, facilitating immune responses in various tissues.
- The interaction between MHC Class II molecules and CD4+ T helper cells is crucial for the activation of the adaptive immune response. The antigenic peptides presented by MHC Class II molecules to TH cells help in coordinating immune responses and initiating appropriate effector mechanisms.
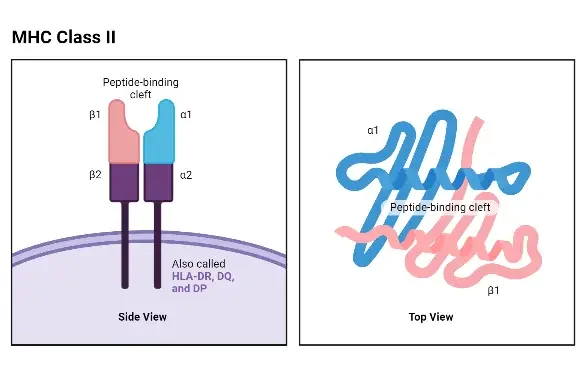
Structure of MHC Class II
- The structure of Major Histocompatibility Complex II (MHC Class II) molecules consists of two polypeptide chains, known as the alpha (α) chain and the beta (β) chain. Both humans and mice have similar-sized α and β chains, with molecular weights ranging from 32 to 34 kDa for the α chain and 29 to 32 kDa for the β chain. Each chain is controlled by a separate gene, resulting in polymorphic variations in both the α and β chains.
- Some MHC Class II loci, particularly the β genes, can be tandemly duplicated. This means that instead of having one gene per homologous chromosome, a cell can possess two or three copies of the β gene. Consequently, a single cell can express multiple allelic products of each MHC Class II locus. This tandem duplication phenomenon enables a cell to simultaneously express up to 20 different MHC Class II gene products. For instance, a cell can express allelic products identified as HLA-DRα1– HLA-DRβ1, HLA-DRα2 – HLA-DRβ2, HLA-DRα1 – HLA-DRβ2, HLA-DRα2 – HLA-DRβ1, and so on.
- The structure of the α and β chains of MHC Class II molecules shares similarities with the α chain of MHC Class I molecules. Both chains can be divided into the peptide-binding domain, the immunoglobulin-like domain, the transmembrane domain, and the cytoplasmic domain.
- One notable difference is that the peptide-binding cleft in Class II molecules is formed by both the alpha and beta chains. Although the alpha and beta chains are positioned closely together, they are not physically bound to each other. Consequently, the peptide-accommodating cleft in Class II MHC molecules has an “open” or “hole-like” floor. This structural feature allows MHC Class II molecules to accommodate larger peptides compared to the peptides that fit into MHC Class I molecules.
- The immunoglobulin-like domain of MHC Class II molecules contains a binding site for the T-cell accessory molecule CD4. However, this site cannot bind the CD8 molecule, which is associated with MHC Class I molecules.
Major Histocompatibility Class III (MHC Class III)
- Major Histocompatibility Complex Class III (MHC Class III) genes are responsible for encoding a diverse range of secreted proteins that play critical roles in immune functions. These proteins include components of the complement system, which is a group of proteins involved in the body’s defense against pathogens.
- The complement system consists of a cascade of proteins that work together to eliminate pathogens, enhance phagocytosis, and promote inflammation. Some of the proteins encoded by MHC Class III genes, such as C2, C4, and factor B, are part of the complement system. These proteins participate in the activation and regulation of the complement cascade, helping to clear pathogens and promote immune responses.
- In addition to complement components, MHC Class III genes also encode various cytokines and other molecules involved in inflammation. Cytokines are signaling proteins that mediate immune responses and regulate the communication between immune cells. Examples of cytokines encoded by MHC Class III genes include tumor necrosis factor alpha (TNF-α) and lymphotoxin alpha (LT-α). These cytokines play crucial roles in initiating and modulating inflammatory responses, which are essential for combating infections and promoting tissue repair.
- MHC Class III genes contribute significantly to the immune system’s ability to mount effective immune responses against pathogens. The secreted proteins encoded by these genes participate in key immune processes, including complement activation, inflammation, and cytokine-mediated signaling. Understanding the functions and regulation of MHC Class III genes and their protein products is crucial for unraveling the complexities of the immune system and developing therapies targeting immune-related disorders.
Antigen Processing and Presentation
- Antigen processing and presentation are essential steps in the immune response that enable T lymphocytes to recognize and respond to protein antigens. This process involves the degradation of antigens into peptides, their association with Major Histocompatibility Complex (MHC) molecules, and their subsequent display on the cell membrane.
- Antigen processing begins with the internalization of the protein antigens by antigen-presenting cells (APCs), such as macrophages, dendritic cells, and B cells. Within these cells, the antigens are broken down into smaller peptide fragments through enzymatic degradation, a process known as antigen processing.
- Once the antigens are processed into peptides, they associate with MHC molecules within the cell’s cytoplasm, forming a peptide-MHC complex. In the case of MHC Class I molecules, the peptides derived from endogenous antigens are processed within the cytoplasm of the cell. These endogenous antigens can include tumor proteins, viral proteins, bacterial proteins, or cellular proteins. The Class I MHC molecules bind these peptides and present them on the cell surface. This process is known as the cytosolic pathway.
- On the other hand, MHC Class II molecules bind peptides derived from exogenous antigens that are taken up by the cell through phagocytosis or endocytosis. These exogenous antigens can include extracellular bacteria, viruses, or other foreign particles. The antigens are processed within the endocytic pathway, where they are degraded into peptides. The resulting peptides associate with Class II MHC molecules and are transported to the cell membrane, where they are displayed for recognition by CD4+ T helper cells. This process is called the endocytic pathway.
- By presenting antigen-derived peptides, both MHC Class I and Class II molecules play crucial roles in activating T lymphocytes. The interaction between the T cell receptor on T lymphocytes and the peptide-MHC complex on antigen-presenting cells is central to initiating an immune response against specific antigens. This recognition triggers a cascade of immune reactions, leading to the activation and proliferation of T lymphocytes and the coordination of various immune effector mechanisms.
- Antigen processing and presentation ensure that T lymphocytes can recognize a diverse array of antigens, whether they originate from intracellular sources (MHC Class I pathway) or extracellular sources (MHC Class II pathway). This process is fundamental for the adaptive immune response and plays a critical role in immune surveillance, defense against infections, and the elimination of abnormal or infected cells.
1. Cytosolic pathway – Endogenous antigen
- The cytosolic pathway is responsible for processing and presenting endogenous antigens using Class I MHC molecules. This pathway involves several steps that culminate in the display of antigenic peptides on the cell surface for recognition by cytotoxic T lymphocytes.
- The process begins with the degradation of intracellular antigen proteins into short peptides. This degradation occurs in the cytosol and is mediated by a proteolytic system that involves the attachment of a small protein called ubiquitin to the target proteins. The ubiquitin-protein conjugate is then recognized and degraded by a large protease complex known as the proteasome. The proteasome consists of four rings of protein subunits and has a central channel where the degradation of the ubiquitin-protein complex takes place.
- Following degradation, the resulting peptides need to be transported from the cytosol to the rough endoplasmic reticulum (RER) where Class I MHC molecules are located. This transportation is facilitated by a transporter protein called TAP (transporter associated with antigen processing). TAP is a membrane-spanning heterodimer composed of two proteins, TAP1 and TAP2, and it mediates the ATP-dependent transport of peptides from the cytosol into the RER. TAP proteins preferentially transport peptides of 8-10 amino acids in length, which is optimal for binding to Class I MHC molecules. Peptides with hydrophobic or basic carboxyl-terminal amino acids, which are preferred anchor residues for Class I MHC molecules, are favored by TAP for transport.
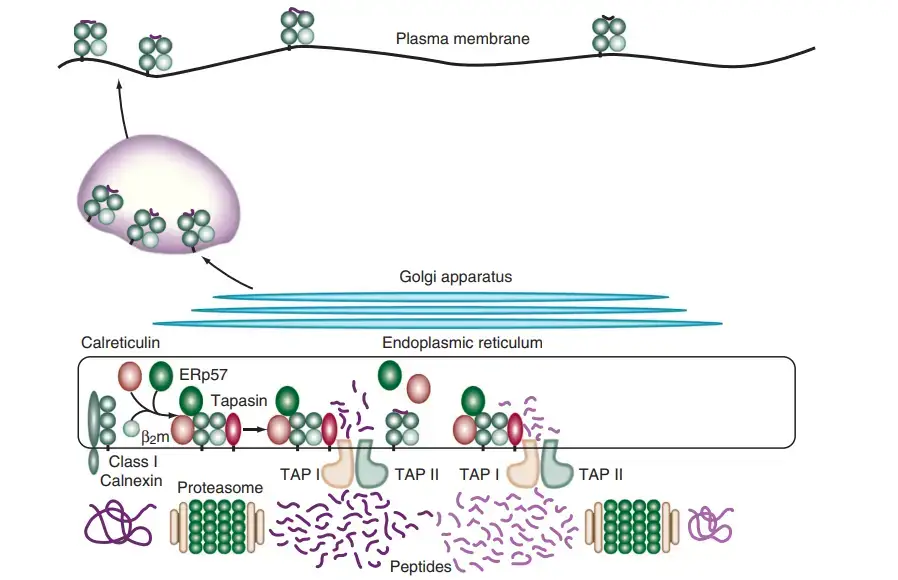
- Once the peptides reach the RER, they encounter chaperone molecules that assist in their folding and assembly with Class I MHC molecules. The alpha and beta-2-microglobulin components of Class I MHC molecules are synthesized on the rough endoplasmic reticulum. Calnexin, a resident membrane protein of the ER, associates with the Class I alpha chain and promotes its folding. When the beta-2-microglobulin binds to the alpha chain, calnexin is released, and the Class I molecule associates with the chaperone calreticulin and tapasin.
- Tapasin, a TAP-associated protein, brings the TAP transporter into proximity with the Class I molecule, allowing it to acquire an antigenic peptide. The physical association between the alpha chain-beta-2-microglobulin heterodimer and the TAP protein promotes peptide capture by the Class I molecule before the peptides are exposed to the RER. Peptides that fail to bind to Class I molecules are rapidly degraded.
- The formation of disulfide bonds during the maturation of Class I chains is facilitated by an additional chaperone protein called ERp57, which associates with calnexin and calreticulin complexes. Although the precise role of ERp57 in the Class I peptide assembly and loading process is not fully understood, it is believed to contribute to the maturation of Class I chains.
- Once the Class I molecule has acquired an antigenic peptide, it gains increased stability and dissociates from calreticulin and tapasin. It then exits the RER and proceeds to the cell surface via the Golgi apparatus. The loaded Class I MHC molecules are displayed on the cell membrane, allowing them to present the antigenic peptides to cytotoxic T lymphocytes for immune recognition and response.
- In summary, the cytosolic pathway is responsible for processing and presenting endogenous antigens using Class I MHC molecules. This pathway involves the degradation of antigen proteins, facilitated by the proteasome, followed by the transport of peptides into the RER via TAP proteins. The peptides are then assembled with Class I MHC molecules with the assistance of chaperone molecules, leading to the display of peptide-MHC complexes on the cell surface for immune surveillance and response.
2. Endocytic Pathway – Exogenous antigen
- The endocytic pathway is responsible for processing and presenting exogenous antigens using Class II MHC molecules. This pathway involves the internalization of antigens by antigen-presenting cells (APCs) through processes such as phagocytosis, pinocytosis, or receptor-mediated endocytosis.
- APCs like macrophages can internalize antigens by both phagocytosis and endocytosis, while other APCs, such as B cells, primarily internalize antigens through receptor-mediated endocytosis using antigen-specific membrane antibody receptors. Once the exogenous antigen is internalized, it undergoes degradation into peptides within the compartments of the endocytic processing pathway.
- The endocytic pathway consists of several acidic compartments, including the early endosome (pH 6.0-6.5), late endosome or endolysosomes (pH 5.0-6.0), and lysosomes (pH 4.5-5.0). As the antigen moves through these compartments, the pH progressively decreases, creating an environment conducive to the activity of hydrolytic enzymes present in the lysosomes. These enzymes, including proteases, nucleases, glycosidases, lipases, phospholipases, and phosphatases, break down the antigen into smaller oligopeptides comprising 13-18 amino acid residues that can bind to Class II MHC molecules.
- Transport vesicles facilitate the movement of the peptides from one compartment to the next within the endocytic pathway. Once the peptides reach the final compartments, they can be recycled back to the cell periphery by fusing with the plasma membrane, allowing for the recycling of surface receptors.
- To prevent the binding of MHC II molecules to the same set of antigenic peptides as MHC I molecules, mechanisms exist to regulate peptide binding. When MHC II molecules are synthesized within the rough endoplasmic reticulum (RER), they associate with a protein called the invariant chain (Ii, CD74). The invariant chain interacts with the peptide-binding cleft of the MHC II molecules, preventing endogenously derived peptides from binding while the MHC II is in the RER. The invariant chain is also involved in the folding of MHC II molecules, their exit from the RER, and their routing to the endocytic processing pathway via the trans-Golgi network and endocytic vesicles.
- As the MHC II-invariant chain complexes progress through the endocytic pathway, the invariant chain is gradually degraded, leaving a short fragment known as the CLIP (Class II-associated invariant chain peptide) bound to the MHC II molecule within the endosomal compartment. CLIP occupies the peptide-binding groove of the MHC II molecule, preventing premature binding of antigenic peptides.
- The exchange of CLIP for antigenic peptides is facilitated by a molecule called HLA-DM. HLA-DM catalyzes the exchange reaction, promoting the binding of antigenic peptides to the MHC II molecule. HLA-DO, a molecule similar in structure to HLA-DM, modulates the function of HLA-DM, potentially affecting the efficiency of the exchange reaction.
- In summary, the endocytic pathway is involved in the processing and presentation of exogenous antigens using Class II MHC molecules. Antigens are internalized by APCs through various endocytic mechanisms, undergo degradation within acidic compartments, and eventually bind to MHC II molecules after the removal of the invariant chain fragment CLIP. The exchange of CLIP for antigenic peptides is facilitated by HLA-DM, while HLA-DO helps regulate the function of HLA-DM in this process.
Presentation of Non-peptide antigens
- Non-peptide antigens can also elicit immune responses and are recognized by a specific subset of T-cell receptors known as δγ-TCR. These T-cell receptors are dimers of αβ and δγ chains and are derived from glycolipids found in bacterial pathogens like Mycobacterium tuberculosis.
- The presentation of non-peptide antigens is mediated by a group of molecules called CD1, which are nonclassical class I molecules. The CD1 family of molecules associates with β2-microglobulin and shares structural similarities with MHC class I molecules. In humans, there are five CD1 genes (CD1A, CD1B, CD1C, CD1D, and CD1E), although CD1E has not been identified yet. These genes are located on chromosomes and not within the MHC I region.
- The CD1 molecules are divided into two groups based on sequence homology. Group 1 includes CD1A, CD1B, CD1C, and CD1E, while CD1D belongs to group 2. Different species have varying numbers of CD1 genes. For example, rodents only have group 2 CD1 genes, whereas humans and rabbits have five genes, encompassing both group 1 and group 2 CD1 types.
- The sequence identity between CD1 molecules and classical class I MHC molecules is relatively lower compared to the sequence identity among class I MHC molecules themselves. CD1D1, for instance, exhibits a deeper and more voluminous antigen-binding groove than class I MHC molecules.
- These unique structural features of CD1 molecules allow them to bind and present non-peptide antigens, such as glycolipids derived from bacterial pathogens. The interaction between CD1 molecules and δγ-TCR on T cells enables the recognition and immune response against these non-peptide antigens.
- In summary, non-peptide antigens derived from bacterial pathogens can be recognized by δγ-TCR on T cells. These antigens are presented by CD1 molecules, a family of nonclassical class I molecules. CD1 molecules have distinct structural characteristics and exhibit sequence variations compared to classical class I MHC molecules. Their ability to bind and present non-peptide antigens contributes to the immune response against infections caused by pathogens like Mycobacterium tuberculosis.
Clinical Significance of Antigen processing and presentation
- The process of antigen processing and presentation plays a crucial role in various clinical conditions, including autoimmune diseases. In some cases, antigen-presenting cells (APCs) can present self-antigens, leading to the initiation of an immune reaction against our own tissues. This immune dysregulation can result in autoimmune disorders such as Graves’ disease and rheumatoid arthritis.
- Graves’ disease, for instance, involves the presentation of self-antigens related to the thyroid gland. In this condition, the self-antigen Thyroid-stimulating hormone receptor (TSHR) is presented to T-cells by APCs. This interaction activates B-cells, leading to the production of autoantibodies against TSHRs present in the thyroid. As a consequence, the TSHRs become activated, causing hyperthyroidism and resulting in the enlargement of the thyroid gland, known as goiter.
- This autoimmune process in Graves’ disease highlights the clinical significance of antigen processing and presentation. It demonstrates how the incorrect presentation of self-antigens can trigger an immune response against our own tissues, leading to the development of autoimmune disorders. Similar mechanisms can occur in other autoimmune diseases, where self-antigens are presented to T-cells, resulting in the production of autoantibodies and subsequent tissue damage.
- Understanding the underlying mechanisms of antigen processing and presentation is crucial for unraveling the pathogenesis of autoimmune diseases. It can provide insights into the development of targeted therapies aimed at modulating the immune response and restoring immune tolerance to self-antigens. By regulating antigen presentation, it may be possible to prevent or attenuate the autoimmune response, providing potential therapeutic avenues for managing these complex disorders.
- In summary, the clinical significance of antigen processing and presentation lies in its association with autoimmune diseases. Dysregulated presentation of self-antigens can lead to the activation of the immune system against our own tissues, contributing to conditions such as Graves’ disease. Further research into the mechanisms involved in antigen processing and presentation holds promise for the development of novel treatments for autoimmune disorders.
MHC Class I Antigen-Processing Pathway
- In general, antigens presented by MHC class I and class II molecules originate from distinct cellular compartments.
- The antigen processing route for MHC class I antigens begins in the cytosol with the breakdown of an endogenous self-protein. After microbial infection, intracellular proteins produced from microbes enter the MHC class I processing pathway.
- Cross-presentation is a mechanism through which extracellular host or microbe proteins that have been absorbed into membrane-bound compartments via endocytosis or phagocytosis can enter the MHC class I processing pathway.
- Proteasomes breakdown misfolded or unnecessary proteins through proteolysis in the cytoplasm, thereby regulating the protein composition of the cell. These multicomponent proteases have a barrel shape and are composed of four rings containing seven subunits apiece.
- Proteasomes degrade proteins by a variety of methods. Misfolded proteins and faulty ribosomal products, which both fail to assume their native conformation states, may expose peptide sequences that are identified by proteasomes, resulting in their fast destruction.
- Alternatively, numerous proteins destined for fast proteasomal breakdown are conjugated to ubiquitin by enzymes that identify the phosphorylation of particular amino acids.
- Proteasomal breakdown of microbial antigens is essential for the presentation of microbial peptides by MHC class I proteins during microbial infection.
- Some proteasomal subunits are replaced by others that improve the formation of antigenic peptides, and other components are introduced to the ends of the barrel to alter the effectiveness and specificity of protein degradation in activated cells or after exposure to IFN-γ.
- It is unknown if pathogen-derived polypeptides and proteins are degraded selectively and targeted for presentation by MHC class I molecules during an infection.
- Bacterial proteins that enter the cytosol are rapidly degraded due to the presence of unique amino-terminal amino acids or internal amino-acid sequences that promote fast breakdown.
- In the majority of cases, pathogen-derived antigens are probably degraded nonselectively alongside indigenous proteins, and pathogen-derived peptides compete with more abundant endogenous peptides for a binding groove in MHC class I.
- Proteasomes create peptides between 9 and 12 amino acids in length due to the length of the proteasome channel. Transporter Associated with Antigen Processing binds peptides synthesised by proteasomes (TAP).
- This ATP-dependent, heterodimeric transporter efficiently transports peptides from the cytosol to the lumen of the endoplasmic reticulum.
- Peptides less than 6 amino acids or longer than 14 amino acids are poorly transported by TAP.
- TAP is the primary peptide transporter involved in the formation of peptide/MHC class I complexes; animals with genetic deletions of TAP have significantly reduced levels of surface MHC class I and significantly reduced numbers of CD8+ T lymphocytes.
- Rarely identified in people, TAP deficiency is associated with a substantial reduction in circulating CD8+ T lymphocytes and mild immunodeficiency.
- TAP1 and TAP2 molecules carry peptides from the cytosol to the endoplasmic reticulum lumen.
- The development of the peptide loading complex involves the association of newly synthesised MHC class I molecules with TAP in the endoplasmic reticulum and the recruitment of many additional endoplasmic reticulum resident proteins and chaperones (PLC).
- The PLC consists of the MHC class I/β2-microglobulin complexes coupled to tapasin, which serves as a molecular adapter for TAP, as well as calreticulin and thiol reductase ERp57.
- The primary function of β2-microglobulin and the PLC is to maintain the shape of the MHC class I peptide-binding groove that favours the binding of high-affinity peptides. Due to the fact that TAP delivers numerous peptides into the endoplasmic reticulum lumen that are too long to fit into the MHC class I peptide–binding groove, the endoplasmic reticulum resident proteases ERAP1 and ERAP2 trim peptides prior to their final incorporation into the MHC class I peptide–binding groove.
- If the affinity between peptide and MHC class I is high enough, the PLC releases the MHC class I/β2-microglobulin/peptide complex, allowing it to reach the cell surface via the Golgi complex. If the affinity between peptide and MHC class I is low, the MHC class I heavy chain undergoes re-glycosylation of an N-linked glycan, which redirects MHC class I molecules into the PLC for peptide exchange and blocks their release into the secretory pathway.
- Inflammation controls the antigen processing pathway of MHC class I. IFN-γ in particular is a cytokine with numerous effects on the MHC class I pathway.
- IFN-γ increases the transcription of numerous components of the MHC class I pathway, including MHC class I molecules, TAP, tapasin, and a number of proteasome components. Three proteasome subunits, LMP-2, LMP-7, and MECL, are specifically induced and replace three subunits of the core proteasome complex.
- IFN-γ generates other accessory proteins that affect proteasome efficiency and specificity, with PA28, a six-subunit activator that forms rings that can cap the ends of the proteasome, playing a key role.
- PA28 can enhance the presentation of MHC class I–restricted, virus-derived epitopes to CD8+ T lymphocytes.
- In the presence of an infection, the MHC class I antigen processing pathway is amplified, allowing for greater presentation of pathogen-derived peptides to CD8+ T cells.
Viral Intervention with the MHC Class I Antigen-Processing Pathway
- CD8+ T cells and the MHC class I antigen processing pathway are primarily responsible for viral infection defence. Fascinatingly, viral infections have gone to great lengths to subvert the MHC class I antigen-processing pathway, highlighting the significance of this antiviral defence mechanism.
- It was discovered early on that herpesvirus-infected cells downregulate MHC class I expression. Exploration of the mechanism underlying this discovery found numerous viral proteins that inhibit various MHC class I antigen processing pathway steps.
- Herpes simplex virus encodes ICP47, which disrupts human TAP by blocking the peptide transport channel from the cytosolic side.
- The human cytomegalovirus-encoded protein US6 hinders TAP transport by inhibiting the peptide transporter from the endoplasmic reticulum luminal side using a similar method but a completely different protein.
- Additionally, human cytomegalovirus employs a number of additional ways to prevent MHC class I molecules from reaching the cell surface. US3 binds MHC class I molecules in the endoplasmic reticulum, preventing their transport to the cell surface.
- Adenoviruses, which encode the type I membrane protein E3-19K, adopt a similar method. By expressing an endoplasmic reticulum retention motif on its cytoplasmic tail, this protein binds MHC class I molecules in the endoplasmic reticulum lumen and blocks their egress from the endoplasmic reticulum.
- The displacement of MHC class I molecules residing in the endoplasmic reticulum into the cytoplasm, where they are promptly ubiquitinated and destroyed by proteasomes, is an additional method for downregulating surface MHC class I retention. Two human cytomegalovirus-encoded proteins, US2 and US11, are responsible for mediating this process.
- Remarkably, transport of misfolded or otherwise dysfunctional proteins from the endoplasmic reticulum lumen to the cytosol via the Sec61 translocon is a normal mechanism.
- US2 and US11 appear to expedite this process for MHC class I molecules alone. The binding of US2 to MHC class I molecules has been studied by x-ray crystallography.
- Using a distinct mechanism, the Kaposi sarcoma herpesvirus also inhibits surface MHC class I expression. K3 and K5 expressed by the Kaposi sarcoma herpesvirus are ubiquitin ligases that specifically conjugate ubiquitin to the cytoplasmic tails of MHC class I and B7.2 co-stimulatory components.
- Surface MHC class I molecules are rapidly absorbed and destined for lysosomal destruction upon ubiquitination. Additionally, HIV has evolved methods to inhibit surface expression of MHC class I molecules.
- By connecting with the clathrin adaptor complex, the retrovirally generated Nef protein specifically downregulates the production of HLA-A and HLA-B molecules.
- Importantly, the downregulation of MHC class I renders afflicted cells vulnerable to NK cell-mediated lysis. On contact with MHC class I molecules, NK cells express receptors that suppress NK cell activation.
- To prevent NK cell–mediated lysis of virally infected cells, human CMV generates an MHC class I–like protein, UL18, which works as a decoy for the NK cell inhibitory receptor LIR-1, providing the viral pathogen with an additional layer of camouflage.
MHC Class I Cross-Priming
- The antigen processing route for MHC class I has two primary functions. First, it delivers antigens to CD8+ T lymphocytes that are not yet activated, proliferating, or differentiating in a manner that promotes their activation, proliferation, and differentiation.
- Second, it signals cellular infection to activated CD8+ T cells by presenting antigens. DCs mediate the first function largely, if not solely.
- Any infected cell that expresses MHC class I can execute the second function. Different antigen processing criteria apply in these two instances. As discussed in the preceding sections, the normal MHC class I antigen processing pathway applies to the second function.
- Pathogen-derived antigens are degraded and displayed on the cell surface in the presence of MHC class I molecules when cells are directly infected.
- The presentation of MHC class I antigens by DCs is more complicated and is not limited to indigenous cytosolic proteins.
- Due to the fact that CD8+ T-cells are rarely infected directly, the major route for CD8+ T-cell priming involves uptake of debris from infected cells by DCs and re-presentation of pathogen-derived peptides by an antigen-processing pathway involving endocytosis and TAP-mediated transport of antigen into the endoplasmic reticulum.
- The CD8+ fraction of DCs is especially efficient at taking up and delivering exogenous antigens to the MHC class I antigen processing pathway.
- Antigen-containing phagosomes in DCs fuse with endoplasmic reticulum membranes, which recruits retrotranslocation machinery that shuttles misfolded proteins or antigens from the phagosome lumen into the cytosol, where they are degraded by proteasomes and enter the conventional MHC class I processing pathway.
- This convoluted route for CD8+ T-cell priming guarantees that CD8+ T-cell priming happens in a regulated manner and is likely a critical barrier to prevent excessively powerful CD8+ T-cell responses to systemic viral infections.
MHC Class II Antigen-Processing Pathway
- The antigen processing pathway of MHC class II provides peptides to CD4+ T cells. Although there are similarities to the MHC class I antigen processing system, there are significant differences.
- Firstly, the majority of peptides presented by MHC class II molecules originate from extracellular proteins endocytosed by MHC class II– expressing cells.
- During transport to the cell surface, MHC class II molecules also display peptides from membrane or secretory proteins that have been degraded in endosomal compartments.
- Regarding antimicrobial responses, the MHC class II antigen-processing pathway has been associated mostly with the response to extracellular pathogens and vacuolar-dwelling pathogens, such as S. typhimurium and M. tuberculosis.
- Due to the fact that CD4+ T-cell responses are also necessary for the complete priming, activation, and differentiation of CD8+ T-cell responses, it is difficult to directly attribute immunological vulnerability to CD4+ T-cell deficit.
- The HIV epidemic has made the ramifications and complications of CD4+ T-cell depletion evident.
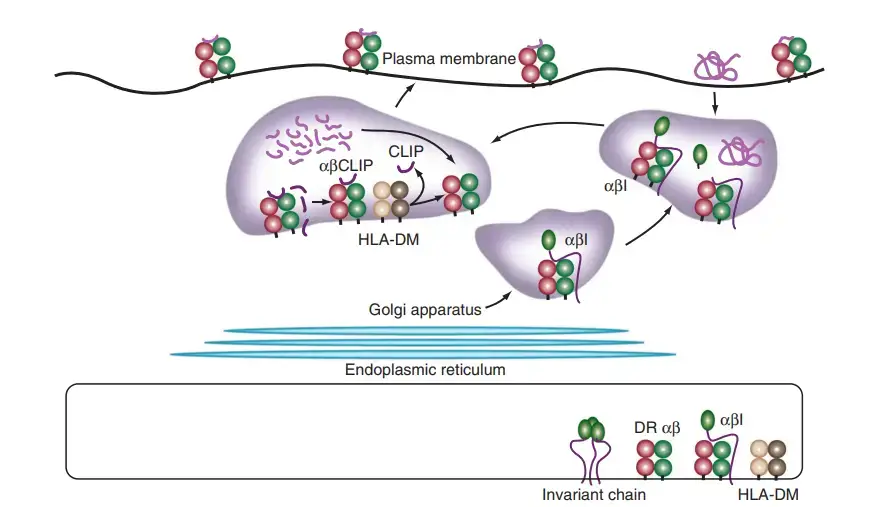
- The initial stage in the processing of MHC class II antigens is the translocation and assembly of MHC class II α- and β-chains in the endoplasmic reticulum, a mechanism regulated by a chaperone, the invariant chain.
- In the endoplasmic reticulum, MHC class II molecules do not bind antigenic peptides. The α- and β-chains fold by substituting the membrane-bound protein invariant chain for peptide.
- As the complex exits the endoplasmic reticulum, traverses the Golgi complex, and travels to the endosomal compartments, a fragment of the invariant chain fills the MHC class II groove.
- On acidification of the endosomal compartment, proteases such as cathepsin D and cathepsin B are activated and destroy all portions of the invariant chain excluding the section that is protected by the MHC class II groove.
- During the degradation process, MHC class II molecules might interact with endocytosed antigens in the acidified endosome compartment known as MIIC.
- The invariant chain segment is replaced by a proteolytically produced peptide in a complex, topologically demanding sequence of events.
- Similar to MHC class I molecules, MHC class II molecules are selective with regard to peptide binding; however, because the groove is open and can accommodate peptides in different registers, the range of peptides that are bound is expanded and the binding rigour is loosened.
- HLA-DM, an MHC-encoded, MHC-class II-like protein that dwells in MIICs, accelerates the binding mechanism of MHC class II peptides.
- Recent crystallographic investigations demonstrate that HLA-DM stabilises empty MHC class II proteins in a conformation that favours the insertion of a high-affinity peptide. HLA-DM catalyses the extraction of the invariant chain peptide fragment from the MHC class II molecule.
- In addition to proteases, GILT (gamma interferon–inducible lysosomal thioreductase) is involved in the denaturation of certain antigens prior to their breakdown and presentation by MHC class II molecules.
- GILT has also been linked to the activation of the primary secreted virulence factor of the bacterial pathogen L. monocytogenes, listeriolysin-O, offering an exceptional example of microbial exploitation of antigen-processing pathways.
- Upon peptide attachment in the MIIC, MHC class II molecules travel to the cell surface, where CD4+ T lymphocytes can detect the MHC class II/peptide complex. MHC class II molecules undergo re-internalization, and it is possible that these complexes recycle to endosomal compartments and acquire new peptides before returning to the cell surface.
- Uncertainty surrounds the contribution of this pathway to the MHC class II antigen processing pathway during immunological responses to infection.
What is CD1?
- The CD1 family contains antigen-presenting molecules that are structurally similar to MHC class I molecules and are associated with β2-microglobulin.
- Human chromosome 1’s CD1 locus has five different genes that code for the proteins CD1a through CD1e.
- Consistent with their structural resemblance to MHC class I, CD1 molecules are often strongly expressed on antigen-presenting cells, present antigens for TCR recognition, and interact with T lymphocytes. CD1 molecules differ significantly from MHC molecules in the class of displayed antigens.
- In contrast to the peptide antigens presented by the MHC, CD1 proteins present lipid and glycolipid antigens to T cells, a discovery that dramatically increased the number of antigens recognised by T lymphocytes.
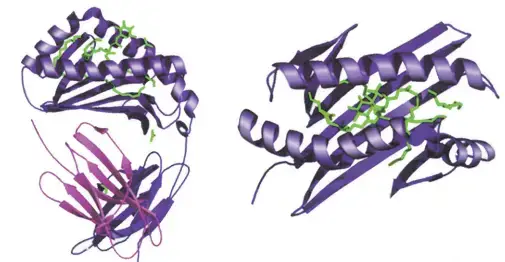
- The illustration depicts the structure of the human CD1b molecule displaying ganglioside GM2.
- This section discusses the antigens given by CD1, the lymphocytes that identify these antigens, and the significance of this antigen-presenting system in the defence of the host against specific infectious illnesses.
CD1 Protein Structure
- CD1 proteins are transmembrane proteins with a brief intracellular domain. The extracellular component of CD1 consists of three antigen-binding domains, while particular patterns in the intracellular region regulate CD1 trafficking to intracellular compartments (see later discussion).
- The antigen-binding groove formed by the extracellular domains of CD1 molecules is structurally similar to the peptide-binding groove of the MHC.
- Consistent with CD1’s function of lipid presentation, the antigen-binding groove has several hydrophobic pockets that can accept the aliphatic chains of lipid antigens.
- The three-dimensional structure of mouse CD1266 and human CD1b265 reveals a complex network of hydrophobic channels capable of accommodating various lipids with differing aliphatic chain lengths.
- In contrast to the five CD1 isoforms found in humans, mice lack CD1a, CD1b, and CD1c and possess two copies of the CD1d gene.
- This significant difference between mouse and human CD1 hampers the experimental analysis of CD1 function since genetically modified mice cannot be used to examine the function of CD1a, CD1b, and CD1c.
Antigens Presented by CD1
- T lymphocytes are presented with lipid and glycolipid antigens from various bacteria and fungi by CD1. CD1-presented antigens include diacylglycerols from S. pneumoniae, a fungal glycosphingolipid (i.e., asperamide B), and a marine sponge-derived synthetic glycolipid, -galactosyl ceramide.
- These last three chemicals are delivered to NKT cells in a CD1-restricted way, and -galactosyl ceramide has been utilised extensively to explore the effect of CD1d-mediated NKT-cell activation on the host’s defence against infections and malignancies.
- CD1 is essential for delivering mycobacterial lipids to T lymphocytes, particularly glycosylated and free mycolic acids and lipoarabinomannan, two key lipid and glycolipid components of M. tuberculosis cell envelope.
- Further investigations of the structure of CD1-presented lipids indicated that T-cell recognition of CD1b-presented glycolipids was extraordinarily sensitive to the fine structure of the carbohydrate head group, but rather insensitive to structural changes in the lipid tail.
- Together, these observations and the subsequent characterization of CD1c-presented mycobacterial isoprenoid glycolipids have led to a model of CD1 lipid antigen presentation in which the hydrophilic head group is exposed and available for TCR interactions while the hydrophobic lipid tails of the antigen are bound within the hydrophobic pockets of the CD1 protein.
Cell Biology of CD1 Antigen Processing and Loading
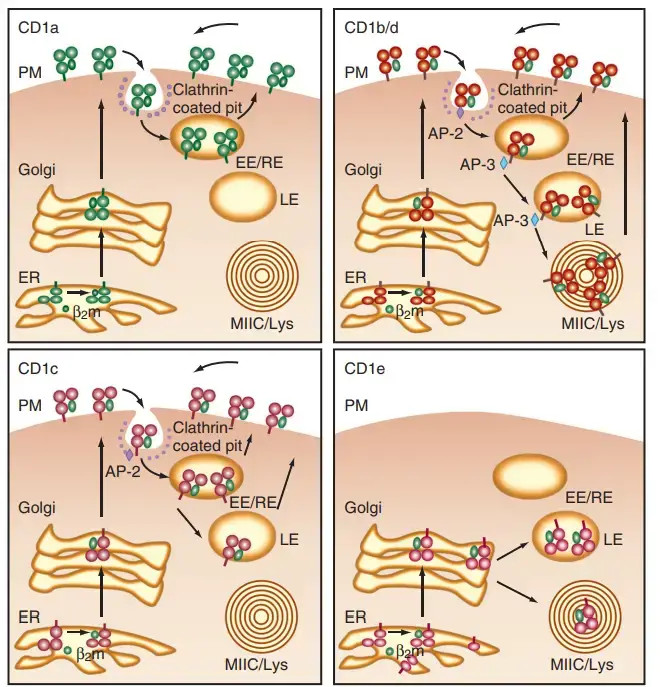
- CD1 isoforms are abundant in diverse intracellular compartments, indicating that each isoform of CD1 has evolved to detect microbial antigens that are present in unique regions of the endosomal lysosomal network.
- The figure provides a summary of CD1 trafficking pathways. Endocytosis results in the internalisation of all CD1 isoforms at the cell surface. C D1a is primarily found in early endosomes, CD1c in late endosomes, and CD1b/d in both late endosomes and lysosomes.
- Specific amino acid residues in the short intracellular tails of CD1 isoforms bind to cytosolic adaptor molecules that mediate organelle trafficking, thereby targeting these isoforms to their respective compartments.
- Although the specific physiological ramifications of this trafficking pattern for the immune response are still being explored, each CD1 isoform may survey a unique intracellular compartment for unique lipid structures from unique pathogens.
What is antigen processing and presentation?
Antigen processing and presentation is a crucial immune process in which cells capture, process, and display antigens to activate T-cells, leading to an immune response.
What are the main cells involved in antigen processing and presentation?
Antigen-presenting cells (APCs) such as dendritic cells, macrophages, and B cells are primarily responsible for antigen processing and presentation.
How are antigens processed within cells?
Antigens can be processed within cells through two main pathways: the cytosolic pathway (for endogenous antigens) and the endocytic pathway (for exogenous antigens).
What are MHC molecules and their role in antigen presentation?
Major Histocompatibility Complex (MHC) molecules are cell surface proteins that bind and display antigens to T-cells. MHC class I presents endogenous antigens, while MHC class II presents exogenous antigens.
How do MHC class I molecules present antigens?
MHC class I molecules present peptides derived from endogenous antigens that are processed within the cytoplasm of the cell. These peptides are then displayed on the cell surface to activate CD8+ cytotoxic T-cells.
How do MHC class II molecules present antigens?
MHC class II molecules present peptides derived from exogenous antigens that are internalized through phagocytosis or endocytosis. These peptides are processed within endocytic compartments and displayed on the cell surface to activate CD4+ helper T-cells.
What is the role of T-cell receptors (TCRs) in antigen recognition?
T-cell receptors (TCRs) are proteins expressed on the surface of T-cells that recognize and bind to specific antigens displayed by MHC molecules. TCRs play a crucial role in initiating the immune response.
Can non-peptide antigens be presented by MHC molecules?
Yes, non-peptide antigens, such as glycolipids, can be recognized and presented by nonclassical MHC molecules called CD1 proteins.
How does antigen processing and presentation contribute to autoimmune diseases?
In autoimmune diseases, the presentation of self-antigens can lead to the activation of T-cells against our own tissues, resulting in autoimmune reactions and tissue damage.
What is the importance of studying antigen processing and presentation?
Studying antigen processing and presentation helps us understand the mechanisms behind immune responses, including the development of protective immunity and autoimmune disorders. It also provides insights for designing vaccines and developing immunotherapies for various diseases.
References
- Kindt, T., Goldsby, R., Osborne, B., Kuby, J. and Kuby, J. (2007). Kuby immunology. New York: W.H. Freeman.
- Natarajan K, Li H, Mariuzza RA, Margulies DH. MHC class I molecules, structure and function. Rev Immunogenet. 1999;1(1):32-46. PMID: 11256571.
- Li XC, Raghavan M. Structure and function of major histocompatibility complex class I antigens. Curr Opin Organ Transplant. 2010 Aug;15(4):499-504. doi: 10.1097/MOT.0b013e32833bfb33. PMID: 20613521; PMCID: PMC3711407.
- Natarajan, K & Li, Hongmin & Mariuzza, RA & Margulies, David. (1999). MHC class I molecules, structure and function. Reviews in immunogenetics. 1. 32-46.
- Wieczorek, M., Abualrous, E. T., Sticht, J., Álvaro-Benito, M., Stolzenberg, S., Noé, F., & Freund, C. (2017). Major Histocompatibility Complex (MHC) Class I and MHC Class II Proteins: Conformational Plasticity in Antigen Presentation. Frontiers in Immunology, 8. doi:10.3389/fimmu.2017.00292
- Janeway CA Jr, Travers P, Walport M, et al. Immunobiology: The Immune System in Health and Disease. 5th edition. New York: Garland Science; 2001. The major histocompatibility complex and its functions. Available from: https://www.ncbi.nlm.nih.gov/books/NBK27156/
- Hohl, T. M. (2015). Cell-Mediated Defense against Infection. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases, 50–69.e6. doi:10.1016/b978-1-4557-4801-3.00006-0
- Mak, T. W., & Saunders, M. E. (2006). MHC: The Major Histocompatibility Complex. The Immune Response, 247–277. doi:10.1016/b978-012088451-3.50012-0
- The Major Histocompatibility Complex. (2014). Primer to the Immune Response, 143–159. doi:10.1016/b978-0-12-385245-8.00006-6
- Rammensee, H.G. (1993). Structure and Function of MHC Class I Molecules. In: Eibl, M.M., Huber, C., Peter, H.H., Wahn, U. (eds) Symposium in Immunology I and II. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-78087-5_9
Потрясающий обзор) Очень мне помог при разборе этой темы, спасибо 🙂 Иммунология сложная, но жутко интересная ^,^
Большое спасибо