What is Gel Filtration Chromatography?
- Gel Filtration Chromatography can define as a separation technique which is based mainly on molecular size and sometimes shape of molecules in solution.
- It is used for separation of biomolecules like proteins, polysaccharides, enzymes etc. depending on their different hydrodynamic volume.
- The stationary phase is composed of porous beads made from materials like dextran, agarose, or polyacrylamide, which contain different pore sizes.
- Molecules are separated as they are passed by the column containing these beads, small molecules enters inside the pores while large molecules are excluded and elute faster.
- So the elution order is opposite to most chromatographic techniques, where small molecules elute last and large ones first.
- The mobile phase used is usually an aqueous buffer, which maintain pH and ionic strength suitable for the biological sample stability.
- No chemical interaction occurs between solute molecules and stationary phase; hence separation purely depends on size exclusion mechanism.
- This technique often used for determination of molecular weight, desalting of protein solutions, and for purification of macromolecules.
- Examples of matrices used are Sephadex G-75, Sephacryl S-200, and Superdex 75 etc., each with defined fractionation range.
- The process is simple and mild, because the biological activity of sample is not affected during separation, that’s why it is mostly applied in biochemical and biotechnological research.
- Flow rate and column dimension also affect the resolution, too high flow rate may cause overlapping of peaks or poor separation.
- Temperature sometimes also influence the viscosity of solvent and diffusion of molecules inside the gel pores.
- In short, Gel Filtration Chromatography is considered one of the most gentle and reliable methods for macromolecular separation and analysis.
Principle of Gel Filtration Chromatography – Gel Filtration chromatography principle
The principle of Gel Filtration Chromatography (GFC) can define as separation of molecules mainly depending by their molecular size / shape when passed by a porous gel medium.
The stationary phase used is made of porous beads (like dextran, agarose or polyacrylamide) that contain pores of defined diameters, which allow smaller molecules to enter while larger ones are excluded.
The separation occur because molecules of different sizes move by different paths inside the column, causing different elution times.
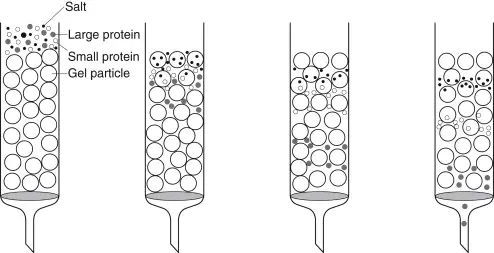
Large molecules cannot enter the pores, so they are excluded from gel and travel around the beads, they elute first from column.
Smaller molecules penetrate into many pores, resulting in longer paths, therefore they elute later.
Molecules of intermediate size enter partially into pores, hence elute between large and small molecules, showing gradual separation pattern.
The mobile phase, usually buffer or solvent, flows continuously through the column carrying molecules according to their exclusion behavior.
No adsorption or ionic interaction happen between solute and stationary phase, that’s why separation is purely based on size exclusion mechanism.
The process depends on hydrodynamic volume not exactly on molecular weight, though often correlated closely.
The elution volume (Ve) for each molecule is determined, which relates with their size, and can used to estimate molecular mass by calibration curve.
Within the same experimental condition, molecules with similar size elute together forming single peak on chromatogram.
The principle therefore rely on differential penetration of molecules into gel pores depending by their dimension and shape.
It’s a very gentle method, as no chemical modification of sample occur, thus biological activity of proteins / enzymes etc. is maintained.
In short, Gel Filtration Chromatography separate molecules only on basis of their physical size differences under mild condition without affecting their nature.
Components of Gel Filtration Chromatography
- Column – It is usually made of glass or sometimes steel, which used for holding the stationary phase. The column length and diameter depend on separation requirement, sometimes longer column gives better resolution but slow speed.
- Stationary Phase / Matrix – The matrix consist of porous beads like Sephadex, Sepharose, or Bio-Gel P, which act as sieving material. The pore size determines which molecules can enter or excluded, so correct gel must be chosen for molecular weight range.
- Mobile Phase – A buffer solution is used for eluting the sample, it maintains pH and ionic strength for stability of biomolecules. Sometimes distilled water or phosphate buffer are used (pH 7.0–7.4) depending by sample type.
- Sample Injector – By this part, the sample solution introduced into top of column, usually by syringe or injector loop. Care is taken to avoid disturbing the gel surface else band broadening occur.
- Detector – A UV–Visible detector mostly used for detecting eluted components by measuring absorbance at specific wavelength (like 280 nm for proteins). Some systems may have refractive index detectors or conductivity sensors etc.
- Recorder / Data System – The output from detector transmitted to recorder or computer, producing chromatogram peaks that represent separated molecules.
- Fraction Collector – The effluent from column collected into small tubes or vials at fixed time intervals (or volume). Each fraction may contain different molecular size components, which analyzed later.
- Pump – In modern system, peristaltic or piston pump is used to maintain constant flow rate of mobile phase by column, ensuring reproducible elution time.
- Reservoir – The solvent/buffer stored in reservoir flask or bottle, from which it continuously supplied to the pump and then to column. Sometimes degassed before use to avoid air bubble formation.
- Support Stand / Clamp – The column usually fixed vertically on stand or frame to maintain stable operation, any vibration can affect flow pattern and separation efficiency.
Types of Gel Filtration Chromatography
1. Analytical Gel Filtration Chromatography –
This type mainly used for studying purity, molecular weight determination, and structural properties of biomolecules.
In this method, small amount of sample is applied to the column and separation is based on molecular size / shape differences.
The elution volume measured precisely, which helps for estimation of molecular weight by calibration curve.
It’s mostly used in biochemical analysis, protein folding study and for checking aggregation state of macromolecules.
2. Preparative Gel Filtration Chromatography –
This type used when large amount of sample need to be separated or purified for further experimental use.
Columns are usually larger, and flow rate is maintained higher but still controlled to avoid band broadening.
The aim here is to collect fractions which contain pure and active biomolecules without denaturation.
Mostly used for purification of proteins, enzymes, nucleic acids etc., for biochemical / pharmaceutical work.
3. Group Separation Chromatography –
In this type separation is done between two groups of molecules having very large difference in size.
For example, separation of salts from proteins or removal of small buffer components.
The smaller molecules get entered into pores of gel while larger molecules excluded and eluted earlier.
This method usually called as desalting or buffer exchange chromatography also.
4. Fractionation Chromatography –
In this type molecules are separated having slight differences in molecular weight or hydrodynamic radius.
High resolution gels (like Sephacryl S-300 or Superdex 200) used for fine separations.
Used for complex protein mixture or polymer molecular weight distribution determination.
Each type of Gel Filtration Chromatography has been designed according to requirement of separation scale, resolution, and sample nature etc.
Steps of Gel Filtration Chromatography
1. Preparation of Gel Matrix – In this step, the gel (like Sephadex, Sepharose, or Agarose) is allowed to swell by soaking in buffer or distilled water for few hours till complete hydration. The swollen gel is then degassed to remove trapped air bubbles, because bubbles disturb the flow and separation efficiency.
2. Column Packing – The gel slurry prepared earlier is carefully poured into the chromatography column, usually with gentle stirring or slow flow of buffer to avoid channel formation. The bed is allowed to settle uniformly, and excess liquid drained until compact packing achieved.
3. Equilibration of Column – The packed column is washed / equilibrated with several column volumes of buffer to establish steady state conditions similar to that of sample environment. The pH and ionic strength of the equilibration buffer must be same as the sample buffer to prevail unwanted interactions.
4. Sample Application – The sample mixture containing molecules of different sizes is carefully layered on top of the gel bed without disturbing it. Small sample volume (less than 5% of bed volume) gives sharper resolution, large volume may cause band broadening.
5. Elution / Separation – Buffer (mobile phase) is continuously added on the top, and fractions start to elute according to size of molecules. Large molecules cannot enter into gel pores, so they elute first, while small molecules enter into the pores and elute later. Elution carried out under gravity flow or by using peristaltic pump for constant flow rate.
6. Detection / Fraction Collection – The eluted fractions collected at regular intervals, and absorbance usually measured at 280 nm for proteins. Each fraction analyzed separately to identify which one contain desired molecule.
7. Regeneration and Storage of Column – After experiment, column washed thoroughly with buffer or distilled water to remove remaining substances. Sometimes it stored in buffer containing small amount of preservative like sodium azide (0.02%) to avoid microbial growth.
These are the main steps in Gel Filtration Chromatography procedure, where separation mainly depends on molecular size difference without any chemical interaction.
Factors affection on Resolution of gel filtration
Particle Size of Gel – Resolution is highly affected by the particle size of the gel beads, smaller particles give better separation but they also increase column pressure and slow flow rate, which sometimes cause band spreading.
Column Length – Longer column provides more separation distance, so molecules get better resolved, though it also prolongs elution time. A shorter one may reduce resolution drastically.
Flow Rate – If the mobile phase flow too fast, the molecules have not enough time for equilibrium between inside and outside pores, leading to poor resolution. When flow is very slow, diffusion may occur that cause band broadening.
Sample Volume – The injected sample volume should be small (about 1–5% of total bed volume), large sample load decrease resolution because of overlapping zones between components.
Pore Size of Gel / Fractionation Range – The proper selection of gel with suitable pore size is crucial, if pores are too large or too small for molecular size range then proper separation not achieved.
Temperature – Temperature affect viscosity of solvent and diffusion rate, higher temperature reduce viscosity but may denature biomolecules like proteins, so optimal condition must maintained (around 25°C mostly).
Buffer Composition – Ionic strength and pH of buffer influence interaction of sample with gel, though gel filtration mainly size-based, weak ionic interactions can still occur if buffer not optimized.
Column Packing – Improperly packed column cause channeling or uneven flow, which lower resolution. The gel should be uniformly settled without air bubbles or cracks.
Diffusion and Dispersion Effects – During passage through column, molecular diffusion and eddy diffusion both reduce the sharpness of peaks, leading to poor resolution of components.
Viscosity of Sample – Highly viscous samples (like concentrated protein or polysaccharide) slow down migration and cause uneven flow, which prevail proper separation.
Elution Volume – The difference between elution volumes of solutes decide the resolution, so accurate control of flow rate and collection fraction is important.
Column Diameter – For analytical separation small diameter is preferred, while for preparative purpose wider column used but may reduce efficiency due to uneven flow path.
Applications of Gel Filtration Chromatography
- Gel filtration chromatography is widely applied for protein purification, where the separation of proteins is done according to their molecular size / shape.
- It is used for desalting and buffer exchange, since small salts or buffer molecules are allowed to enter the pores, while large protein molecules are excluded from it.
- This method is applied for molecular weight determination of biological macromolecules, the elution volume is correlated with log of molecular weight.
- In enzyme study, it’s often used to determine the quaternary structure, for example whether enzyme exists as monomer or dimer etc.
- Separation of biopolymers like polysaccharides, nucleic acids, lipoproteins are performed by this technique effectively.
- Gel filtration has been used in removal of aggregated proteins, which may form during storage or by denaturation.
- It is also used by researchers for fractionation of serum proteins, which help in diagnostic or biochemical analysis.
- Within the field of biotechnology, it’s employed for purification of recombinant proteins after expression in E. coli or yeast system.
- The technique is applied for analysis of protein–ligand interactions, since it allows separation of free ligand from the complexed form easily.
- For virus purification, gel filtration is used to separate viral particles from smaller cellular debris or unreacted chemicals.
- In pharmaceutical industry, it is used for quality control, to check the aggregation state and stability of biologics.
- Such as, in production of monoclonal antibodies (mAbs), gel filtration helps to remove unwanted fragments or dimers.
- It is sometimes used to study denaturation/renaturation process of proteins, by analyzing their size change upon refolding.
- Peptide purification and removal of low molecular weight impurities are done through small scale gel filtration columns.
- For chromatography calibration, gel filtration columns are used with standard proteins to plot calibration curve (Kav vs log MW).
- In food chemistry, separation of polysaccharides and oligosaccharides fractions is done by this technique for composition study.
- It has been used for liposome purification where large vesicles are separated from unencapsulated drug molecules.
- Gel filtration is useful for radiolabeled compound purification, removing unbound isotope from the labeled macromolecule.
- For DNA–protein complex study, it helps to separate bound and free DNA fragments effectively.
- In general, it’s a very flexible and sturdy method, suitable for both analytical and preparative purpose, though resolution sometimes depend by column length, flow rate and particle size of gel.
Advantages of Gel Filtration Chromatography
- It can allow biomolecules to be separated under very mild conditions so that their activity is preserved and intact, the buffer / solvent conditions can be varied freely.
- Sample loss is minimised because the stationary matrix is inert and there is no strong binding of target molecules (so recovery is high).
- It may be used for rapid desalting or buffer-exchange, and this is much faster than dialysis (minutes vs hours) for certain low-molecular-weight contaminants.
- Narrow elution bands are produced which means less dilution of the target molecule and better sensitivity when downstream work is required.
- It is robust and flexible so that a wide variety of solvent/ buffer/ ionic-strength/ temperature conditions can be used without major effect on resolution.
- Large molecules, aggregates or complexes are excluded from pores and elute early so you can separate size-based groups in a pretty straightforward manner.
- It does not require ligand or charge interactions (unlike affinity or ion-exchange methods) so fewer changes in sample conditions are needed, making downstream steps simpler.
Limitations of Gel Filtration Chromatography
- It is often found that the resolution is rather low when molecules have very similar size, the peaks are broad and overlap easily.
- Large sample volume loadings cannot be used effectively because the sample‐to‐column bed volume ratio must be small for acceptable separation, thus throughput is limited.
- Dilution of the target molecule often occurs as the eluent volume is large relative to the sample, making downstream concentration steps necessary.
- The molecular weight range over which good separation occurs is narrow, so molecules outside the fractionation range will elute poorly or as single lumps.
- The technique does not give information about chemical structure or composition, only gives size‐based separation; so further methods will be needed for full characterization.
- It is sensitive to hydrodynamic shape of molecules: if a molecule is non‐globular then it may elute anomalously, and they may mis‐estimate molecular weight when assuming globular shape.
- Sample viscosity, particulates or aggregates may hamper performance (flow irregularities / bed packing issues) and such imperfections reduce performance.
FAQ
What is gel filtration chromatography?
Gel filtration chromatography, also known as size-exclusion chromatography, is a technique used for the separation and purification of biomolecules based on their size. It relies on the differential partitioning of molecules between a porous stationary phase (gel matrix) and a mobile phase (buffer) as they pass through a chromatography column.
How does gel filtration chromatography work?
In gel filtration chromatography, a gel matrix with pores of controlled size is packed into a column. When a sample containing molecules of different sizes is applied to the column, smaller molecules can enter the pores of the gel matrix, leading to slower elution, while larger molecules cannot enter the pores and are eluted first. Thus, the molecules are separated based on their size.
What is the purpose of gel filtration chromatography?
The main purpose of gel filtration chromatography is to separate and purify biomolecules based on their size. It is particularly useful for removing contaminants, separating different forms of biomolecules (e.g., aggregates, monomers), and obtaining fractions of specific sizes.
What are the advantages of gel filtration chromatography?
Gel filtration chromatography offers several advantages, including the ability to separate molecules under gentle conditions that maintain their stability and activity. It does not require any specific binding interactions, allowing for high recoveries of activity. Gel filtration chromatography is also relatively easy to perform, reliable, and scalable.
What are the limitations of gel filtration chromatography?
Despite its advantages, gel filtration chromatography has some limitations. It has lower resolution compared to other chromatographic techniques because none of the molecules are retained by the column. Proteolysis can be a problem when separating proteins, as the target protein can become a substrate for proteases present in the mixture, resulting in reduced recovery of activity. Gel filtration columns require large volumes of eluent, leading to higher running costs.
What types of biomolecules can be separated using gel filtration chromatography?
Gel filtration chromatography can be used to separate a wide range of biomolecules, including proteins, peptides, nucleic acids, carbohydrates, and lipids. It is particularly effective for separating proteins and peptides based on their molecular size.
What factors should be considered when choosing a gel filtration chromatography media?
Several factors should be considered when selecting a gel filtration chromatography media, including the fractionation range (size range of molecules that can be separated), size exclusion limit (maximum size of molecules that can enter the gel matrix pores), operating pressure, flow rate, sample viscosity, pH range, tolerance for organic solvents and additives, and operating temperature. The choice of media depends on the specific requirements of the separation and the properties of the biomolecules being studied.
Can gel filtration chromatography be used for preparative-scale purifications?
Yes, gel filtration chromatography can be scaled up for preparative-scale purifications. Larger columns with higher bed volumes are used to accommodate larger sample volumes and achieve higher yields of purified biomolecules.
Can gel filtration chromatography be used for analytical purposes?
Yes, gel filtration chromatography is commonly used for analytical purposes. It can provide information about the size distribution and oligomeric state of biomolecules, as well as their relative molecular masses.
Are there any alternative techniques to gel filtration chromatography?
Yes, there are alternative chromatographic techniques for biomolecule separation, such as ion exchange chromatography, affinity chromatography, hydrophobic interaction chromatography, and reversed-phase chromatography. The choice of technique depends on the specific properties of the biomolecules and the separation requirements.
- Prapulla, S.G. (2014). Encyclopedia of Food Microbiology || FERMENTATION (INDUSTRIAL) | Recovery of Metabolites. , (), 822–833. doi:10.1016/B978-0-12-384730-0.00109-9
- Stellwagen, Earle (2009). [Methods in Enzymology] Guide to Protein Purification, 2nd Edition Volume 463 || Chapter 23 Gel Filtration. , (), 373–385. doi:10.1016/S0076-6879(09)63023-8
- Evans, David R.H. (2009). [Methods in Enzymology] Guide to Protein Purification, 2nd Edition Volume 463 || Chapter 9 Concentration of Proteins and Removal of Solutes. , (), 97–120. doi:10.1016/S0076-6879(09)63009-3
- Ó’Fágáin, C., Cummins, P. M., & O’Connor, B. F. (2017). Gel-Filtration Chromatography. Methods in molecular biology (Clifton, N.J.), 1485, 15–25. https://doi.org/10.1007/978-1-4939-6412-3_2
- https://www.slideshare.net/asabuwangwa/gel-permeation-chromatography-gpc
- https://library.um.edu.mo/ebooks/b28050630.pdf
- https://www.slideshare.net/shovameghbalika/gel-chromatography
- https://kirschner.med.harvard.edu/files/protocols/GE_gelfiltration.pdf