Gerhardt’s test is a widely used diagnostic method to detect acetoacetic acid, a key ketone body, in urine. The principle of Gerhardt’s test revolves around its reaction with ferric chloride (FeCl₃), where acetoacetic acid binds to iron ions, forming a distinctive burgundy or red-colored complex. Commonly referred to as the Gerhardt ferric chloride test, this chemical assay helps identify elevated ketone levels, often linked to metabolic conditions like diabetic ketoacidosis. A Gerhardt’s test positive result occurs when the characteristic color change appears, signaling excess ketones in urine. However, the Gerhardt test has limitations—it does not react with other ketones like acetone or beta-hydroxybutyric acid. Despite this, it remains a valuable tool for healthcare providers monitoring ketone bodies in urine, especially in emergencies. Understanding the Gerhardt’s test reaction and its clinical applications is essential for accurate diagnosis and patient care.
What is Gerhardt’s test?
Gerhardt’s test is a chemical procedure historically used in clinical settings to detect the presence of acetoacetic acid, a type of ketone body, in urine samples. This test plays a role in identifying conditions like diabetic ketoacidosis or metabolic imbalances where excessive ketone production occurs. The process involves adding a solution of ferric chloride to a small volume of urine. If acetoacetic acid is present, the iron in the reagent reacts with it, producing a distinct reddish or burgundy color. However, the test requires careful interpretation because other substances, such as salicylates (found in aspirin) or certain medications, can trigger similar color changes, leading to false-positive results. To improve accuracy, some protocols recommend boiling the urine sample first—since acetoacetic acid is heat-stable, while interfering compounds like aspirin byproducts may degrade, helping distinguish true positives. Despite its utility in earlier medical practice, Gerhardt’s test has largely been replaced by modern dipstick methods (e.g., nitroprusside-based tests) that are quicker, more specific, and capable of detecting multiple ketone types. Nevertheless, understanding this test remains relevant for educational purposes, illustrating foundational diagnostic techniques and the importance of considering contextual factors—like medication history—when interpreting lab results. Its limitations, including insensitivity to beta-hydroxybutyrate (the dominant ketone in severe cases), also highlight the evolution of clinical tools toward more comprehensive analyses.
Objective
- To detect the presence of ketone bodies within the supplied urine sample.
Gerhardt’s test Principle
Gerhardt’s test works on the idea of a chemical interaction between ferric ions (Fe³⁺) and acetoacetic acid, a ketone body generated in aberrant metabolic conditions such as uncontrolled diabetes or malnutrition. Urine containing ferric chloride solution causes the iron in the reagent to interact especially with the enol tautomer, a reactive form, of acetoacetic acid. This reaction creates a persistent, water-soluble iron-acetoacetate complex with a clearly reddish-brown or burgundy color. The test depends on this color change as a visual clue of ketone content. Still, the process takes place in an acidic environment—which urine naturally maintains—which guarantees the enol form of acetoacetic acid stays active for binding. Its specificity is a major drawback; it does not react with other ketones such as beta-hydroxybutyrate, more plentiful in severe ketoacidosis. False positives can also be produced by drugs include phenols or salicylates, aspirin metabolites, which mimic this response. Urine samples are occasionally cooked ahead of time to solve this; acetoacetic acid is stable under heat, whereas interfering chemicals may break down and increase dependability. Though mostly out-of-date, the test’s concept emphasizes early diagnosis techniques based on observable biochemical interactions, in contrast to today’s enzyme-based dipstick techniques that detect several ketone forms with greater accuracy. Knowing this process helps one to appreciate previous clinical methods and the development of diagnostic specificity.
Gerhardt’s test reaction
- When 10% ferric chloride (FeCl₃) is added to urine, the Fe³⁺ ions react with the enol tautomer of acetoacetic acid.
- This forms a water-soluble ferric-acetoacetate complex, resulting in a burgundy-red or port wine color
- Gerhardt’s test detects acetoacetic acid in urine by reacting with ferric chloride (FeCl₃), producing a port wine or Bordeaux red hue.
- The reaction creates a colorful complex between ferric ions (Fe³⁺) and acetoacetic acid, signifying ketone molecules.
- Acetone does not react with ferric chloride, therefore acetoacetic acid decomposes into acetone and carbon dioxide when heated. The red hue disappears while boiling, confirming its presence.
- It only detects acetoacetic acid, not other ketone molecules as acetone or β-hydroxybutyrate.
- Salicylates, which form colorful complexes with ferric chloride and do not disintegrate when heated, might cause false positives.
Requirements For Gerhardt’s test
- Reagents:
- Ferric chloride solution (10%): This is the primary reagent. The iron (Fe³⁺) ions in the solution react with acetoacetic acid to form a reddish-brown complex.
- Distilled water: Used for dilution or preparing controls if needed.
- Sample Preparation:
- Fresh urine sample: Acetoacetic acid degrades over time, so a freshly collected urine specimen is critical. Stale samples may yield false negatives.
- Boiling setup: A heat source (e.g., Bunsen burner, hot plate) and test tubes are required to boil the urine. Boiling helps eliminate interfering substances like salicylates (e.g., aspirin metabolites), which can cause false positives. After boiling, the sample is cooled before testing.
- Equipment:
- Test tubes or containers: To hold the urine sample during heating and testing.
- Pipettes/droppers: For precise addition of ferric chloride to the urine.
- Color comparison tools: A white background or reference chart to accurately observe color changes.
- Safety Measures:
- Protective gear: Gloves and goggles to avoid contact with chemicals or biohazardous urine.
- Ventilation: Ensure proper airflow if open flames (e.g., Bunsen burner) are used for boiling.
- Procedural Considerations:
- Control testing: Running a parallel test with a known negative sample helps validate results.
- Medication history: Awareness of the patient’s drug use (e.g., aspirin, phenothiazines) is crucial, as certain compounds mimic acetoacetic acid’s reaction.
- Limitations Awareness:
- Gerhardt’s test does not detect beta-hydroxybutyrate, the predominant ketone in severe ketoacidosis. Clinicians must pair results with clinical context or modern tests (e.g., nitroprusside dipsticks) for a complete assessment.
Gerhardt’s test Procedure
- To prevent contamination and guarantee sample integrity, gather 3–5 ml of fresh mid‑stream urine in a sterile glass test tube.
- Using a pipette or dropper, dropwise add 5 ml of 10% ferric chloride solution to the urine sample to enable regulated response.
- See a port wine or Bordeaux red tint developing right away to signal acetoacetic acid presence.
- To break down acetoacetic acid and verify specificity, gently heat over a Bunsen burner or place the test tube in a water bath for five minutes.
- Let the solution cool and track the color change; While persistence points to salicylate interference, fading of the red hue indicates acetoacetic acid.
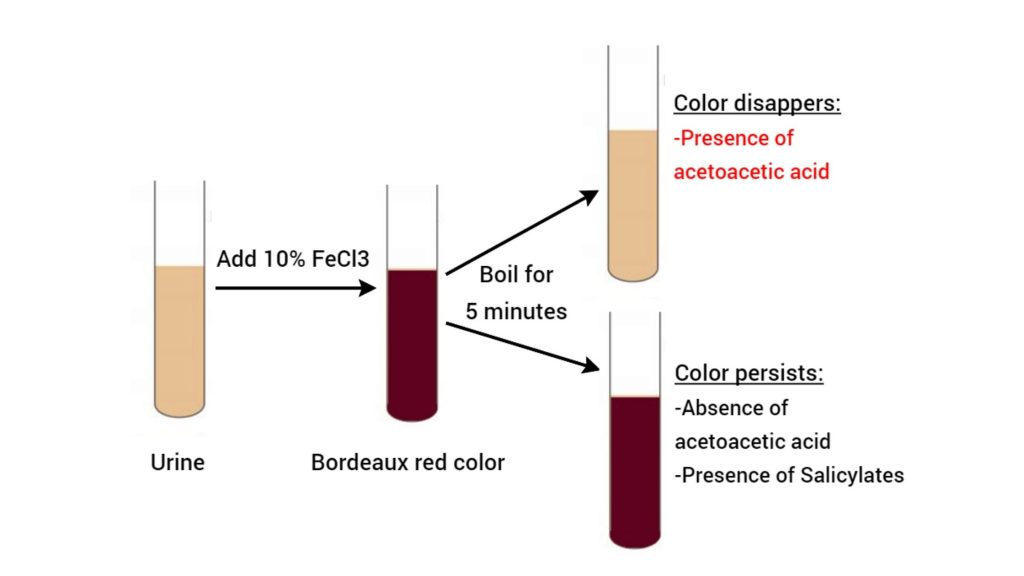
Observations and Results of Gerhardt’s test
When performing Gerhardt’s test, the key observation revolves around a color change in the urine sample after adding ferric chloride (FeCl₃). Here’s how results are interpreted:
- Gerhardt’s test Positive Result:
- A burgundy or reddish-brown color develops immediately or within a few minutes of adding the reagent. This indicates the presence of acetoacetic acid, a ketone body.
- Clinical Relevance: A positive result suggests conditions like diabetic ketoacidosis, starvation, or metabolic stress where the body breaks down fats for energy, releasing ketones into the urine.
- Gerhardt’s test Negative Result:
- No color change (urine remains pale yellow) or a transient color that fades quickly. This implies no detectable acetoacetic acid in the sample.
- False Positives:
- Certain substances can mimic acetoacetic acid’s reaction, such as salicylates (aspirin metabolites), phenols, or drugs like levodopa. These produce similar reddish hues, complicating interpretation.
- Confirmation Step: To rule out false positives, the urine sample is boiled and cooled before retesting. Acetoacetic acid is heat-stable, so the color persists post-boiling. Interfering compounds (e.g., aspirin byproducts) often degrade, eliminating the false signal.
- False Negatives:
- Degraded samples: Acetoacetic acid breaks down over time, especially in improperly stored urine. Fresh samples are critical.
- Low concentration: The test is less sensitive to trace amounts of acetoacetic acid compared to modern dipstick methods.
Key Limitations:
- Beta-hydroxybutyrate (BHB): Gerhardt’s test does not detect BHB, the primary ketone in severe ketoacidosis. A negative result does not rule out dangerous ketosis if BHB dominates.
- Subjectivity: Color interpretation can vary between observers, requiring careful comparison against controls or standardized charts.
Uses of Gerhardt’s test
- Detects acetoacetic acid in urine to diagnose ketonuria.
- Assists in identifying diabetic ketoacidosis by detecting elevated ketone levels.
- Monitors metabolic disturbances during prolonged fasting or starvation.
- Evaluates patients on high-fat, low-carbohydrate diets for ketosis.
- Differentiates between types of ketone bodies in urine analysis.
- Serves as a qualitative test for acetoacetic acid in clinical laboratories.
Limitations of Gerhardt’s test
- Only detects acetoacetic acid, not beta-hydroxybutyrate and acetone, which are common ketone bodies in diabetic ketoacidosis.
- Ketone concentrations below 25–50 mg/dL may not be detected, causing false-negative findings in early or moderate ketosis.
- Due to similar color responses, salicylates, antipyrines, and para-aminosalicylic acid might cause false positives.
- Phosphates can precipitate and conceal color change, requiring cautious interpretation.
- Not suited for identifying ketosis in extended fasting or alcohol-induced ketoacidosis patients when beta-hydroxybutyrate dominates.
- The urine sample must be boiled, which might degrade acetoacetic acid to acetone and lower test accuracy.
- Less accurate than enzymatic assays and dipstick tests, which are more sensitive and specific for all ketone bodies.
Precautions For Gerhardt’s test
- To avoid errors, clean all glassware, especially test tubes, before and after the experiment.
- To avoid chemical burns and inhalation, use gloves and safety goggles when handling all chemicals, especially concentrated nitric acid and ferric chloride.
- To prevent contamination, use pipettes or droppers to transfer liquids and use disposable gloves while handling urine samples.
- Heat samples in test tube holders to avoid burns and guarantee stability during boiling.
- Ketone bodies decompose or get contaminated during storage, decreasing test accuracy. Use freshly voided urine samples.
- Salicylates can cause similar color changes, causing false-positive readings.
- If testing is not possible immediately, store urine samples in tight containers and refrigerate to reduce ketone body loss from evaporation or bacteria.
- To maintain laboratory safety and prevent environmental contamination, dispose of chemical reagents and biological samples according to institutional guidelines.
- For proper interpretation and replication, scrupulously record all observations and any deviations or abnormalities.
- Understand that Gerhardt’s test only detects acetoacetic acid and may miss other ketone bodies like beta-hydroxybutyrate, requiring further tests for complete analysis.
FAQ
What is Gerhardt’s test?
Gerhardt’s test is a laboratory test used to qualitatively detect the presence of ketone bodies in urine.
What are ketone bodies?
Ketone bodies are a byproduct of fat metabolism and include acetone, acetoacetic acid, and beta-hydroxybutyrate.
What can cause ketonuria?
Ketonuria, the presence of excess ketone bodies in urine, can be caused by conditions such as starvation, uncontrolled diabetes, persistent vomiting, and a high-fat, low-carbohydrate diet.
How does Gerhardt’s test work?
Gerhardt’s test is a type of nitroprusside test that detects acetoacetic acid in urine. It is not considered very sensitive, as it can only detect levels of acetoacetic acid between 25 to 50 mg/dl.
What is the relationship between ketosis and diabetic ketoacidosis?
Ketosis is the presence of ketone bodies in the body, while diabetic ketoacidosis is a condition where the body produces excess ketone bodies due to uncontrolled diabetes. Ketosis can also occur due to starvation, a high-fat, low-carbohydrate diet, or persistent vomiting.
What is the color formed when ferric chloride reacts with acetoacetic acid in Gerhardt’s test?
When ferric chloride reacts with acetoacetic acid in Gerhardt’s test, a port wine or Bordeaux red color is formed.
Can acetone be detected using Gerhardt’s test?
No, acetone cannot be detected using Gerhardt’s test as it does not react with ferric chloride.
What happens to acetoacetic acid when it is boiled during Gerhardt’s test?
When acetoacetic acid is boiled during Gerhardt’s test, it loses carbon dioxide and transforms into acetone.
What does the disappearance of the color during Gerhardt’s test indicate?
The disappearance of the color during Gerhardt’s test indicates that acetoacetic acid is present within the sample being tested.
What is the significance of the color disappearance in a urine test?
The disappearance of color in a urine test indicates the presence of Acetoacetic acid in the urine sample.
What does a persistent color mean in a urine test?
The persistence of color in a urine test indicates the absence of Acetoacetic acid in the urine sample.
Can Acetoacetic acid be found in a negative urine test result?
No, Acetoacetic acid cannot be found in a negative urine test result, as the persistent color indicates its absence.
Is the disappearance of color the only indicator of Acetoacetic acid in a urine test?
Yes, the disappearance of color is the only indicator of Acetoacetic acid in a urine test, as per the given text.
What are the possible reasons for a false negative result in a urine test?
The possible reasons for a false negative result in a urine test could be an error in the testing process, such as not following the instructions correctly, or a low concentration of Acetoacetic acid in the urine sample.
- https://medicalstudyzone.com/gerhards-test-ketone-bodies/
- https://laboratorytests.org/gerhardts-test/
- https://www.histopathology.guru/test-for-ketone-bodies-in-urine/
- https://labpedia.net/diabetic-ketoacidosis-and-ketone-bodies-and/
- https://laboratoryintern.com/gerhardts-test-explained/
- https://laboratorytests.org/rotheras-test/
Thanks ,the content has been helpful to writing my report and being on top