What is Hydrophobic Interaction Chromatography (HIC)?
- In HIC, separation is done by interaction between hydrophobic regions on protein surface and hydrophobic ligands attached to chromatographic matrix (like butyl, octyl, or phenyl groups).
- The sample is usually applied in high salt concentration (like (NH₄)₂SO₄) that promote hydrophobic interactions.
- Proteins with higher hydrophobic surface areas bind more strongly to the column matrix.
- Elution is achieved by gradual decrease in salt concentration, which reduces hydrophobic interactions and releases bound proteins.
- It’s considered a mild technique, because protein structure and activity are generally maintained due to aqueous environment.
- It’s mostly used for purification of antibodies, enzymes, and recombinant proteins.
- HIC is often used after ion exchange chromatography and before gel filtration in multistep purification processes.
- The main principle depends on reversible interaction between hydrophobic areas on biomolecules and stationary phase ligands.
- Column materials often based on agarose, dextran, or synthetic polymers that are functionalized with hydrophobic groups.
- The technique provide high resolution and selectivity for separation based on surface hydrophobicity differences among proteins.
Principle of Hydrophobic Interaction Chromatography (HIC)
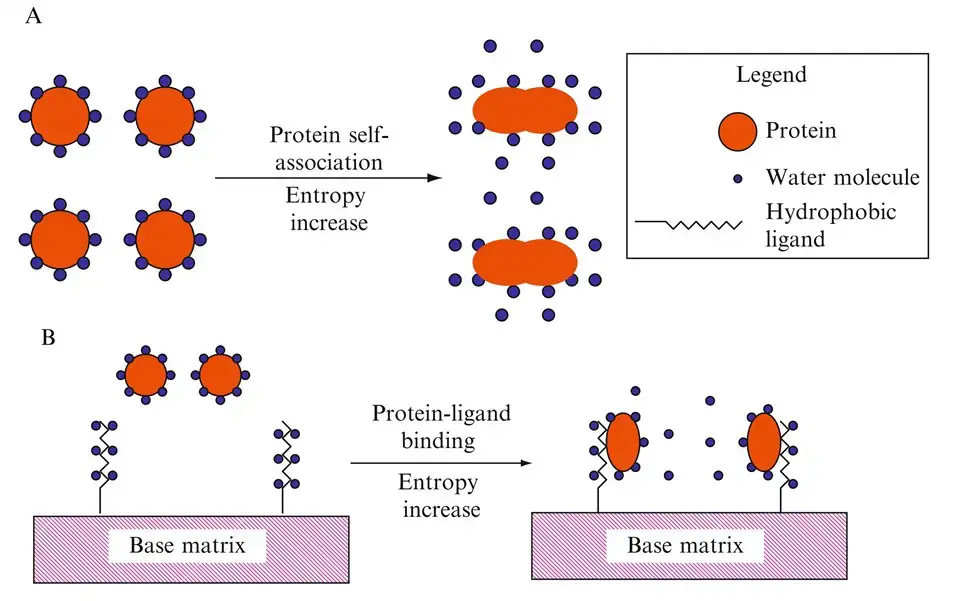
- The principle of Hydrophobic Interaction Chromatography (HIC) based on the interaction between hydrophobic regions on protein molecules and hydrophobic ligands attached to the chromatographic matrix.
- In presence of high salt concentration, hydrophobic interactions among non-polar groups are promoted, which cause protein to bind with matrix surface.
- The salt ions from solution reduce the solvation of hydrophobic patches on protein, thus they get exposed and interact with hydrophobic groups (like butyl, octyl, phenyl).
- When salt concentration is gradually decreased, the hydrophobic interactions become weaker and proteins elute from column depending upon their surface hydrophobicity.
- The process mainly depends on the “salting-out effect”, which cause water molecules to be excluded from hydrophobic surfaces, enhancing interaction strength.
- Proteins with more hydrophobic surface areas bind strongly, while less hydrophobic proteins elute earlier.
- The principle rely on reversible and non-covalent interaction, hence protein structure and biological activity are mostly preserved.
- In HIC separation, the retention time of protein molecules determined by the degree of hydrophobicity and salt gradient used.
- Thus, the basic idea is that hydrophobic interactions between stationary phase and protein molecules are enhanced by high salt, and reduced by low salt, leading to separation.
Protocol of Hydrophobic Interaction Chromatography (HIC)
- The HIC column is equilibrated with a high-salt buffer (for example ~1.5-2.0 M ammonium sulfate) so that strong hydrophobic interactions may be promoted.
- The sample is prepared by adjusting its salt concentration to match the equilibration buffer and the pH is set (often around pH 6-8) so that the target protein is stable.
- The sample is loaded onto the column slowly, allowing the hydrophobic patches of the protein surface to interact with the hydrophobic ligands (butyl, octyl, phenyl) on the matrix.
- After loading, a wash step is performed using the same high-salt buffer (or slightly reduced salt) to remove unbound or loosely bound contaminants, while the more hydrophobic species remain retained.
- Elution is initiated by gradually decreasing the salt concentration (or changing buffer composition) so that proteins are eluted in order of increasing hydrophobicity (i.e., less hydrophobic come off earlier, more hydrophobic later).
- A gradient elution (for example from 1.5 M to 0 M salt) may be used to improve resolution of proteins with similar hydrophobicity.
- Fractions are collected during elution and each fraction is analysed (by UV absorbance at 280 nm, SDS-PAGE, etc) to locate the target protein and assess purity.
- Column regeneration is done by washing with low-salt buffer or water, followed by a cleaning solvent (mild organic or detergent) to remove any residual bound materials or aggregates.
- The column is stored in a suitable buffer (often with a preservative) when not in use, to maintain the media’s performance for next runs.
- Optimization is required: factors like salt type/concentration, ligand type, buffer pH, temperature all affect binding/elution in HIC and must be tuned for each protein system.
Factors that Affect Hydrophobic Interaction Chromatography (HIC)
- The salt concentration in the mobile/starting buffer is a big determinant of retention, because when high salt is used more hydrophobic regions are exposed and binding is stronger so proteins stay longer.
- The type of salt / ionic species has strong effect, for example ammonium sulphate often used because of strong “salting-out” effect, while NaCl or acetate salts give different selectivity and binding strength.
- The ligand type / hydrophobic moiety on the stationary phase (for example butyl, octyl, phenyl) matters a lot because a longer/more hydrophobic chain will retain more strongly, while weaker ligand gives less retention.
- The matrix / support material and its ligand density / surface area also affect binding, because more surface + more ligand = greater contact and retention, but sometimes steric hindrance reduces effect.
- The pH of buffer influences protein charge/solvation and thus hydrophobic patches exposure, so the retention shifts when pH changes (even if protein remains folded) .
- The temperature of the system is important: higher temp often increases hydrophobic interaction (makes protein hydrophobic patches more accessible) but risk of protein denaturation or changes in conformation exists.
- The gradient slope / salt reduction rate during elution matters: a shallow gradient (slow decrease) gives better separation of proteins with similar hydrophobicity while a steep gradient may collapse resolution.
- The flow rate of mobile phase influences contact time: if flow is too fast then less time for hydrophobic interaction so weaker retention or lower resolution will occur.
- The protein’s intrinsic surface hydrophobicity (exposed hydrophobic patches, amino acid composition) is key: even under identical conditions proteins differ because they have different hydrophobic character.
- The presence of organic modifiers / other additives can alter solvation/hydrophobic interaction (for example low concentration of organic solvent can reduce binding) which changes retention.
Uses of Hydrophobic interaction chromatography
- Protein purification is carried out by HIC in many cases where other methods (ion-exchange, size-exclusion) don’t separate well, especially when proteins have similar charge/size but different hydrophobic patches.
- Use in impurity removal is found by HIC, where product-related impurities or aggregate species are separated out because they differ in hydrophobicity from target species.
- In therapeutic proteins (like monoclonal antibodies, bispecifics) HIC is used for characterizing variants and for polishing steps in downstream process; it gives non-denaturing separation of species differing in hydrophobicity.
- Analytical use is done by HIC, for example to assess the hydrophobicity of a biomolecule (for example after mutation, chemical modification) and to separate heterogeneities in a sample (for example variants of mAbs).
- In bioprocess applications HIC is used at different stages: capture, intermediate, polish – it is versatile and hardy method used in production scale and in lab scale.
Advantages of Hydrophobic interaction chromatography
- Selective separation is achieved because HIC exploits the hydrophobicity of biomolecules rather than just charge or size, and this gives an extra dimension of purity.
- Mild/non-denaturing conditions can be used so that native state of protein is more likely retained, and activity is preserved.
- Resolution between very similar variants (for example isoforms or closely related species) can be improved, because subtle changes in hydrophobic surface patches are discriminated.
- Versatility is offered, because many proteins (enzymes, antibodies, recombinant proteins) can be handled, and they can be purified from complex mixtures etc.
- Orthogonal selectivity is provided (meaning different from Ion Exchange or Size Exclusion) which helps in multi-step purification schemes to clear impurities that other methods cannot remove.
- Scalability and reuse-potential are possible, since well-designed HIC resins allow for multiple runs and larger volumes (when optimized) so process cost may be lowered.
Limitations of Hydrophobic interaction chromatography
- The sample‐preparation step often must be very careful (for example high salt addition), and the technique can demand lots of optimization for salt type & concentration.
- Proteins with very low exposed hydrophobic patches may not bind well, so poor retention or resolution are encountered for those low hydrophobicity ones.
- The high salt concentrations used for binding can cause problems with protein solubility/ stability (such as precipitation or aggregation) and may require extra care.
- The specificity is lower compared to affinity methods because HIC relies on hydrophobic surface patches rather than a unique molecular recognition, so the separation of very similar molecules may falter.
- The resin (stationary phase) fouling / non-specific binding is a risk because hydrophobic ligands may trap unintended molecules and cleaning/regeneration may be more frequent.
- Scale-up (large scale purification) is more challenging because packing and flow/hydrodynamics of HIC columns under high salt may not scale linearly and cost may rise.
- Some additives/detergents that are used for protein solubilization interfere with hydrophobic interactions and reduce performance or require compromise conditions.
FAQ
What is Hydrophobic Interaction Chromatography (HIC)?
HIC is a chromatographic technique used for the separation and purification of biomolecules, particularly proteins, based on their hydrophobic properties.
Which types of biomolecules can be separated using HIC?
HIC is primarily used for the separation and purification of proteins. However, it can also be applied to the separation of other biomolecules and organic compounds with hydrophobic groups.
What factors affect the separation in HIC?
Factors such as the type of ligand, ligand density, degree of substitution, temperature, pH, and salt concentration can affect the separation efficiency in HIC.
What is the typical salt concentration used in HIC?
HIC is typically performed using high-salt buffers, such as 1-2 M ammonium sulfate or 3 M sodium chloride, to promote the hydrophobic interactions between the proteins and the matrix.
How are proteins eluted in HIC?
Proteins are eluted from the HIC column by decreasing the salt concentration, either using a decreasing salt gradient or by incorporating mild organic modifiers or detergents in the elution buffer.
What are the advantages of using HIC?
HIC allows for the separation of proteins in their native state without denaturation. It can be used for the isolation of protein complexes and the study of protein folding and unfolding.
What is the principle behind HIC?
The principle of HIC is based on the exposure of hydrophobic regions in proteins when the solvation of the proteins is reduced by high salt concentrations. These exposed hydrophobic regions interact with the hydrophobic ligands in the chromatography matrix.
What is the binding capacity of HIC media?
The binding capacity of HIC media refers to the maximum amount of protein that can be bound to the matrix. It is influenced by factors such as the type of ligand, ligand density, and the degree of substitution.
How does HIC work?
HIC utilizes the reversible interaction between hydrophobic ligands in the chromatography matrix and hydrophobic regions of proteins. Proteins are adsorbed onto the hydrophobic ligands in a high-salt buffer and can be eluted using a decreasing salt gradient.
Can HIC be combined with other chromatographic techniques?
Yes, HIC can be used in combination with other chromatographic techniques, such as ion exchange chromatography or size exclusion chromatography, to achieve higher purification levels and resolve complex mixtures of biomolecules.