What is Mass Spectrometry (MS)?
- Mass Spectrometry (MS) refer as a analytical technique which used for measuring the mass-to-charge (m/z) ratio of ions in a sample.
- It used for determining the molecular weight and structural information of compounds (organic, inorganic etc.).
- In this method, the sample is ionized by several ways (like electron impact, electrospray, MALDI etc.), producing charged particles/ions.
- Those ions are then separated according to their m/z values by an analyzer system.
- A detector used for recording the intensity of each ion, producing a mass spectrum (graph of intensity vs. m/z).
- The resulting mass spectrum provide information about the composition and structure of the molecule.
- The process of ionization, separation and detection happen in high vacuum chamber so contamination can prevail.
- Common parts of a MS instrument are: ion source, mass analyzer, and detector.
- Identification of unknown compounds is done by comparing their spectra with standard/reference spectra.
- It’s widely used by research fields like proteomics, pharmacology, environmental chemistry, etc.
- Sometimes fragmentation patterns are used for structural elucidation which is very helpful in complex organic molecules.
- In general, MS considered as a sensitive, precise, and fast technique but it require skilled handling and calibration.
- The mass spectrometer itself they are quite delicate and must be operated under controlled condition (temperature, pressure).
- Data interpretation sometimes become difficult when overlapping peaks or adduct formation occurs.
- Overall, Mass Spectrometry is viewed as one of the most dependable tools for molecular identification / quantification in modern analytical science.
Definition of Mass Spectrometry (MS)
Mass Spectrometry (MS) is an analytical technique used to identify the chemical composition of a sample by measuring the mass-to-charge ratio of its ions, resulting in a mass spectrum that reveals the molecular and structural information of the sample.
Principle of Mass Spectrometry (MS)
- Mass Spectrometry (MS) refer as technique for measurement of mass-to-charge (m/z) ratio of ions.
- Molecules are ionized (made charged) in ion source like EI (electron impact), ESI (electrospray ionization) , or MALDI, and then gas-phase ions are produced.
- The produced ions are accelerated by an electric field, so kinetic energy is imparted to ions, and they travel into analyzer region.
- Separation of ions is performed according to their m/z, by analyzers (like quadrupole, TOF, magnetic sector), which cause different trajectories / flight-times.
- The detector then measure intensity of arriving ions, a mass spectrum is produced (intensity vs m/z), and peaks are recorded.
- The relation KE = (1/2)mv² = zV is used, where m mass, v velocity, z charge, V potential; when V constant, lower m/z travel faster, and arrive earlier.
- Fragmentation of molecular ions is often induced, fragment patterns are interpreted for structural information, they are compared with libraries or predicted patterns.
- Ionization efficiency and fragmentation tendency are influenced by chemistry of analyte, solvent, matrix (MALDI matrix), and source conditions, so calibration and tuning are required.
- Accurate mass measurement is obtained by high-resolution analyzers, exact mass is used to infer molecular formula, isotopic patterns help confirm composition.
- Quantification is achieved by comparing signal intensity to standards or internal standards, but matrix effects sometimes cause signal suppression, and careful prep is needed.
- Data analysis/software are used for peak picking, deconvolution, and identification, and spectra are catalogued in libraries; the operator often will check peaks manually, for sure.
- The overall principle thus rely on conversion of neutral molecules to ions, separation by m/z under electric/magnetic influence, and sensitive detection of those ions.
- The instrument they are operated under high vacuum (to reduce collisions), maintenance and calibration are required frequently, it is delicate but powerful.
- In short, MS allow accurate molecular weight determination, structural clues from fragments, and quantitative data, and it’s widely used in proteomics, metabolomics, pharmaceutics etc., prevails in many labs.
Working of Mass Spectrometry (MS)
- Sample Introduction – The sample (solid, liquid or gaseous form) is first introduced into the ion source of the mass spectrometer, usually under vacuum condition so that contamination is avoided but sometimes it prevail due to moisture.
- Ionization – Molecules of the sample are converted into charged ions by several ionization methods like Electron Ionization (EI), Electrospray Ionization (ESI), Matrix-Assisted Laser Desorption/Ionization (MALDI) etc. The process of ionization depend by type of sample, for example volatile sample use EI but large biomolecules prefer MALDI or ESI.
- Acceleration – The ions produced are then accelerated by an electric field toward the mass analyzer, this acceleration gives them kinetic energy which depend on their charge. In this step, all ions are given same kinetic energy but because they have different mass/charge values, their velocities become different.
- Deflection / Separation – In analyzer section, the accelerated ions are separated according to their mass-to-charge (m/z) ratio using magnetic or electric field, lighter ions bend more strongly than heavy ions. The analyzer type used may be Time of Flight (TOF), Quadrupole, Ion trap, or Magnetic sector, each one having their own accuracy & resolution.
- Detection – After separation, ions are detected by a detector (like electron multiplier or photomultiplier tube) which measures the number of ions hitting it, producing signals proportional to their abundance.These signals are converted to digital data by computer, generating a mass spectrum, which is a plot of ion intensity vs. m/z ratio.
- Data Analysis – From the spectrum, peaks are identified that correspond to molecular ion and fragment ions, the molecular weight and structure of compound are determined. Sometimes isotopic peaks are also seen which help in confirming elemental composition of molecule, so they are used in structural elucidation.
The whole procedure take place under high vacuum (10⁻⁶–10⁻⁸ torr) for prevent collision between ions and air molecules, otherwise accuracy decrease.
In many instruments calibration is required before each run to maintain precision of m/z scale.
The overall working thus involves steps: Ionization → Acceleration → Separation → Detection → Data interpretation, all under vacuum environment.
Hence, by this sequence of operations, Mass Spectrometry (MS) provide both qualitative and quantitative information about analyte compounds in mixture.
Instrumentation of Mass Spectrometry (MS)
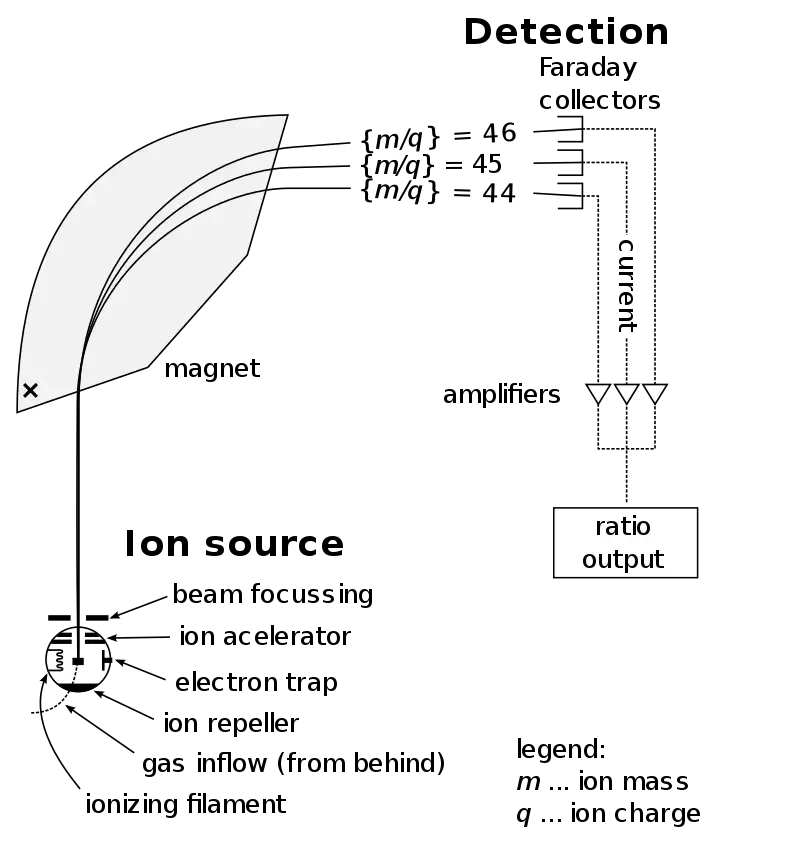
1. Sample Inlet – The sample is first introduced into the instrument by a device called Molecular leak that allows the molecules to pass slowly from a large reservoir into the ionization chamber at very low pressure. Usually a steady stream of gas/vapor is maintained so that the amount of sample entering remains constant for analysis. Sometimes for non-volatile samples, direct insertion probe or GC/LC interfaces are used too.
2. Ionization – In this region, atoms or molecules are ionized mostly by knocking out electrons so that they become positive ions (mainly +1 charge). Ionization is done by different methods like Electron Ionization (EI-MS), Chemical Ionization (CI-MS) and Desorption Techniques (FAB) etc. The selection of ionization method depend on sample type and thermal stability. Often a beam of electrons is used to bombard the molecules, causing fragmentation which help in structure determination later.
3. Acceleration – After ionization, the produced ions are accelerated by passing through a series of three slits which carry different voltages (decreasing order). Middle slit have intermediate voltage while the final one is at zero volt potential. All ions are thus given same kinetic energy before entering the analyzer region. It ensures that separation later depend only on mass-to-charge (m/z) ratio and not energy difference.
4. Deflection – The accelerated ions then enter a magnetic field where they are deflected according to their mass and charge. The lighter ions are deflected more than heavier ones. Also ions with higher positive charge (+2, +3) deflect more strongly than single charged ions. The path curvature depends on magnetic strength and the velocity of ions. By adjusting the magnetic field strength, different masses can be focused one by one onto the detector.
5. Detection – When ions reach the detector, their impact is measured based on the m/e (mass/charge) ratio. Upon hitting the metal surface of the detector, each ion is neutralized as an electron jumps from the metal onto the ion. This creates a small current pulse which is amplified and recorded electronically. The intensity of these pulses represents ion abundance and forms the basis of mass spectrum.
Types of Mass Analyzers / Detectors –
(a) Magnetic sector mass analyzer – uses magnetic deflection to separate ions based on momentum.
(b) Double focusing analyzers – combine electric and magnetic fields for higher resolution.
(c) Quadrupole analyzer – uses oscillating electric fields to filter ions of particular m/z.
(d) Time of Flight (TOF) – ions with different masses reach detector at different times.
(e) Ion Trap analyzer – ions are trapped and released sequentially for measurement.
(f) Ion Cyclotron analyzer – measure ion motion frequency in magnetic field for very high accuracy.
6. Vacuum System – All these components operate under a high vacuum (10⁻⁶ to 10⁻⁸ torr) to avoid collision of ions with air molecules. Vacuum is maintained by rotary, diffusion, and turbo molecular pumps. Without proper vacuum, ion loss and signal noise increase drastically.
7. Data Handling / Recording System – The signals from detector are converted to digital form and displayed as mass spectrum (intensity vs m/z graph). Modern MS is computer controlled and software is used for data storage, mass calibration, and interpretation. Peak intensities show relative abundance and help identify the compound.
Mass Spectrometry Diagram
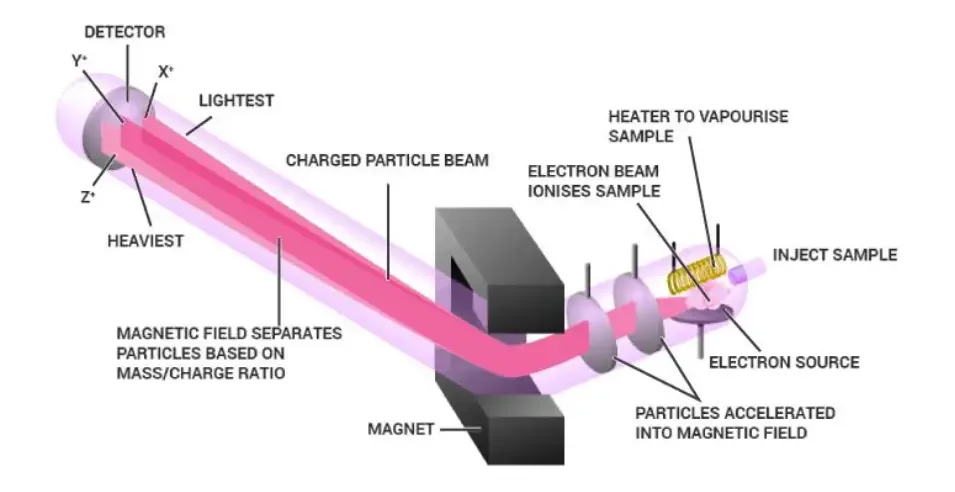
How Does Mass Spectrometry Work?
- In Mass Spectrometry (MS), a sample is first ionized so that charged particles are formed, which can then be analyzed.
- The ionization can done by several methods – like Electron Ionization (EI), Electrospray Ionization (ESI), or Matrix-Assisted Laser Desorption/Ionization (MALDI) etc.
- During ionization, molecules are converted into ions, sometimes they also broken into fragments, producing pattern that’s characteristic for each compound.
- The ions are then accelerated by electric field, their speed depend on the mass/charge (m/z) ratio.
- Lighter ions moves faster, while heavier ones move slower, this difference used for separation.
- Separation happen by analyzer part of MS, which can be Quadrupole, Time-of-Flight (TOF), Ion trap, Orbitrap, or Magnetic sector analyzer.
- Inside analyzer, ions are separated according to their m/z value, producing unique spectrum for each compound.
- The detector (like Electron Multiplier) measure intensity of each ion, converting it into electrical signal.
- These signals are recorded and shown as a Mass Spectrum, where x-axis show m/z ratio and y-axis show intensity.
- From the pattern of peaks, molecular weight and structure of unknown compound can be determined.
- Calibration of instrument is important, otherwise small drift occur by temperature or magnetic field change.
- Mostly, the whole process occur under vacuum conditions to prevail interference of air molecules.
- In many systems, computer software used to interpret data automatically, but still expert analysis required for complex spectra.
- The results from MS can combined with other methods (like GC-MS or LC-MS) for better identification and quantification.
- Finally, Mass Spectrometry works on a simple idea – ions are made, they are separated, then detected and the data interpreted to know what’s inside a sample.
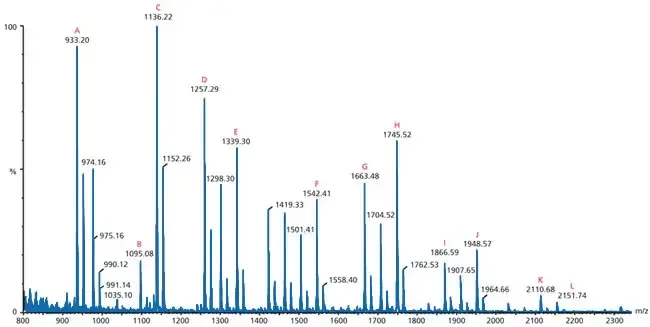
How To Read A Mass Spectrometry Spectrum
- In Mass Spectrum, the x–axis show the mass-to-charge ratio (m/z), while y–axis indicate relative abundance or intensity.
- The spectrum consist of many peaks, each peak represent ions that were detected with specific m/z value.
- The base peak is the tallest one, and it’s taken as 100% intensity, other peaks are compared with it in relative %.
- Usually, the molecular ion peak (M⁺) represent the intact molecule without fragmentation, showing molecular weight of compound.
- Sometimes M⁺ peak is small or missing when molecule fragment easily, which make interpretation little harder.
- Peaks with smaller m/z correspond to fragment ions, produced when molecule break into smaller parts during ionization.
- Pattern of fragmentation give important clues about structure, position of functional group, and type of bonds.
- On left side of spectrum smaller ions are found, while on right side heavier ions occur, that’s normal in most cases.
- The isotope peaks (like ¹³C, ²H, ³⁷Cl) appear very near to M⁺ peak, showing natural isotopic abundance of elements.
- Difference between isotope peaks can used to identify number of certain atoms (like one Cl show M+2 peak).
- For organic compound, fragments like CH₃⁺ (m/z 15), C₂H₅⁺ (m/z 29), C₃H₇⁺ (m/z 43) are often observed etc.
- The cluster of peaks sometimes due to adduct ions (Na⁺, K⁺, NH₄⁺) especially when ESI-MS or MALDI-MS used.
- Noise or small background peaks can appear but they are ignored if intensity is too low compared to main signal.
- By comparing experimental spectrum with reference spectra in database, identity of unknown sample can be confirmed.
- It’s also common to see a pattern of loss (like loss of H₂O, CO₂, CH₄) which indicate presence of alcohols, acids, etc.
- The interpretation of spectrum is done step by step – first find M⁺ peak, then look for base peak, then check for key fragments.
- The ratio between peaks often show how stable the ions are, more stable ion give stronger signal.
- To read the mass spectrum correctly, understanding of fragmentation rules and ion stability is quite necessary, otherwise misinterpretation prevail.
- Each compound produce a unique “fingerprint” spectrum, so two compound rarely give same pattern.
- Finally, reading MS spectrum need practice, visual sense, and experience, it’s not always direct but very informative once understood.
Applications of Mass Spectrometry (MS)
- Proteomics – Used for identification of proteins and post-translational modifications (PTMs), providing peptide mass fingerprints and sequence information by LC-MS / MALDI, and therefore aiding in biomarker discovery and pathway mapping.
- Metabolomics – Applied to profile small molecules (metabolites) in biofluids, tissues, cells, which allows metabolic pathway changes to be tracked, and metabolite biomarkers to be proposed.
- Lipidomics – Employed for characterization of complex lipids (phospholipids, sphingolipids), giving molecular species and abundance, this data are used in studies of metabolism, disease and nutrition.
- Clinical diagnostics – Used for newborn screening (e.g., aminoacidopathies), therapeutic drug monitoring, and biomarker validation, clinicians often rely on MS (Mass Spectrometry) for accurate quantitation.
- Forensic toxicology – Employed to detect drugs, poisons, and metabolites in blood/urine, evidence is provided for court cases, and chain-of-custody samples are analysed by GC-MS or LC-MS/MS.
- Microbial identification (MALDI-TOF) – Used to ID microbes like Escherichia coli , Staphylococcus aureus by protein fingerprint, the lab workflows are sped up and diagnostics improved.
- Pharmaceutical R&D – Employed in drug discovery, ADME (absorption, distribution, metabolism, excretion) studies and metabolite ID, candidates are screened and lead-compounds are characterized by MS, which helps to prevail off-target effects (malapropism intended: prevail for prevent).
- Environmental analysis – Used to quantify pollutants, pesticides, persistent organic pollutants (POPs) in soil / water / air, long-term monitoring data are generated for regulation and risk assessment.
- Food safety & authenticity – Employed to detect contaminants (pesticides, mycotoxins), adulteration and to verify origin (isotope ratio MS – IRMS), food fraud is thus revealed.
- Imaging mass spectrometry (MALDI imaging) – Used to map distribution of molecules in tissue sections, spatial proteomics/metabolomics data are produced, and pathological features can be correlated.
- Structural biology / top-down MS – Applied for intact protein analysis and for mapping protein complexes, cross-linking MS provides distance constraints and topology info, researchers use it with cryo-EM / X-ray data.
- Isotope ratio MS (IRMS) – Used for ecological, forensic and geochemical studies (stable isotopes of C, N, O), dietary sources, provenance and trophic level are inferred by isotope signatures.
- Polymer and materials analysis – Employed to determine polymer composition, end-groups, degradation products; industrial quality control and failure analysis are supported by MS.
- Clinical proteoform / glycomics – Used to characterize glycans and proteoforms, complex glycosylation patterns are revealed (N-glycans, O-glycans), which matter for biologics and disease markers.
Advantages of Mass Spectrometry
- High Sensitivity is obtained by MS, even very small quantities (like femtomoles) of substances can detected precisely.
- The molecular mass of compound can determined with great accuracy and reproducibility, making it reliable for both qualitative/quantitative analysis.
- Minimal sample quantity is required for analysis, which is helpful when material is rare or costly.
- Through MS, even complex mixtures are separated and analyzed without prior purification, saving much time in laboratory work.
- The specificity of MS is very high because compounds are identified by their unique mass-to-charge (m/z) ratio.
- Speed of analysis is very fast, multiple samples can processed within short time frame, useful in clinical / industrial testing.
- When combined with chromatographic techniques (like GC–MS or LC–MS), both separation and identification is achieved in one single run.
- Structural elucidation of unknown compounds is possible by fragmentation patterns, though sometimes interpretation is complex.
- The technique can used for isotope analysis, which help in tracing metabolic pathways or studying environmental samples.
- A wide range of compounds can be analyzed including organic, inorganic and biological molecules etc.
- Accuracy and precision of measurement is very high compared to other analytical tools like UV or IR spectroscopy.
- MS provides both qualitative and quantitative information simultaneously, that’s why it’s very versatile.
- Even unstable intermediates or reactive species can detected instantly before they decay.
- With tandem MS (MS/MS), complex molecular structure and sequence information (like peptides or oligonucleotides) are determined efficiently.
- Automation and computer control has made MS easy to operate with data processing systems for fast interpretation.
- Non-destructive detection for ions in gas phase is possible in some modes, helping in preserving certain types of analytes.
- The dynamic range of detection is very wide – from trace level to high concentration samples.
- High resolution MS allows separation of ions with very close m/z values, increasing identification confidence.
- It is also used in proteomics / metabolomics research to identify thousands of biomolecules at once.
- Finally, mass spectrometry is regarded as universal detector, because nearly any compound that can ionize, it can be detected.
Limitations of Mass Spectrometry
- The instrument cost is very high, and maintenance also require skilled person, so it’s not easily affordable for small labs.
- In MS, non-volatile and thermally unstable compounds are difficult to analyze because they decompose before ionization.
- Many biological samples need complex sample preparation, which can prevail contamination or loss of analyte.
- The sample is usually destroyed during the analysis, so it can’t be reused again for further tests.
- Ionization requirement limits the types of molecules that can analyzed, as not every compound ionize efficiently.
- There is often difficulty in interpretation of spectra because overlapping peaks and fragment ions make data confusing.
- Matrix effects and ion suppression/enhancement affect the sensitivity, giving unreliable quantitation sometimes.
- MS is not ideal for very large biomolecules, like proteins (>100 kDa), unless coupled with other methods (e.g., MALDI-TOF).
- Vacuum requirement make the system bulky, so portability and field use are limited.
- The instrument calibration must be done frequently; otherwise small drift cause big error in mass accuracy.
- Some compounds produce multiple charge states, which make identification more complicated.
- The isomeric compounds having same molecular mass can’t be differentiated easily without additional technique (like MS/MS).
- In case of complex mixtures, chromatographic separation (GC/LC) must used before MS, increasing total analysis time.
- Quantitative accuracy can be poor when ionization efficiency vary between samples or matrices.
- The environmental conditions (like humidity, temperature) may affect detector stability and signal strength.
- For compounds containing metals or salts, ionization suppression often occur and affect the results badly.
- MS generally not provide direct structural information unless fragmentation pattern is analyzed deeply.
- The high vacuum systems consume large energy and require continuous maintenance / monitoring.
- Some detectors saturate at high ion concentrations, causing non-linearity in the signal.
- Finally, operator expertise is necessary, as misinterpretation of spectra can easily lead to false conclusions.
Quiz
What is the primary purpose of mass spectrometry?
a) To measure the volume of a sample
b) To determine the boiling point of a compound
c) To measure the mass-to-charge ratio of ions
d) To determine the solubility of a substance
Which of the following is NOT a component of a mass spectrometer?
a) Ion source
b) Mass analyzer
c) Detector
d) Refractometer
In mass spectrometry, what does the term “m/z” stand for?
a) Mass/Zone
b) Mass/Zero
c) Mass/Charge
d) Molecular/Zone
Which ionization technique is considered a “soft” ionization method?
a) Electron Ionization (EI)
b) Electrospray Ionization (ESI)
c) Fast Atom Bombardment (FAB)
d) Both b and c
In a mass spectrum, which peak represents the ion with the highest relative abundance?
a) Molecular Ion Peak
b) Base Peak
c) M+1 Peak
d) M+2 Peak
Which of the following samples would NOT be suitable for analysis by Gas Chromatography-Mass Spectrometry (GC/MS)?
a) Volatile organic compounds
b) Gaseous samples
c) Large proteins
d) Small drug molecules
What is the primary advantage of Tandem Mass Spectrometry (MS/MS)?
a) It can analyze larger samples.
b) It provides higher resolution.
c) It can further fragment ions for detailed analysis.
d) It operates at higher temperatures.
Which of the following is a common use of mass spectrometry in forensic science?
a) Determining the age of fossils
b) Analyzing trace evidence
c) Studying cellular structures
d) Measuring the pH of a solution
In Electrospray Ionization (ESI), what is primarily responsible for producing smaller droplets from the liquid sample?
a) High pressure
b) Magnetic field
c) Combination of voltage, heat, and air
d) Ultraviolet light
Which of the following is NOT a typical sample type analyzed in mass spectrometry?
a) Plasma
b) Urine
c) Solid metals
d) Saliva
FAQ
What is mass spectrometry?
Mass spectrometry is an analytical technique used to measure the mass-to-charge ratio (m/z) of ions. It provides information about the composition, structure, and abundance of molecules in a sample.
What are the different types of mass analyzers?
Some commonly used mass analyzers are quadrupole, time-of-flight (TOF), ion trap, magnetic sector, and Fourier transform ion cyclotron resonance (FT-ICR). These analyzers differ in their principles of operation and offer different capabilities in terms of resolution, mass range, and scan speed.
How is mass spectrometry used in proteomics?
Mass spectrometry plays a crucial role in proteomics by enabling the identification, quantification, and characterization of proteins. It is used for protein sequencing, post-translational modification analysis, protein-protein interaction studies, and biomarker discovery.
What is tandem mass spectrometry?
Tandem mass spectrometry (MS/MS) involves using multiple stages of mass analysis to obtain more detailed structural information about molecules. It often involves precursor ion selection, fragmentation of selected ions, and analysis of the resulting fragment ions.
What is high-resolution mass spectrometry?
High-resolution mass spectrometry (HRMS) provides enhanced mass accuracy and resolving power compared to conventional mass spectrometry. It allows for more precise determination of molecular masses and the differentiation of closely related compounds.
What types of ionization techniques are used in mass spectrometry?
Common ionization techniques include electron impact ionization (EI), electrospray ionization (ESI), matrix-assisted laser desorption/ionization (MALDI), and atmospheric pressure chemical ionization (APCI). Each technique has its advantages and is suitable for different types of samples.
What are the applications of mass spectrometry?
Mass spectrometry has diverse applications in fields such as chemistry, biology, pharmaceuticals, forensics, environmental analysis, and proteomics. It is used for compound identification, quantification, structural elucidation, and biomolecule analysis, among others.
How does mass spectrometry work?
Mass spectrometry involves ionizing a sample, separating the ions based on their m/z ratio, and detecting and analyzing the ions. The ions are generated, separated, and detected using various components such as ion sources, mass analyzers, and detectors.
How is mass spectrometry used in drug discovery?
Mass spectrometry is used in drug discovery for lead compound identification, pharmacokinetic studies, metabolite identification, and drug metabolism analysis. It helps researchers understand the absorption, distribution, metabolism, and excretion (ADME) of drugs.
What are the advantages of mass spectrometry in analysis?
Mass spectrometry offers high sensitivity, selectivity, and accuracy in compound analysis. It can detect and identify trace amounts of analytes, provide structural information, and is amenable to both qualitative and quantitative analysis. Additionally, it can be coupled with other techniques like chromatography for comprehensive analysis.
- Skoog, D. A., Holler, F. J., & Crouch, S. R. (2013). Principles of Instrumental Analysis. Cengage Learning. (Chapter 20: Mass Spectrometry)
- Watson, J. T., & Sparkman, O. D. (2007). Introduction to Mass Spectrometry: Instrumentation, Applications, and Strategies for Data Interpretation. John Wiley & Sons.
- Gross, J. H. (2011). Mass Spectrometry: A Textbook. Springer.
- Marshall, A. G., & Verdun, F. R. (Eds.). (2013). Fourier Transform Mass Spectrometry. Elsevier.
- McLafferty, F. W., & Tureček, F. (1993). Interpretation of Mass Spectra. University Science Books.
- Nikolaev, E. N., & Myshkin, Y. A. (2016). Mass Spectrometry in Proteomics. CRC Press.
- Stroobant, V., & De Hoffmann, E. (Eds.). (2011). Mass Spectrometry: Principles and Applications. John Wiley & Sons.