Measurement of Bacterial Growth
- Bacterial Growth is a biological process that involves increasing cell number, cell mass, and cell activity.
- We can thus measure the bacterial growth through measuring cell activity, cell mass and counting cell numbers.
- Two methods can be used to count cells: directly by microscopy, using an electronic particle counter or indirectly using a colony counting.
- You can measure cell mass directly by using weighing, measuring cell nitrogen or indirectly by turbidity.
- You can measure cell activity indirectly by relating the level of biochemical activity with the size of the population.
Why Should We Measure Bacterial Growth?
The following are reasons we measure bacterial growth:
- To count the number of cells within a media.
- To measure cell mass.
- To measure cell activity.
Methods used for Measurement of Bacterial Growth
There are many methods available to measure bacterial growth. Each one is discussed below.
Cell Number count
The cell number counting is done by two following methods;
- Direct Method
- Indirect Method
A. Direct Method
The following methods are used for direct count of bacterial cells;
- Direct Microscopic Count/Counting chamber
- Electronic Enumeration of Cell Numbers
a. Direct Microscopic Count/Counting chamber
- Direct counts in a counting chamber, such as the Petroff-Hausser chamber, are the best way to determine microbial populations.
- Counting chambers are made up of specially designed slides, coverslips, and a space between them that creates a chamber with known depth.
- This slide is precisely ruled into squares of 1/400 mm2 area. A glass cover slip rests 1/50mm above the slide so that the volume is 1/20,000mm3 (1/20.000.000cm3).
- A suspension of unstained bacteria can also be counted inside the chamber using a phase contrast microscope.
- You can calculate the number of microorganisms present in a sample by taking the volume of the chamber and the dilutions of the sample prior to counting.
- An example: Five bacteria are found in every square ruled. This is 5 X 20,000,000 or 108 bacteria per milliliter.
Advantages of Direct Microscopic Count
- This method is simple, cheap, and quick.
- It also provides information on the size and morphology microorganisms.
- The Petroff-Hausser counting chamber makes it easy to count Bacteria accurately and quickly.
- It is possible to quickly and easily perform direct microscopic measurements using very little equipment.
- If they are properly diluted, very dense suspensions can still be counted.
Disadvantages of Direct Microscopic Count
- It is impossible to count the number of bacteria in a suspension, such as at the beginning or end of a growth curve.
b. Electronic Enumeration of Cell Numbers
- Electronic counters, such as the Coulter counter, can also be used to count microorganisms.
- This method involves placing the bacterial suspension in an electronic particle counter. The bacteria are then passed through a small orifice measuring 10-30 um in diameter.
- This orifice connects two counter compartments that contain an electrically conductive solution.
- Each bacterium that passes through the orifice experiences a temporary increase in the electrical resistance.
- This creates an electric signal that is counted automatically.
Advantages of Electronic Enumeration of Cell Numbers
- This is a fast method.
- It is easy to use
Disadvantages of Electronic Enumeration of Cell Numbers
- This requires advanced electronic equipment.
- Clogged orifices are more common.
- It is impossible to tell if the cells being counted have survived.
B. Indirect Method
The Indirect Method of bacterial cell count is done by this following method;
a. The Plate-Count Method
- This allows you to determine the number of cells which will multiply under certain conditions.
- In a Petri dish, a measured amount of the bacterial suspension will be added.
- The agar medium, which is kept at 45°C, is then added to the mixture. Finally, the plate is rotated to mix the two.
- The medium will solidify and the organisms will be trapped in the gel.
- Each organism reproduces itself, growing until there is a visible colony.
- A colony count on the plate shows the viable microbial population.
- The original sample is often diluted to reduce the number of colonies that form on the plate. This usually results in a range of 30-30%. This range allows for accurate counting and minimizes the chance of interference with the growth of other organisms.
- Colonies can be counted by lighting them from below (dark field illumination). This makes them easily visible and often a magnifying lens is used. There are many electronic methods that can be used to count colonies.
- The plate-count method is routinely used with satisfying results to estimate bacterial populations in milk, food, and other materials.
Procedure of Plate-Count Method
- Culture of bacteria, or any other sample containing strains of bacteria in suspension
- 1 ml was transferred to 99 mg dilution empty; 1 ml was transferred to 2d99 ml dilution blank: 1 ml was transferred to 3d99 ml delution blank.
- Inoculum is added to each plate by alternate addition. Each plate should contain 15-20 ml of agar media. To ensure that the inoculum is evenly distributed throughout the medium, rotate each plate gently.
- Place plates inverted in an incubator for at least 24 hours.
- The plate contains between 30 and 300 colonies. After that, the number of cells is counted.
- Calculation of Count: Number of colonies counted on plate X dilution of sample = number of bacteria per ml.
Advantages of Plate-Count Method
- It is simple to use and can be used to measure populations of any size.
- Because it can count very few organisms, it has the advantage that it is sensitive. If a specimen has less than one bacterium per liter, then one colony should form upon plating 1 ml.
- Can differentiate between dead and alive cells. Colonies cannot be formed by dead cells.
Disadvantages of Plate-Count Method
- The plate-count method has one limitation. Only bacteria that can grow in the medium and conditions of incubation used will be counted. This is an important point to remember if you are trying to count a mixture of bacteria.
- One limitation is that not all viable organisms that are capable of growing in the provided culture conditions will necessarily produce one colony. One colony can be formed from one cell if the bacterial suspension contains no aggregates. However, this is possible if there are cells that have a tendency for accumulation. The resulting counts, such as cocci in clusters or chains (staphylococci), or pairs (diplococci), will be lower than the individual cells. This is why “counts” are sometimes reported as colonies per milliliter, rather than the number of bacteria per liter.
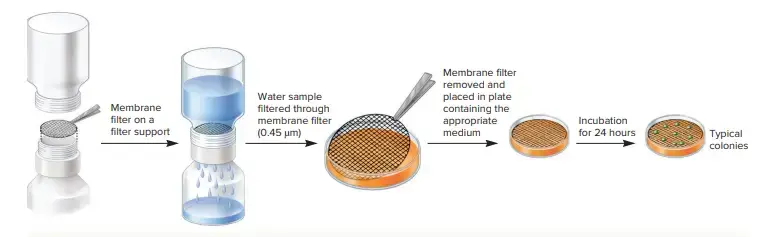
b. Membrane-Filter Count
- Direct counts of bacteria found in aquatic samples are often determined after they have been captured on membrane filters.
- The membrane filter technique involves filtering the sample first through a black membrane filter made of polycarbonate.
- Next, the bacteria is stained with fluorescent nucleic acids such as DAPI or acridine orange and microscopically observed.
- You can also use fluorescently-labeled dyes, which are specific to members of a taxon.
- The membrane filter’s black background makes it easy to see the stained cells and you can count them with an epifluorescence microscope.
- Direct cell count of environmental samples almost always results in higher cell densities that methods that rely upon culturing. Because only a small fraction (about 1%) of cells that grow in nature can be grown in the laboratory, this is why it is so important.
Advantages of Membrane-Filter Count
- It is easy to use.
- Not expensive.
Disadvantages of Membrane-Filter Count
- It can not distinguish between live and dead cells.
Cell Mass Measurement
The measurement of cell mass can be done by two methods such as;
- Direct Methods
- Indirect Methods
Direct Methods of Cell Mass Measurement
It is accomplished by this following methods;
a. Determination of Nitrogen Content
- Protein is the major component of cell material. Because nitrogen is a part of protein, it is possible to measure a bacterial population (or cell crop) in terms of bacterial Nitrogen.
- Bacteria have an average of 14 percent nitrogen per dry weight, but this number can vary due to cultural differences or differences among species.
- This technique measures growth. First, harvest the cells and rinse them with water. Then you can do a quantitative chemical analysis to determine the nitrogen content.
Disadvantages of Determination of Nitrogen Content Method
- The process of determining bacterial nitrogen is laborious. It can only be done on samples that are free from all other sources.
- The method can only be used for highly concentrated populations. This and other reasons make this method a preferred choice for research.
b. Determination of the Dry Weight of Cells
- To determine the population size, there are also methods that can measure changes in cell mass. The determination of microbial dry mass is one approach. This method is the best for measuring the mass of cells.
- This method involves the collection of cells in liquid medium. They are then dried in an oven and weighed.
Advantages
- It’s a very simple process.
- This technique is especially useful for measuring the growth rate of filamentous mushrooms.
- However, dry weight measurement is a reliable and accurate way to measure growth in many organisms. It is often used in research.
Disadvantages
- It is not recommended to use it with dense suspensions. Cells must be free from any extraneous matter.
- It can be time-consuming and sensitive. It is possible to centrifuge hundreds of milliliters to obtain sufficient bacteria culture because the bacteria is so small.
- The amount of living material within cells may not always be indicated by dry weight. For example, the intracellular reserve material poly-s-hydroxybutyrate can accumulate in Azotobacter beijerinckii at the end of the log phase of growth and during the stationary phase and finally can comprise up to 74 percent of the dry weight of the cells; thus, the dry weight may continue to increase without corresponding cell growth.
Indirect Methods of Cell Mass Measurement
The Indirect Method of Cell Mass Measurement is accomplished by measuring the turbidity of a cell.
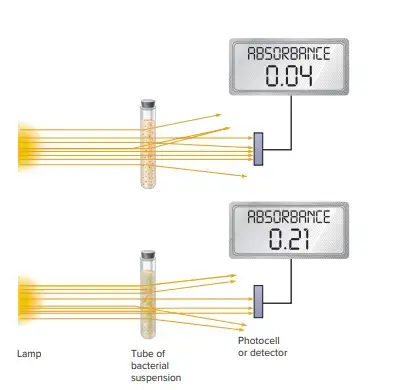
a. Turbidimetrie Methods
- Spectrophotometry is a more sensitive and rapid method to measure cell mass.
- For turbidimetric measurements on cell mass, a spectrophotometer (or colorimeter) can be used.
- The fact that microbial cells scatter light is the basis of spectropotometry. The number of microbial cells within a population is roughly constant. Therefore, scattering light is directly proportional and indirectly related to cell numbers.
- The medium becomes slightly cloudy or turbid when the bacteria concentration reaches approximately a million (106) cell/milliliter.
- Increases in concentration lead to greater turbidity and less light being transmitted through the medium.
- A spectrophotometer can measure the extent of light scattering, i.e. the decrease in transmitted sunlight. This is known as the absorbance (optical density) of the medium.
- Absorbance is linearly related with cell concentration at absorbance levels below about 0.5. This value must be exceeded by the sample. If it exceeds that, it should first be diluted and then absorbance measured.
- As long as there is enough turbidity to be able to measure the population, it can easily be measured.
Advantages of Turbidimetrie Methods
- Turbidimetry can be used to quickly and easily track growth.
Disadvantages of Turbidimetrie Methods
- The culture must be dense enough that it registers some turbidity.
- It is possible that it may not be possible for cultures to be measured in cultures that have been grown in darkened media or contain suspended material.
- Turbidity can also be caused by dead cells as well as living ones.
b. Measurement of a Specific Chemical Change Produced on a Constituent of the Medium
- This method can be used to estimate cell mass by taking a species that makes an organic acid from glucose fermentation.
- It is assumed that acid production under certain conditions and for a set time period is proportional to the size of the bacterial population.
- The measurement of acid or other end products is an indirect method of measuring growth. It is only applicable in very special cases.
Cell Activity Measurement
a. The Membrane Filtration Procedure
- Another common plating method traps bacteria in water samples using a membrane filter.
- After the filter has been placed on an agar media or a pad soaked in liquid media, it is incubated until each colony forms.
- The colony count is a measure of the number and type of microorganisms present in the sample. Selective media can also be used to identify specific microorganisms. This method is particularly useful for analyzing water purity.
- These filters are known to have a uniform porosity that is small enough to trap microorganisms.
- This method is especially useful in determining the amount of bacteria present in large samples that have a small number of viable cells.
- The membrane with trapped bacteria is placed on a special plate that has a pad containing the medium.
- To make it easier to identify certain kinds of organisms, special media and dyes are available. The organisms form colonies during incubation and appear on the membrane’s surface.
b. MPN (Most probable number) test
- This method involves making many copies of several dilutions in a culture and adding them to tubes that contain a suitable liquid growth medium.
- Each tube is checked after incubation to see if there has been any growth.
- The assumption is that the last tube of the series that shows growth was inoculated using between 1-10 cells and the next tube with between 11-100 cells.
- If there is no growth, the tube is presumed to not have received any cells.
- This allows you to estimate the number of cells present in your original sample.
- When a select medium is available that supports the growth of a particular type of microbe, MPN values are used most often.
- It is used to assess microbial densities in water samples.
| Method | Some Applications | Manner in which Growth Is Expressed |
| Microscopic count | Enumeration of bacteria in vaccines and cultures | Number of cells per ml |
| Electronic enumeration | Same as for microscopic count | Same as for microscopic count |
| Plate count | Enumeration of bacteria in milk, water, foods, soil, cultures, etc. | Colony-forming units per ml |
| Membrane filter | Same as plate count | Same as plate count |
| Turbidimetric measurement | Microbiological assay, estimation of cell crop in broth, cultures, or aqueous suspensions | Optical density (absorbance) |
| Nitrogen determination | Measurement of cell crop from heavy culture suspensions to be used for research in metabolism | Mg nitrogen per ml |
| Dry weight determination | Same as for nitrogen determination | Mg dry weight of cells per ml |
| Measurements of biochemical activity e.., acid production by cultures | Microbiological assays | Milliequivalents of acid per ml or per culture |