The Gelatin Hydrolysis Test is a biochemical method used to identify if a bacterium can produce gelatinase enzyme. It is the process in which gelatin, a protein derived from collagen, is hydrolyzed into smaller peptides and amino acids, and these smaller molecules is easily absorbed by the bacterial cells. Gelatin molecules are large, so bacteria that use this nutrient secrete extracellular gelatinase to break it down, and this is referred to as gelatin liquefaction.
It is an important test in microbiology because it helps in distinguishing different bacterial species, and among the important example is the differentiation of Staphylococcus aureus which produces gelatinase, from Staphylococcus epidermidis which does not. Some other bacteria like Serratia, Proteus and Bacillus also show positive reactions.
In this test, the organism is inoculated into nutrient gelatin medium and incubated, and the tubes is often kept for several days because gelatin hydrolysis can be slow. It is the process where temperature control is important since gelatin melts above 25°C naturally, so the final observation is always done after placing the tubes in a refrigerator.
If the medium remains liquid even after cooling, the gelatin is hydrolyzed and the reaction is positive. If the medium becomes solid again on cooling, then gelatinase is absent and the reaction is negative. There is also a plate method where the colonies are grown on agar and then a reagent such as ammonium sulfate is added, and in this step a clear zone around the colony indicates hydrolysis.
Purpose of gelatin hydrolysis test
There are the different purpose of the Gelatin hydrolysis test such as;
- To detects the ability of bacteria to produce gelatinases.
- To identify the Serratia, Pseudomonas, Flavobacterium, and Clostridium.
- To distinguishes the gelatinase-positive, pathogenic Staphylococcus aureus from the gelatinase-negative, nonpathogenic Staphylococcus epidermidis.
- To detect the Gram-positive, spore-forming, rod-shaped, aerobic or anaerobic bacteria such as Bacillus anthracis, B. cereus, B. subtilis, Clostridium perfringens and C. tetani.
- Used to differentiate genera of gelatinase-producing bacteria such as Serratia and Proteus from other members of the family Enterobacteriaceae.
Principle of Gelatin hydrolysis test
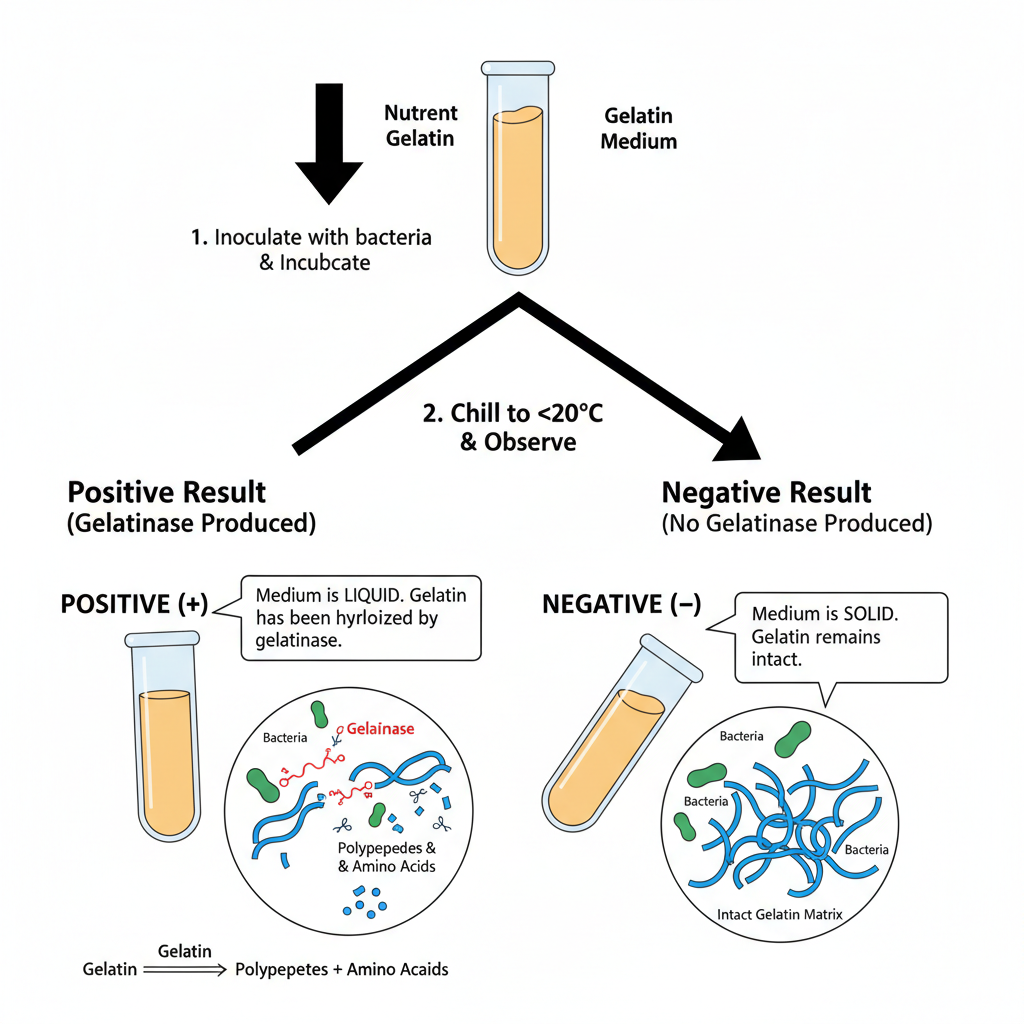
Gelatin Hydrolysis Test is based on the ability of some bacteria to produce extracellular enzyme called gelatinase. This enzyme hydrolyzes gelatin into smaller units. Gelatin is a protein derived from collagen in animal connective tissue. The molecules of gelatin is large and cannot pass through bacterial cell membrane. Bacteria secrete gelatinase to break down gelatin outside the cell. It is the process where peptide bonds in gelatin is cleaved. This results in formation of polypeptides and amino acids. These smaller products is absorbed by bacteria for nutrition and metabolism.
In the test, nutrient gelatin medium is used which solidifies at low temperature. The medium contains gelatin as solidifying agent. It forms gel below 20–25°C. When gelatinase is produced, it acts on gelatin in the medium. The hydrolysis destroys the structure of gelatin. The medium loses its ability to form gel. After incubation, tubes is chilled in refrigerator. If hydrolysis occurs, medium remains liquid even after cooling. This is referred to as positive result. If no gelatinase is produced, medium resolidifies upon cooling. This indicates negative result.
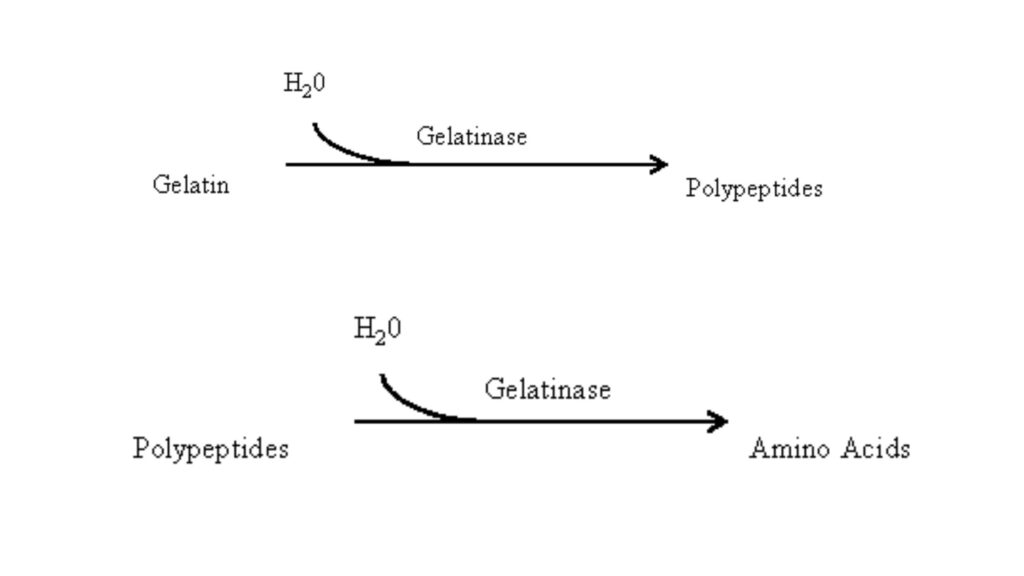
The reaction is as follows– gelatin + gelatinase → polypeptides + amino acids. The change in physical state of medium is observed visually. Some bacteria shows slow hydrolysis over several days. The test distinguishes gelatinase positive organisms from negative ones.
Microorganisms Tested in Gelatin Hydrolysis Test
Gelatin Hydrolysis Test is used to detect gelatinase production in various bacteria. The test differentiates organisms based on liquefaction of medium. Some microorganisms is positive while others is negative.
Gelatinase Positive Microorganisms These bacteria produce gelatinase enzyme. The medium liquefies after incubation and cooling.
- Staphylococcus aureus: This pathogen produces gelatinase. It distinguishes from non-pathogenic species.
- Bacillus species: Several endospore forming rods is positive.
- Bacillus anthracis (causes anthrax)
- Bacillus cereus
- Bacillus subtilis
- Clostridium species: Anaerobic spore formers shows strong hydrolysis.
- Clostridium perfringens (causes gas gangrene)
- Clostridium tetani
- Enterobacteriaceae (some genera): Used for differentiation.
- Serratia species (e.g., Serratia marcescens, Serratia liquefaciens)
- Proteus species (e.g., Proteus vulgaris, Proteus mirabilis)
- Pseudomonas species:
- Pseudomonas aeruginosa
- Pseudomonas fluorescens (differentiates from P. putida)
- Enterococcus faecalis: Some strains produce GelE gelatinase. It relates to virulence.
- Flavobacterium: This genus produces gelatinases.
Gelatinase Negative Microorganisms These organisms do not produce gelatinase. The medium resolidifies after cooling.
- Staphylococcus epidermidis: Used as negative control for S. aureus.
- Escherichia coli: Common Enterobacteriaceae member shows no hydrolysis.
- Pseudomonas putida: Differentiates from P. fluorescens.
- Enterobacter aerogenes: Typically negative in the test.
Requirement
- Media
- Nutrient Gelatin Medium (Stab Method)
- Gelatin 120 g/L (this is the substrate).
- Peptone 5 g/L.
- Beef extract 3 g/L.
- Distilled water (1000 mL).
- pH is adjusted to 6.8 (± 0.2) at 25°C.
- Nutrient Gelatin Agar (Plate Method)
- Nutrient agar base 23 g/L.
- Gelatin 8 g/L mixed in the base.
- Alternative formulation– tryptose 20 g, beef extract 3 g, gelatin 10 g, manganese sulfate 0.01 g, agar 15 g.
- Stone Gelatin Agar (Staphylococcus Medium 110) is also used for some organisms.
- Nutrient Gelatin Medium (Stab Method)
- Reagents
- Saturated ammonium sulfate solution (used to flood the agar surface for precipitation of unhydrolyzed gelatin).
- Mercuric chloride (HgCl₂) solution used earlier for precipitating gelatin.
- Tannic acid solution (used in some older plate protocols).
- Supplies and Equipment
- Inoculating needle (used for stabbing tubes).
- Inoculating loop (used for streaking plates).
- Test tubes (13 × 100 mm screw-cap tubes).
- Petri dishes.
- Pipettes.
- Incubator (kept at 35–37°C or at 25°C depending on organism).
- Refrigerator or ice bath (important for checking solidification after incubation).
- Control Strains
- Positive controls– Bacillus subtilis, Staphylococcus aureus, Serratia marcescens.
- Negative controls– Escherichia coli, Staphylococcus epidermidis.
Nutrient Gelatin Media Preparation Steps
Ingredients (For 1 litre medium)
These are the main ingredients used for preparing nutrient gelatin medium–
- Gelatin – 120 g (acts as substrate and solidifying agent).
- Peptone – 5 g.
- Beef extract – 3 g.
- Distilled water – 1000 mL.
- Final pH adjusted to 6.8 ± 0.2 at 25°C.
Preparation Steps
1. Mixing
It is the process where all dehydrated components (nearly 128 g) is suspended into 1000 mL distilled water. The ingredients are mixed to form a uniform suspension.
2. Dissolving
The mixture is heated gently with agitation. It is kept around 50°C because gelatin dissolves slowly and need warmth to go into solution. Sometimes it is boiled lightly for complete dissolution of commercial gelatin powder.
3. Dispensing
Around 2–3 mL of the medium is dispensed into clean test tubes (13 × 100 mm). These tubes are kept upright because these will form deep tubes after cooling.
4. Sterilization
The tubes are autoclaved at 121°C for 15 minutes (15 psi). During autoclaving, gelatin melts and forms a clear solution.
5. Cooling
The tubes are cooled in upright position. It is the step where the medium solidifies into a deep. No slants are prepared. The gelatin becomes firm after complete cooling.
6. Storage
The prepared tubes are stored at 2°C–8°C. These are not used if the medium shows contamination, drying or discoloration.
Nutrient Gelatin Agar (Plate Method)
This method uses agar to solidify the medium and gelatin concentration is low.
Ingredients
- Nutrient agar base – 23 g/L.
- Gelatin – 8 g to 10 g added per litre.
Preparation
It is the process where the agar base and gelatin is dissolved in distilled water, heated and autoclaved at 121°C for 15 minutes. The molten medium is poured into sterile Petri plates to solidify.
Procedure of Gelatin Hydrolysis Test

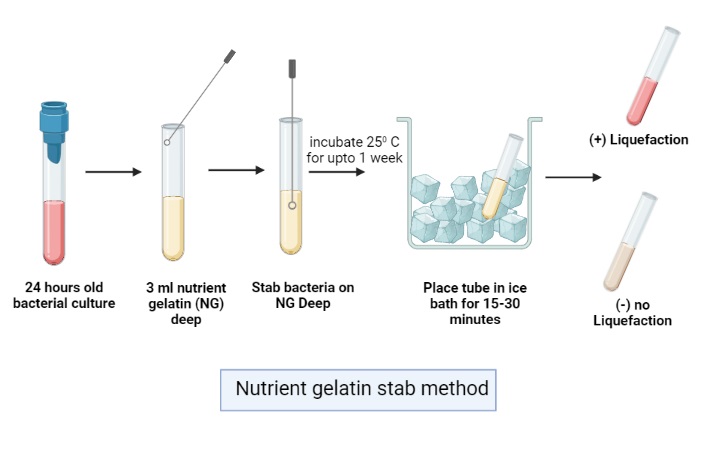
A. Nutrient Gelatin Stab Method
- Preparation and Inoculation
- A nutrient gelatin tube is taken which contains gelatin, beef extract and peptone.
- An inoculating needle is used to pick a heavy inoculum from 18–24 hours culture.
- It is stabbed into the centre of the medium reaching the bottom.
- Sometimes 4–5 drops of broth culture is also added for inoculation.
- Incubation
- The inoculated tube is incubated along with one uninoculated control tube.
- It is incubated at 35–37°C or even at 25°C depending on the organism.
- Incubation may continue for 24–48 hours but slow producers may need 14 days.
- Chilling Step
- Gelatin melts at above 25°C, therefore after incubation the medium remains liquid even without enzyme action.
- The tubes are kept in ice bath for 15–30 minutes or sometimes in refrigerator (4°C) for about 30 minutes.
- This step allow unhydrolysed gelatin to re-solidify.
- Observation and Interpretation– Tubes are tilted gently to see whether the medium is solid or liquid.
– Positive test– the medium remains liquid even after chilling but the control tube becomes solid.
– Negative test– solidification of gelatin is seen.
– Tubes are not shaken because hydrolysis starts near surface and shaking can mix liquid portion with solid portion below.
B. Nutrient Gelatin Plate Method
- Inoculation– A heavy inoculum is stabbed onto nutrient gelatin agar plate. This medium is nutrient agar containing around 8 g/L gelatin.
- Incubation– The plate is incubated at 35–37°C for 24–48 hours.
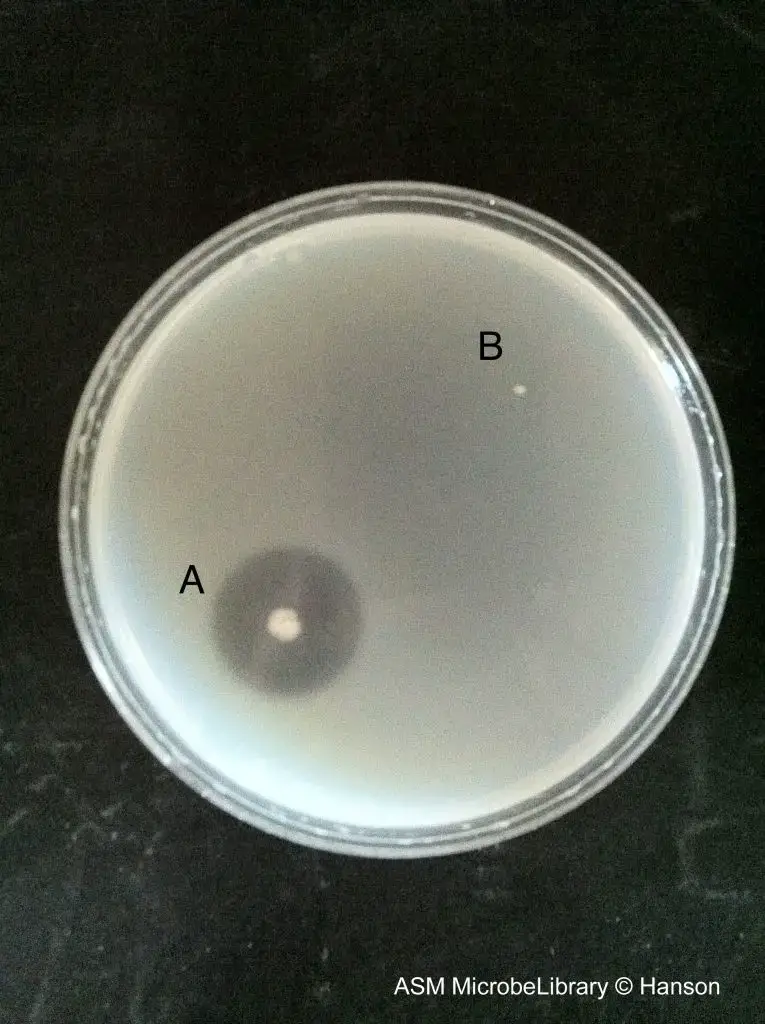
- Flooding (Visualization Step)– The plate is flooded with ammonium sulfate (saturated) or acidic mercuric chloride solution. These reagents form cloudy precipitate with unhydrolysed gelatin.
- Observation– Clear zones are seen around the colonies where gelatin is hydrolysed.
– Positive test– a clear halo appears against opaque background.
– Negative test– no clear zone is found and the medium stay uniformly opaque.
Result Interpretation of Gelatin Hydrolysis Test
A. Interpretation in Nutrient Gelatin Stab Method (Tube Test)
- Positive Result (Liquefaction)
- The medium remains liquid after the chilling step.
- It is the indication that gelatin is hydrolysed by gelatinase enzyme.
- The peptide bonds of gelatin is broken and the medium cannot re-solidify again.
- Negative Result (Solidification)
- The gelatin becomes solid after refrigeration or ice bath cooling.
- It shows that gelatin is intact and no enzyme activity is present.
- Cold Shock Requirement
- Gelatin melts at above 20–25°C so tubes removed from incubator always appears liquid.
- Tubes are kept in ice bath for 15–30 minutes (or refrigerator for 30 minutes) to confirm the actual state.
- If liquefaction is due to heat, the medium solidifies again. If due to enzyme action, it remains liquid.
- Control Tube
- The uninoculated control must solidify after chilling.
- If the control stays liquid then medium may be defective or the chilling time was not sufficient.
- Incubation Duration
- Slow gelatinase producers need long incubation sometimes up to 14 days.
- The final negative result is recorded only after repeated checking with proper chilling.
- Do Not Shake
- Tubes are not shaken because surface liquefaction is common.
- Mixing of liquefied portion with solid part may lead to false negative reading.
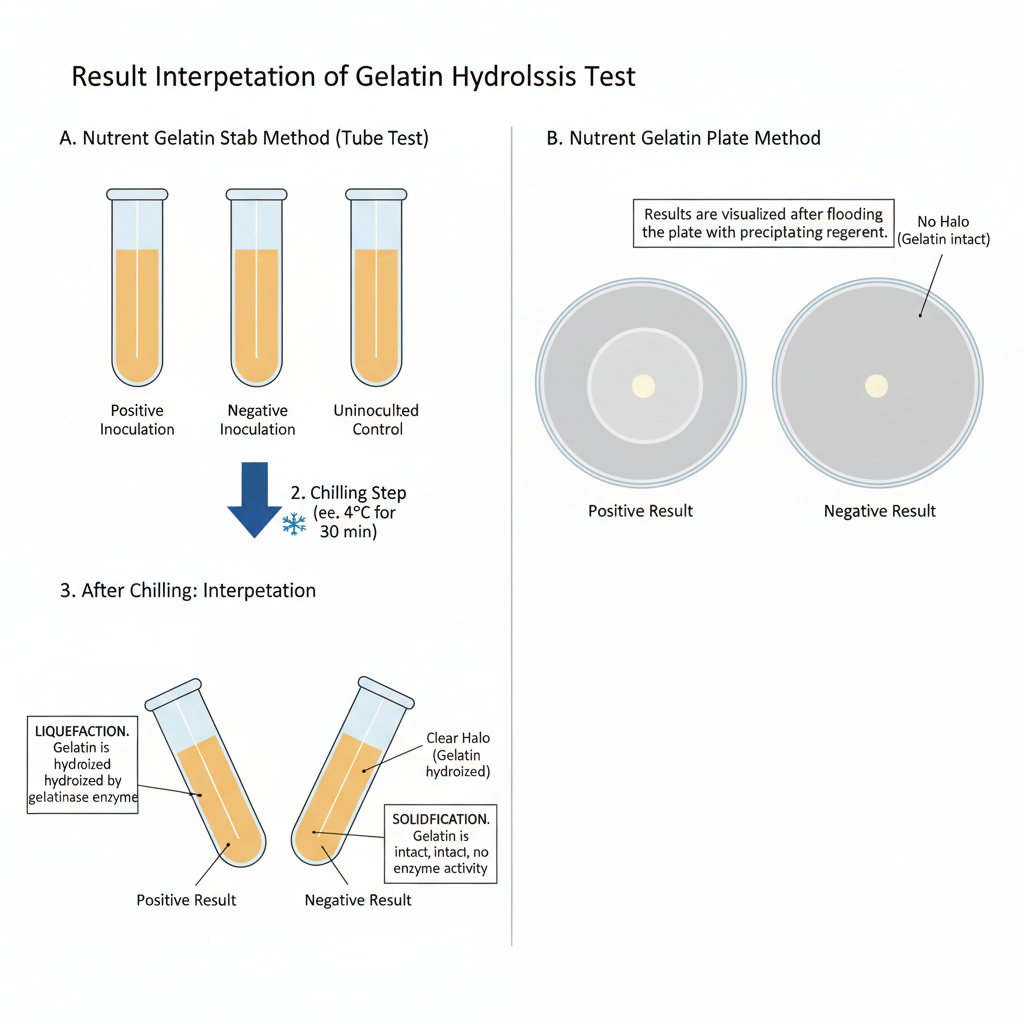
B. Interpretation in Nutrient Gelatin Plate Method
- Positive Result (Clear Zone)– A clear halo is formed around the colony after flooding with precipitating reagent. It is the region where gelatin is hydrolysed and cannot precipitate.
- Negative Result (Opaque Medium) – No clear zone is observed, medium stays uniformly opaque. Unhydrolysed gelatin forms cloudy precipitate with the reagent.
C. Important Points for Interpretation
- Halo Formation Only After Reagent– On plates the zone becomes visible only after flooding with ammonium sulfate or mercuric chloride.
- Consistency of Background– A uniformly opaque background confirms the gelatin is still present.
- Reliability Depends on Proper Control and Cooling – Correct interpretation is only possible when control tube and cooling step behave normally.
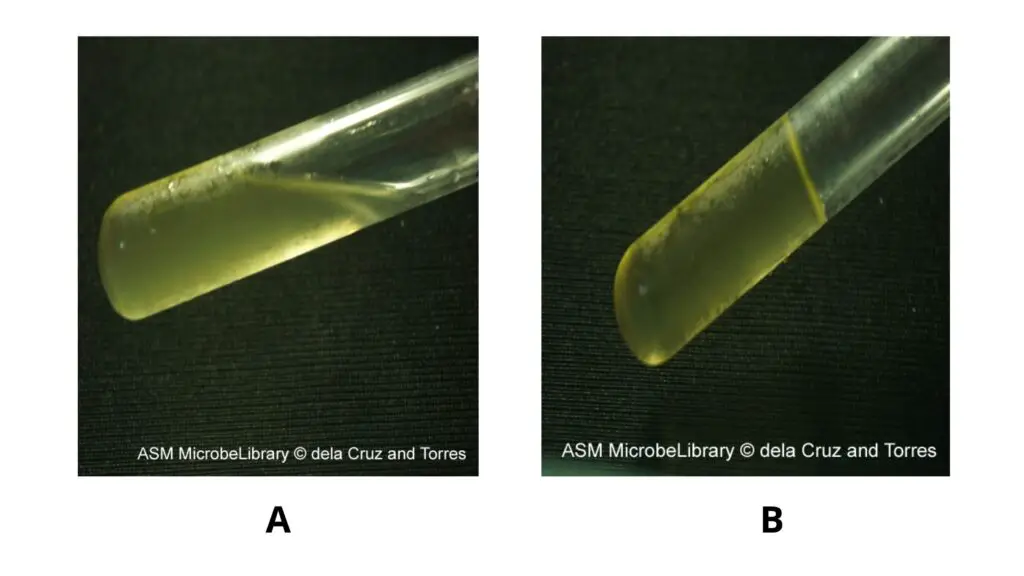
List of common bacteria and their reactions to the gelatin hydrolysis test performed on nutrient gelatin
| Species | Biosafety level | Growth | Liquefaction |
| Bacillus subtilis | 1 | + | + |
| Clostridium perfringens | 1 or 2 | + | + |
| Escherichia coli | 1 | + | – |
| Proteus vulgaris | 1 | + | + |
| Serratia liquefaciens | 1 | + | + |
| Staphylococcus aureus | 1 | + | + |
Quality Control of Gelatin hydrolysis test
For positive and negative Quality Control of Gelatin hydrolysis test, two organisms are used such as;
- Positive Quality Control: Bacillus subtilis
- Negative Quality Control: Escherichia coli
- Uninoculated control tube: medium becomes solid after refrigeration.
Uses of Gelatin Hydrolysis Test
- It is used to detect the ability of microorganisms to produce gelatinase enzyme.
- It is used for identifying bacteria that can hydrolyse gelatin into simple amino acids.
- It helps in differentiating Staphylococcus aureus from Staphylococcus epidermidis as the pathogenic form is gelatinase positive.
- It is used in separating gelatinase producing enteric bacteria like Serratia and Proteus from other members which usually shows negative result.
- It helps in distinguishing fluorescent Pseudomonas fluorescens from Pseudomonas putida as these species shows different gelatin reactions.
- It is used in identifying Gram-positive rods including Bacillus species (B. anthracis, B. cereus, B. subtilis) and Clostridium species (C. tetani, C. perfringens) which generally shows positive reaction.
- It is used to study the virulence characteristics of some organisms because production of gelatinase is linked with tissue invasion.
- It indicates the ability of pathogens to destroy collagen and spread in host tissues.
- It is used to understand biofilm forming ability of Enterococcus faecalis where gelatinase (GelE) is involved.
- It helps in studying immune evasion in some organisms because gelatinase can degrade important immune molecules.
- It is used to examine the translocation potential of bacteria like E. faecalis across the intestinal barrier.
Limitations of Gelatin Hydrolysis Test
- It is affected by temperature because gelatin melts above 20–25°C, so the medium becomes liquid even without enzyme activity.
- It cannot be read immediately after incubation at 35–37°C and the tubes must be cooled to observe true liquefaction.
- It requires long incubation for some organisms as gelatin hydrolysis is slow and results may take many days.
- It cannot be declared negative quickly because some species need more than a week to show liquefaction.
- It may produce false negative result when the tube is shaken while warm because the liquefied layer mixes with solid parts and solidifies again during cooling.
- It is not suitable for fastidious organisms as nutrient gelatin does not support their proper growth.
- It always requires an uninoculated control tube because gelatin quality varies and control must solidify after cooling.
- It is limited in plate method as some organisms, especially anaerobes and fastidious types, cannot be tested properly.
- It may require special reagents in plate method and older methods used toxic precipitating agents.
Advantages of Gelatin Hydrolysis Test
- It is useful for differentiating bacteria based on their ability to produce gelatinase enzyme.
- It helps in separating Staphylococcus aureus from Staphylococcus epidermidis because these species show different gelatin reactions.
- It is important for identifying Enterobacteriaceae members like Serratia and Proteus which usually shows positive result, while E. coli is negative.
- It is used for distinguishing Pseudomonas fluorescens from Pseudomonas putida as these species show opposite reactions.
- It helps in identifying spore-forming rods like Bacillus anthracis, B. cereus, and Clostridium perfringens.
- It indicates virulence because gelatinase breaks collagen and helps organisms to invade host tissues.
- It is useful for studying pathogenicity of organisms like Enterococcus faecalis where gelatinase is involved in biofilm formation and immune evasion.
- It does not require any reagent for interpretation in the stab method as liquefaction itself is the indicator.
- It is comparatively safe because no toxic chemicals are used in the stab method.
- It can give quick results in the plate method as halos appear within 24–48 hours.
- It shows higher sensitivity in plate method using precipitating agents like ammonium sulfate to detect small amounts of gelatinase activity.
FAQ
Q1. What is the Gelatin Hydrolysis Test?
It is a biochemical test used to determine whether an organism can produce gelatinase enzyme that hydrolyses gelatin into simpler compounds.
Q2. What is the purpose of the Gelatin Hydrolysis Test?
It is used to identify and differentiate microorganisms based on their ability to liquefy gelatin and to study their enzymatic activity.
Q3. What is the principle of the Gelatin Hydrolysis Test?
It is the process where gelatin is broken down by gelatinase into polypeptides and amino acids, and the medium becomes liquefied even after cooling.
Q4. How is the Gelatin Hydrolysis Test performed?
A nutrient gelatin medium is stabbed with the test organism and incubated. After incubation the tubes are cooled, and the solid or liquid state is observed.
Q5. How do you interpret the results of the Gelatin Hydrolysis Test?
If the medium remains liquid after cooling, it is considered positive. If the medium becomes solid again, it is negative.
Q6. What does a positive result in the Gelatin Hydrolysis Test indicate?
It indicates that the organism produces gelatinase enzyme and can hydrolyse gelatin.
Q7. What does a negative result in the Gelatin Hydrolysis Test indicate?
It indicates that the organism cannot hydrolyse gelatin and does not produce gelatinase enzyme.
Q8. Which bacteria are positive for the Gelatin Hydrolysis Test?
Bacteria like Staphylococcus aureus, Serratia, Proteus, Pseudomonas fluorescens, Bacillus anthracis, B. cereus, B. subtilis, and Clostridium perfringens show positive reactions.
Q9. What is gelatinase?
It is an extracellular proteolytic enzyme that breaks down gelatin (denatured collagen) into smaller peptides.
Q10. What is gelatin?
Gelatin is a protein derived from collagen found in connective tissues and is used as a solidifying agent in nutrient gelatin medium.
Q11. What are the uses of the Gelatin Hydrolysis Test?
It is used for differentiating species, identifying pathogenic organisms, studying virulence, and detecting gelatinase activity in microbes.
Q12. What media is used for the Gelatin Hydrolysis Test?
Nutrient gelatin medium is mainly used containing gelatin as the solidifying component.
Q13. What are the limitations of the Gelatin Hydrolysis Test?
It is temperature sensitive, requires long incubation, may give false negatives if shaken, does not support fastidious organisms, and needs a control tube for comparison.
Q14. Why is cooling important in the Gelatin Hydrolysis Test?
Cooling is required because gelatin melts at higher temperature and only true hydrolysis remains liquid after cooling.
Q15. What are the different methods for performing the Gelatin Hydrolysis Test?
Two main methods are used—the nutrient gelatin stab tube method and the nutrient gelatin plate method using precipitating agents.
- American Society for Microbiology. (2012, November 1). Protocols: Gelatin hydrolysis test. https://asm.org/protocols/gelatin-hydrolysis-test-protocol
- Aryal, S. (2022, August 10). Gelatin hydrolysis test – Principle, procedure, uses and interpretation. Microbiology Info. https://microbiologyinfo.com/gelatin-hydrolysis-test/
- Creemers, L. B., Jansen, I. D., Docherty, A. J., Reynolds, J. J., Beertsen, W., & Everts, V. (1998). Gelatinase A (MMP-2) and cysteine proteinases are essential for the degradation of collagen in soft connective tissue. Matrix Biology, 17(1), 35–46. https://doi.org/10.1016/s0945-053x(98)90123-8
- dela Cruz, T. E. E., & Torres, J. M. O. (2012, November 1). Gelatin hydrolysis test protocol. American Society for Microbiology. https://asm.org/protocols/gelatin-hydrolysis-test-protocol
Hartline, R. (2023, February 18). 1.25: Gelatin hydrolysis. Biology LibreTexts. https://bio.libretexts.org/Bookshelves/Microbiology/Microbiology_Laboratory_Manual_(Hartline)/01%3A_Labs/1.25%3A_Gelatin_Hydrolysis - Jupe, S. (2011, July 18). Gelatin degradation by MMP1, 2, 3, 7, 8, 9, 12, 13. Reactome Pathway Database. https://reactome.org/content/detail/R-HSA-1454757
- Kart, D., & Kuştimur, A. S. (2019). Investigation of gelatinase gene expression and growth of Enterococcus faecalis clinical isolates in biofilm models. Turkish Journal of Pharmaceutical Sciences, 16(3), 356–361. https://doi.org/10.4274/tjps.galenos.2018.69783
- Pickett, M. J., Greenwood, J. R., & Harvey, S. M. (1991). Tests for detecting degradation of gelatin: Comparison of five methods. Journal of Clinical Microbiology, 29(10), 2322–2325. https://doi.org/10.1128/jcm.29.10.2322-2325.1991
- Reynolds, J. (2024, February 6). 39: Gelatin hydrolysis. Biology LibreTexts. https://bio.libretexts.org/Learning_Objects/Laboratory_Experiments/Microbiology_Labs/Microbiology_Labs_I/39%3A_Gelatin_Hydrolysis
- Sapkota, A. (2022, January 27). Gelatin hydrolysis test- Principle, procedure, types, result, uses. Microbe Notes. https://microbenotes.com/gelatin-hydrolysis-test-principle-procedure-and-result-interpretation/
- Sierra College. (n.d.). Exercise 15-C: Physiological characteristics of bacteria continued: Gelatin liquefaction, TSI, SIM, urea hydrolysis and nitrate reduction [Lab manual]. https://biosci.sierracollege.edu/materials/4/laboratory_syllabus/gelatin_liquefaction_tsi_sim_urea_hydrolysis_and_nitrate_reduction.pdf
- Thurlow, L. R., Thomas, V. C., Narayanan, S., Olson, S., Fleming, S. D., & Hancock, L. E. (2010). Gelatinase contributes to the pathogenesis of endocarditis caused by Enterococcus faecalis. Infection and Immunity, 78(11), 4936–4943. https://doi.org/10.1128/IAI.01118-09
- VUMIE. (2022, June 6). Gelatin hydrolysis test. Virtual Microbiology Lab Simulator Software. https://vumicro.com/docs/gelatin-hydrolysis-test/
- Wikipedia. (n.d.). Gelatinase. Retrieved from https://en.wikipedia.org/wiki/Gelatinase
- Zeng, J., Teng, F., & Murray, B. E. (2005). Gelatinase is important for translocation of Enterococcus faecalis across polarized human enterocyte-like T84 cells. Infection and Immunity, 73(3), 1606–1612. https://doi.org/10.1128/IAI.73.3.1606-1612.2005