What is IR Spectroscopy?
- It is a technique in which infrared radiation interacts with matter (solid, liquid or gas) and the absorption/emission/reflection of that IR light is measured, giving information about molecular structure.
- The method uses light in the infrared region (wavelengths longer than visible light) and molecules are studied by how their bonds vibrate (stretch, bend etc) when IR light of certain frequencies is absorbed.
- Vibrational modes that cause change in the dipole moment of the molecule are “IR active”.
- The result is a spectrum (absorbance or transmittance vs wavenumber or wavelength) often used like a “fingerprint” of a compound.
- It is widely used in chemistry (organic & inorganic) and materials science to identify functional groups or verify substances.
- Quality-control, reaction monitoring, materials verification become easier because the technique is relatively fast and non-destructive in many cases.
- Because each molecule/bond type tends to absorb IR at characteristic frequencies, the method gives insight into molecular bonding, structure, environment.
- A beam of IR light is directed at a sample. The sample absorbs certain frequencies where molecular vibrations resonate with the light energy.
- The bonds in molecules vibrate (stretching, bending, twisting) and those vibrations correspond to specific frequencies of IR light.
- If the vibration leads to a change in dipole moment then absorption happens (non-polar symmetric bonds may not show strong absorption).
- After absorption, the detector records how much light got through (or reflected) at each frequency and generates the spectrum (absorbance vs wavenumber/cm⁻¹).
- Interpretation: The positions (wavenumbers) of peaks tell which bonds/functional groups are present. The intensity/shape may tell about bonding environment, conjugation, hydrogen-bonding etc.
- The discovery of infrared radiation as a form of light (invisible heat beyond red) was first made by William Herschel in 1800 (he used a prism and thermometer) which laid the groundwork.
- The technique of IR spectroscopy (studying molecular absorption of IR) began to be developed in the early 1900s when e.g. William W. Coblentz showed that chemical functional groups absorb specific IR frequencies.
- Instruments (infrared spectrometers) were developed mid-20th century (1940s onwards) and became more widespread in research/industry from 1950s onward.
Principle of IR Spectroscopy
The basic principle is that molecules absorb infrared (IR) radiation when the frequency of the radiation matches a vibration of a chemical bond in the molecule.
Vibrations in molecules (stretching, bending, twisting) are possible because atoms are held by bonds and the atoms move relative to each other (like masses and springs) which leads to quantised vibrational energy levels.
Only those vibrational modes that cause a change in dipole moment of the molecule are IR-active (i.e., only those modes will absorb IR light).
When IR light is passed through (or reflected from) a sample, the sample will absorb light at certain frequencies corresponding to its vibrational modes, and the remaining (transmitted or reflected) light is detected, and the spectrum plotted (absorbance or transmittance vs wavenumber or wavelength).
The position (wavenumber) of an absorption tells which bond / functional group is likely present (because each type of bond has characteristic frequency), and the intensity / shape of the band gives information about environment (bond strength, conjugation, hydrogen bonding etc).
In the theoretical view the vibration frequencies depend on the masses of the atoms, the force constant of the bond (i.e., how stiff the bond is) and the shape of the potential energy surface; so heavier atoms vibrate more slowly, weaker bonds absorb at lower frequency etc.
The technique is called “vibrational spectroscopy” because it deals with transitions between vibrational energy levels (sometimes accompanied by rotational changes) rather than electronic excitations.
The IR region used is often mid-IR (approx. 4000-400 cm⁻¹) where fundamental vibrations occur, rather than near-IR (overtones) or far-IR (low-frequency vibrations) though those regions also exist.
Because each molecule has many vibrational modes, particularly if complex, the resulting spectrum (especially in “fingerprint region”) becomes unique — thus the spectrum can act like a molecular “fingerprint”.
Instrumentation of Infrared Spectroscopy (IR Spectroscopy )
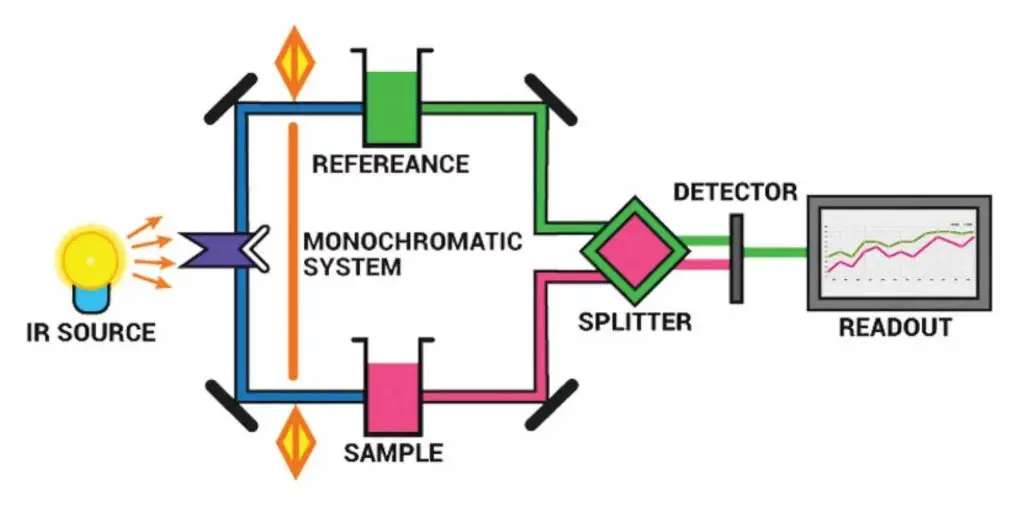
The principal components of IR the spectrometer are:
- Radiation source
- Sample cells and sampling of substances
- Monochromators
- Detectors
- Recorder
- Radiation Source – Nernst glower, Globar (SiC), Tungsten lamp, Mercury arc, Nichrome wire and incandescent lamps are used, the source is expected to give steady and sufficiently intense IR output over desired region, preheating sometimes required.
- Sample Cells / Sampling System – Solid, liquid, gas samples are handled by sample cells and sample holders, solids are prepared by KBr pellet, mull technique, thin films, or run-in-solution; liquids are placed between alkali-halide windows (NaCl, KBr), aqueous solvents avoided, gases are put in long path gas cells.
- Monochromator – Prism, grating or filter based monochromators are used for dispersive systems, prisms are made of KBr / NaCl / CsI and gratings are of alkali-halide coated surfaces, filters (LiF) are applied sometimes, wavelength selection is accomplished by dispersion or interference.
- Interferometer – In FTIR the Michelson interferometer is used, beam is split and recombined (by beam splitter), movable mirror create optical path difference and an interferogram is produced, later Fourier transformed to spectrum.
- Beam Splitter – A beam splitter (Mylar, KBr, CaF₂) is employed to divide beam in interferometer, material choice depend by spectral region and it is critical for performance.
- Optical Components – Mirrors (aluminium or gold coated), lenses (IR transparent like CaF₂), apertures, and windows are used for directing/focusing beam and protecting optics, reflective optics are preferred because glass absorb IR.
- Detectors – Thermal detectors (thermocouple, bolometer, thermistor, Golay cell, pyroelectric) and photon/quantum detectors (PbS, PbSe, MCT – Mercury cadmium telluride) are used, detector selection depend on sensitivity and wavelength region.
- Electronics / Amplifier / Signal Processor – Detector signals are amplified, filtered and digitized, noise reduction and signal averaging circuits are included, microprocessor based signal processing (including Fourier transform) is applied, grounding issues sometimes cause hum or distortions.
- Recorder / Display System – The final spectrum (absorbance or transmittance vs wavenumber cm⁻¹) is recorded by digital recorder or computer display, older instruments used analog chart recorders, printing or file export is usually provided.
- Computer / Data System & Libraries – Control, data acquisition, Fourier transform, baseline correction and library searching are done by computer software, spectral libraries are used for identification though operator judgement still needed.
- Purge / Desiccant System – Optical bench and sample compartment are purged with dry N₂ or air to remove H₂O and CO₂ interferences, desiccants (silica gel, P₂O₅) or gas purge lines are provided, else strong absorption will prevail.
- Sample Accessories / Sampling Interfaces – Accessories like ATR (Attenuated Total Reflectance) crystals (ZnSe, diamond), diffuse reflectance (DRIFTS) cups, specular reflectance attachments, heated cells, and micro-sampling stages are used for convenience and different sample types.
Graph of the IR spectrum
Below is a small example of the typical Infrared Absorption Frequency.
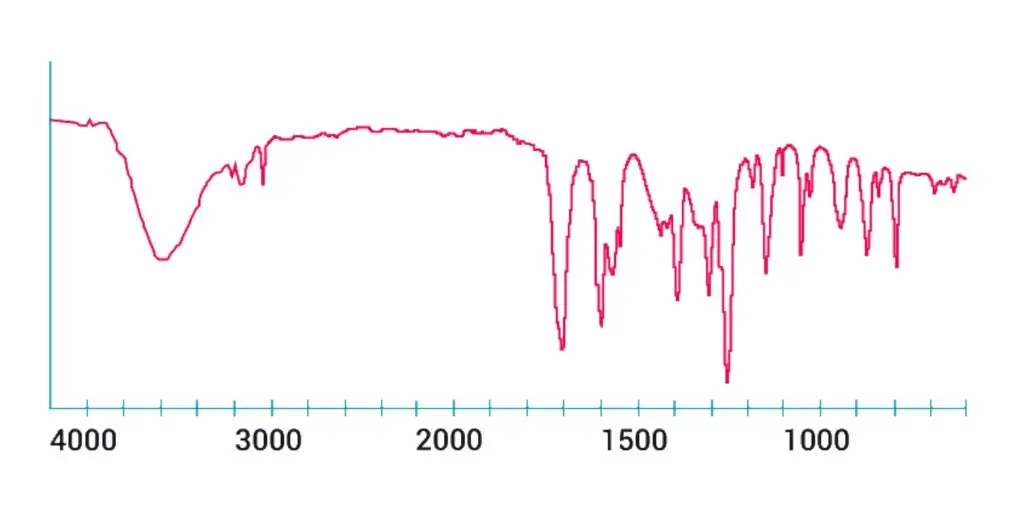
So, IR spectroscopy involves the gathering of absorption data and the analysis of that information as an IR spectrum.
Types of Infrared Spectrophotometry (IR Spectroscopy)
1. Dispersive IR Spectrophotometry – A type where IR radiation is separated by prism or grating and then scanned through wavelengths (or wavenumbers) one by one, the instrument is commonly used previously for IR spectra.
2. Fourier-Transform IR Spectrophotometry (FTIR) – This type uses an interferometer (usually a Michelson interferometer) to collect many wavelengths at once and a computer applies a Fourier transform to get spectrum, it gives higher speed and resolution.
3. Near-Infrared (NIR) Spectroscopy – A type which uses shorter wavelengths (higher energy) IR region (approx 14,000–4,000 cm⁻¹) to measure overtone/combination vibrations of molecules, this is less common for full bond-structure but useful for industrial process control.
4. Mid-Infrared (MIR) Spectroscopy – A main type where the fundamental molecular vibrations are measured (approx 4,000–400 cm⁻¹), widely used for identifying functional groups and bonding in molecules.
5. Far-Infrared (FIR) Spectroscopy – A type covering low energy IR region (approx 400–10 cm⁻¹) which is used for low-frequency vibrations/rotational modes and sometimes intermolecular forces, less common for routine organic analysis but important for materials science.
6. Two-Dimensional IR Spectroscopy (2D IR) – A more advanced type where nonlinear IR pulses are used and spectra are spread in two axes (ω₁ and ω₃) to study coupling between vibrational modes and dynamics on very short timescales (femtoseconds).
Types of Samples used in Infrared Spectroscopy
There are many different types of samples that can be analyzed using infrared (IR) spectroscopy. Some common types of samples include:
Solid Samples – Solid materials (powders, pellets, films) are used and are prepared by grinding/mixing or pressing, the sample is mixed with transparent salt like KBr and pressed into pellet or made into thin film.
Liquid Samples – Liquids or solutions are used, they are held between IR-transparent windows (NaCl, KBr) or a drop placed on ATR crystal; organic solvents (like chloroform) are usually chosen because aqueous solvents would dissolve the windows.
Gas Samples – Gaseous samples are used and special long-path gas cells (with IR-transparent windows) are required because the absorbance is weak; the path length may be many centimetres or more.
Film / Thin-Layer Samples – A variant of solid sample where a thin film (of polymer or deposited solid) is formed on a salt plate or window, the film is analysed directly; the sample state is still solid but special form.
Mixture / Composite Samples – Sometimes the sample is a composite or paste (for example a solid mixed with mulling agent like Nujol) and then placed between transparent plates; this allows solids with limited solubility to be analysed.
Infrared vs Raman spectroscopy
- In Infrared (IR) spectroscopy, absorption of infrared light by molecules are measured, while in Raman spectroscopy, scattering of light (usually from a laser source) are analyzed.
- IR is based on dipole moment change during vibration, whereas Raman depends on polarizability change of molecule.
- Molecules which are active in IR are often inactive in Raman and vice-versa, though some may show both activities sometimes.
- In IR, the source usually a Globar (SiC rod) or Nernst filament, but in Raman, a monochromatic laser (like Ar⁺ or He–Ne laser) are used.
- The IR spectrum is obtained by measuring absorbed radiation, while Raman spectrum is produced from the scattered radiation, that include Stokes and anti-Stokes lines.
- In IR, water strongly absorbs and interferes, but in Raman, water gives very weak scattering, so aqueous samples are studied easily.
- Raman spectra can be taken by glass cells, but IR need special materials (like NaCl, KBr) which transmit IR radiation.
- The IR technique is less useful for symmetric molecules like O₂, N₂, Cl₂, because they show no dipole change, however Raman easily detect them because of polarizability change.
- In Raman method, visible or near IR region of light is used, but in IR spectroscopy, radiation of 4000–400 cm⁻¹ region (mid IR) are used generally.
- Instrumentation of IR involve source, monochromator, detector, recorder, while Raman include laser source, sample illuminator, spectrometer, and detector (CCD).
- Raman lines are weaker than Rayleigh line but they are shifted from incident frequency by vibrational energy of molecules.
- In IR, intensity of absorption band depend upon magnitude of dipole moment change; in Raman, intensity depend upon polarizability change.
- Both methods give information about vibrational energy levels, but they provide complementary data, so both are often used together for complete analysis.
- IR spectra are affected by sample thickness and preparation, Raman mostly not.
- IR cannot used easily with metal surfaces because reflection problem occur, but Raman can study surface (in SERS – Surface Enhanced Raman Spectroscopy).
- For qualitative analysis, both identify functional groups, e.g., –OH, C=O, C–H stretching etc., but selection rules are different.
- Raman spectra lines are sharp and well defined, while IR bands often broad due to hydrogen bonding or overlap.
- IR measurement mostly require dry and clean samples, Raman can handle dirty or turbid ones.
- In IR, calibration often done using polystyrene film, in Raman, internal standard like Si band (520 cm⁻¹) used.
- Therefore, IR and Raman spectroscopy both are vibrational techniques but they operate by different physical principles and are complementary rather than competitive.
Applications of Infrared (IR) Spectroscopy
- IR spectroscopy used for identification of functional groups present in organic or inorganic compounds.
- It can used for structure elucidation because each bond in molecule absorb at different frequency.
- By IR, purity of compound can checked since impurity bands appear at unexpected positions.
- It used for qualitative analysis of solid, liquid, or gaseous samples easily.
- Quantitative estimation of mixture components are done because absorbance obeys Beer–Lambert law.
- In polymer analysis, IR is used to determine composition, additives, and curing extent.
- Pharmaceutical industry uses IR for identification of drugs, intermediates, and to check polymorphism.
- Inorganic complexes like metal carbonyls are analyzed for metal–ligand bonding information.
- IR used to study hydrogen bonding and molecular interactions by shift of absorption bands.
- It applied for detection of impurities and contaminants in chemicals and environment samples.
- IR microscopy is used for surface characterization and micro area analysis.
- It applied for analysis of food products to detect adulteration, moisture, and fats.
- In forensic science, IR help in identification of paints, fibers, drugs, plastics, etc.
- IR spectroscopy used for studying reaction kinetics by recording spectra at different time intervals.
- Environmental monitoring like detection of CO₂, SO₂, CH₄ gases is done by IR absorption.
- In biological field, proteins and lipids structure studied by FTIR (Fourier Transform Infrared) spectroscopy.
- It is also used in quality control of materials to verify composition and detect degradation.
- IR spectroscopy applied in crystallinity determination of polymers or other solids.
- The technique used for identifying unknown compounds by matching spectra with reference library.
- Therefore, IR spectroscopy serve as a versatile and powerful analytical tool in research, industry, medicine etc.
Advantages of Infrared (IR) Spectroscopy
- The IR spectroscopy provides quick and accurate identification of compounds without complex sample preparation.
- It used for both qualitative and quantitative analysis easily by same instrument setup.
- Non-destructive technique so sample mostly remain unchanged after analysis.
- Only small amount of material (even few mg) required for recording spectra.
- Analysis can be done in all states – solid, liquid, gas, or even thin films.
- IR spectra are unique for each compound so it act like molecular fingerprint.
- Operation of instrument is simple and spectra can be obtained rapidly.
- Fourier Transform Infrared (FTIR) instruments provide very high resolution and signal-to-noise ratio.
- IR spectroscopy applicable for both organic and inorganic substances conveniently.
- Water interfere strongly in IR but modern techniques allow correction or use of ATR accessories.
- It allows study of molecular vibrations and functional group identification precisely.
- IR technique can used to monitor reaction progress by measuring band changes with time.
- Method is suitable for opaque or turbid samples when ATR method used.
- Calibration of IR is easy using standard materials like polystyrene film.
- IR spectroscopy equipment need low maintenance and gives reproducible results.
- It is adaptable for remote or automated monitoring systems (like gas analysis).
- In research, the technique gives complementary information with Raman spectroscopy.
- Spectra can stored digitally and compared with large reference libraries for compound identification.
- IR spectroscopy works without chemical reagents, so it’s clean and environment-friendly method.
- Overall, IR spectroscopy offer fast, reliable and versatile analytical advantage for wide range of applications.
Limitations of Infrared (IR) Spectroscopy
- The IR spectroscopy cannot used for analysis of very complex mixtures because spectra overlap badly.
- It not suitable for detecting very low concentration components due to poor sensitivity.
- Water absorption interfere strongly with IR radiation and cause background noise or band masking.
- The method only detect vibration that cause dipole moment change, so symmetric molecules (like O₂, N₂, Cl₂) remain inactive.
- Preparation of sample sometimes difficult since special windows (NaCl, KBr) required for transmission.
- Quantitative analysis less accurate when sample thickness not uniform or scattering occur.
- IR spectra of large molecules often complicated and difficult to interpret correctly.
- The technique provide limited information on structure, it not show connectivity of atoms clearly.
- Metallic surfaces reflect IR strongly, so metallic samples can’t analyzed easily.
- Temperature variations cause change in band position and intensity that reduce accuracy.
- In solid samples, strong absorption or scattering leads to distorted baseline in spectrum.
- IR spectroscopy not used for elements or atomic analysis because only covalent bonds absorb IR radiation.
- Sometimes overtones and combination bands cause confusion during interpretation.
- Calibration and instrument alignment require care, otherwise spectral errors occur.
- Sensitivity for trace analysis lower compared with UV–Vis or Mass spectrometry.
- The IR cannot distinguish between optical isomers (enantiomers) having same functional groups.
- Spectral library matching not always reliable when mixture or impurities present.
- For aqueous samples, special accessories (ATR / reflection cell) required that increase cost.
- IR instruments are sensitive to moisture and require proper maintenance for accuracy.
- Hence, despite its usefulness, IR spectroscopy have certain limitations that restrict its use in few analytical situations.
FAQ
What is the basic principle of infrared spectroscopy?
The basic principle of infrared (IR) spectroscopy is the absorption of infrared light by a sample. Infrared light is a type of electromagnetic radiation with a longer wavelength than visible light. It is absorbed by certain vibrational modes of the bonds between atoms in a molecule.
When infrared light is absorbed by a sample, it causes the bonds in the molecule to vibrate at the same frequency as the absorbed light. The absorbed light is then absorbed as energy by the molecule, and the molecule transitions to an excited state. The molecule will eventually return to its ground state, releasing the absorbed energy as heat or light.
By measuring the absorption of infrared light at different wavelengths, it is possible to determine the vibrational modes of the bonds in the molecule. Each bond in a molecule has a characteristic vibrational frequency, and the absorption of infrared light at these frequencies can be used to identify the molecular structure of the sample.
Infrared spectroscopy is a powerful tool for identifying and analyzing the molecular structure of a wide range of materials, including organic and inorganic compounds, polymers, and biomolecules. It is widely used in a variety of fields, including chemistry, materials science, and biology.
What is infrared spectroscopy used for?
Infrared (IR) spectroscopy is a technique used to identify and analyze the molecular structure of a substance. It involves the absorption of infrared light by a sample, and the measurement of the absorption at different wavelengths. By analyzing the absorption spectrum, it is possible to determine the vibrational modes of the bonds in the molecule, which can be used to identify the molecular structure of the sample.
Infrared spectroscopy is used in a variety of fields, including chemistry, materials science, and biology. It is particularly useful for identifying and analyzing the molecular structure of organic and inorganic compounds, polymers, and biomolecules. It is also used in quality control and research applications, as well as for the analysis of environmental samples and forensic evidence.
What is range of IR spectroscopy?
The range of infrared (IR) spectroscopy is typically defined by the range of wavelengths that can be measured using the instrumentation available. The range of IR spectroscopy is typically between 400 and 4000 cm^-1 (4000 and 400 nm), although some instruments are capable of measuring even lower or higher wavelengths.
The specific range of IR spectroscopy that is used depends on the nature of the sample being analyzed and the vibrational modes of the bonds in the molecule. Different bonds absorb infrared light at different wavelengths, and the absorption spectrum of a molecule can be used to identify the molecular structure of the sample.
For example, the absorption of infrared light by stretching and bending vibrations of bonds in organic molecules is typically in the range of 4000-400 cm^-1 (400-4000 nm). The absorption of infrared light by vibrational modes of inorganic compounds and polymers is typically in the range of 1000-100 cm^-1 (10000-100000 nm). The absorption of infrared light by rotational modes of molecules is typically in the range of 100-10 cm^-1 (100000-1000000 nm).
What instrument is used in IR spectroscopy?
Infrared (IR) spectroscopy is typically performed using a specialized instrument called an infrared spectrometer. An infrared spectrometer consists of three main components: a light source, a sample holder, and a detector.
The light source in an infrared spectrometer is typically a tungsten halogen lamp or a deuterium lamp, which produces a broad spectrum of infrared light. The sample holder is a device that holds the sample and allows infrared light to pass through it. The detector is a device that measures the intensity of the infrared light that is transmitted or absorbed by the sample.
There are several different types of infrared spectrometers, including dispersive spectrometers and Fourier transform spectrometers. Dispersive spectrometers use a prism or grating to separate the different wavelengths of light, while Fourier transform spectrometers use interferometry to measure the spectrum of the sample.
Infrared spectrometers are widely used in a variety of fields, including chemistry, materials science, and biology, to identify and analyze the molecular structure of a wide range of materials, including organic and inorganic compounds, polymers, and biomolecules.
Which type of solvent is used in IR?
Infrared (IR) spectroscopy is a technique that is used to identify and analyze the molecular structure of a substance. In order to prepare a sample for IR spectroscopy, it is often necessary to dissolve the sample in a solvent. The choice of solvent depends on the nature of the sample and the vibrational modes of the bonds in the molecule that are being studied.
In general, it is best to use a solvent that does not absorb infrared light at the wavelengths of interest. This is because the absorption of the solvent can interfere with the measurement of the absorption of the sample.
Common solvents that are used for IR spectroscopy include liquid hydrocarbons (such as hexane, cyclohexane, and toluene), alcohols (such as methanol, ethanol, and isopropanol), and water. In some cases, it may be necessary to use a more specialized solvent, such as dimethylformamide (DMF) or dimethylsulfoxide (DMSO), depending on the nature of the sample.
It is important to note that the choice of solvent can affect the absorption spectrum of the sample. In some cases, the presence of a solvent can alter the vibrational modes of the bonds in the molecule, which can affect the interpretation of the IR spectrum. Therefore, it is important to carefully consider the choice of solvent when preparing a sample for IR spectroscopy.
What is UV and IR range?
The electromagnetic spectrum is the range of all types of electromagnetic radiation, including radio waves, microwaves, infrared (IR) radiation, visible light, ultraviolet (UV) radiation, X-rays, and gamma rays. Each type of electromagnetic radiation has a specific range of wavelengths and frequencies, and is characterized by its unique properties and uses.
The ultraviolet (UV) range is the portion of the electromagnetic spectrum with wavelengths shorter than visible light. UV radiation has wavelengths between 100 and 400 nm, and it is characterized by its ability to cause chemical reactions and damage to living tissues. The UV range is further divided into three regions: UVA (400-315 nm), UVB (315-280 nm), and UVC (280-100 nm).
The infrared (IR) range is the portion of the electromagnetic spectrum with wavelengths longer than visible light. IR radiation has wavelengths between 750 nm and 1 mm, and it is characterized by its ability to be absorbed by certain vibrational modes of the bonds between atoms in a molecule. The IR range is further divided into three regions: near-infrared (NIR, 750-1400 nm), mid-infrared (MIR, 1400-3000 nm), and far-infrared (FIR, 3000-1 mm).
UV and IR radiation have a variety of practical applications, including UV disinfection, UV curing, and IR spectroscopy. They are also used in a variety of scientific and medical instruments, such as UV spectrophotometers and IR cameras.
Can we use water in IR spectroscopy?
Yes, water can be used as a solvent in infrared (IR) spectroscopy. Water is a common solvent for IR spectroscopy because it is widely available, inexpensive, and has a low absorption in the IR region.
However, it is important to note that water can absorb infrared light at certain wavelengths, particularly in the mid-infrared (MIR) region (1400-3000 cm^-1 or 4000-1000 nm). This means that the absorption spectrum of the sample may be affected by the presence of water, particularly if the sample absorbs at the same wavelengths as water.
To minimize the impact of water absorption on the IR spectrum of the sample, it is often necessary to use a deuterated solvent (such as deuterated methanol or deuterated water) or to dry the sample prior to analysis. Deuterated solvents have a lower absorption in the IR region than their non-deuterated counterparts, and they are often used to reduce interference from the solvent in IR spectra. Drying the sample can also help to remove any water that may be present, which can reduce interference from water absorption.
It is also important to carefully consider the nature of the sample when selecting a solvent for IR spectroscopy. In some cases, it may be necessary to use a more specialized solvent, such as dimethylformamide (DMF) or dimethylsulfoxide (DMSO), depending on the nature of the sample. The choice of solvent can affect the absorption spectrum of the sample, and it is important to choose a solvent that does not interfere with the measurement of the absorption of the sample.
How sensitive is IR spectroscopy?
The sensitivity of infrared (IR) spectroscopy depends on a variety of factors, including the nature of the sample, the instrumentation used, and the experimental conditions. In general, IR spectroscopy is sensitive enough to detect small changes in the molecular structure of a sample, and it is often used to identify and quantify trace amounts of compounds in complex mixtures.
One of the main factors that determines the sensitivity of IR spectroscopy is the strength of the vibrational absorption bands in the spectrum of the sample. Strong absorption bands are more likely to be detected than weak absorption bands, and they are generally easier to quantify.
The sensitivity of IR spectroscopy can also be affected by the choice of solvent, the sample preparation techniques, and the instrumentation used. For example, using a deuterated solvent or drying the sample can reduce interference from the solvent, which can improve the sensitivity of the measurement. Using a high-resolution spectrometer or a Fourier transform spectrometer (FTIR) can also improve the sensitivity of the measurement, as these instruments have a higher resolution and can detect smaller changes in the absorption spectrum.
In general, IR spectroscopy is a sensitive and powerful tool for identifying and analyzing the molecular structure of a wide range of materials, including organic and inorganic compounds, polymers, and biomolecules. It is widely used in a variety of fields, including chemistry, materials science, and biology, for a wide range of applications.
Which lamp is used in IR spectroscopy?
Infrared (IR) spectroscopy is typically performed using a specialized instrument called an infrared spectrometer. An infrared spectrometer consists of three main components: a light source, a sample holder, and a detector.
The light source in an infrared spectrometer is typically a tungsten halogen lamp or a deuterium lamp, which produces a broad spectrum of infrared light. The tungsten halogen lamp is a type of incandescent lamp that uses a tungsten filament and a halogen gas to produce light. It has a broad emission spectrum and is capable of producing a wide range of wavelengths in the infrared region.
The deuterium lamp is a specialized type of lamp that uses deuterium, a stable isotope of hydrogen, as the light-emitting element. It produces a spectrum that is rich in ultraviolet and infrared radiation, and it is often used in spectroscopy applications that require a broad spectrum of light.
Both tungsten halogen lamps and deuterium lamps are widely used in IR spectrometry because they produce a broad spectrum of infrared light that is suitable for a wide range of applications. The specific type of lamp used depends on the specific requirements of the measurement, including the wavelength range of interest and the sensitivity of the instrument.
- Kamariotis, A.; Boyarkin, O. V.; Mercier, S. R.; Beck, R. D.; Bush, M. F.; Williams, E. R.; Rizzo, T. R. J. Am. Chem. Soc. 2006, 128, 905
- Peter J. Larkin. “Infrared and Raman Spectroscopy. Principles and Spectral Interpretation” Chapters 1 to 6. Radarweg 29, PO Box 211, 1000 AE Amsterdam, The Netherlands. Elsevier 2011
- Marwa El-Azazy. Introductory Chapter: Infrared Spectroscopy – A Synopsis of the Fundamentals and Applications, Infrared Spectroscopy – Principles, Advances, and Applications. 2018. IntechOpen, DOI: 10.5772/intechopen.82210
- Donald L. Pavia, Gary M. Lampman and George S. Kriz. “Introduction to Spectroscopy. A Guide for Students of Organic Chemistry” Chapter 2. Thompson Learning. United States of America 2001.